Abstract
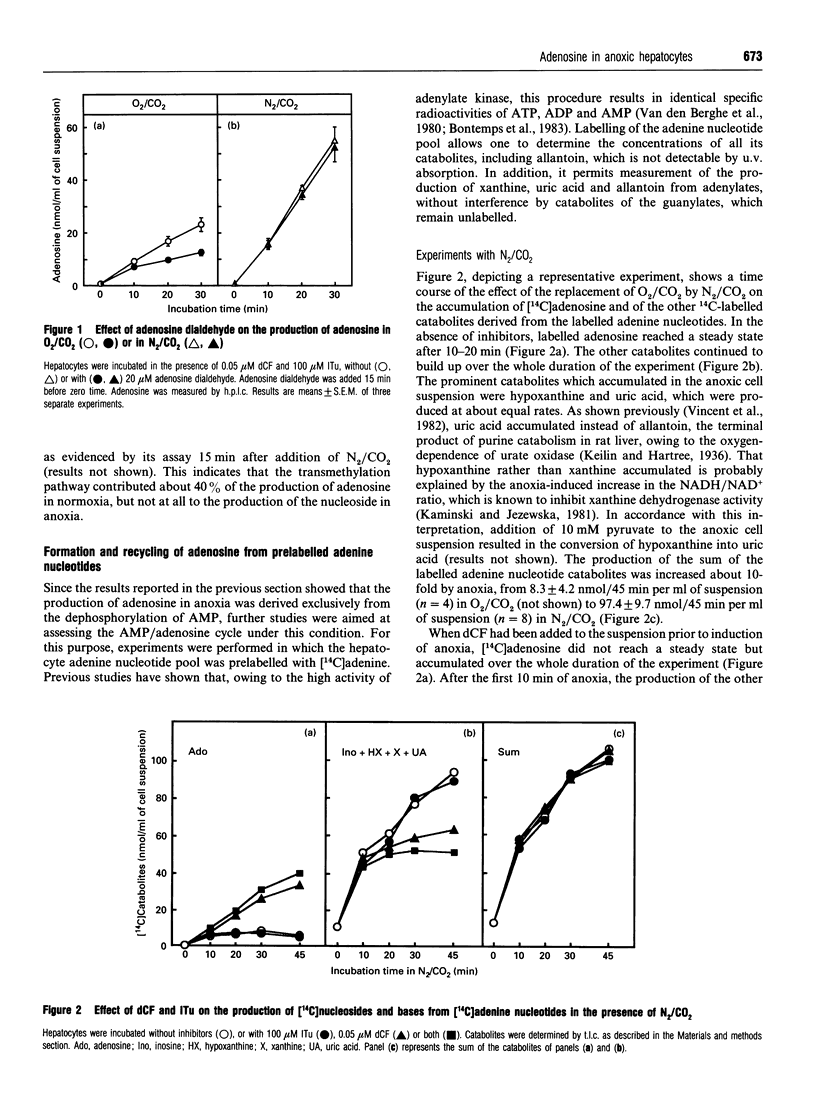
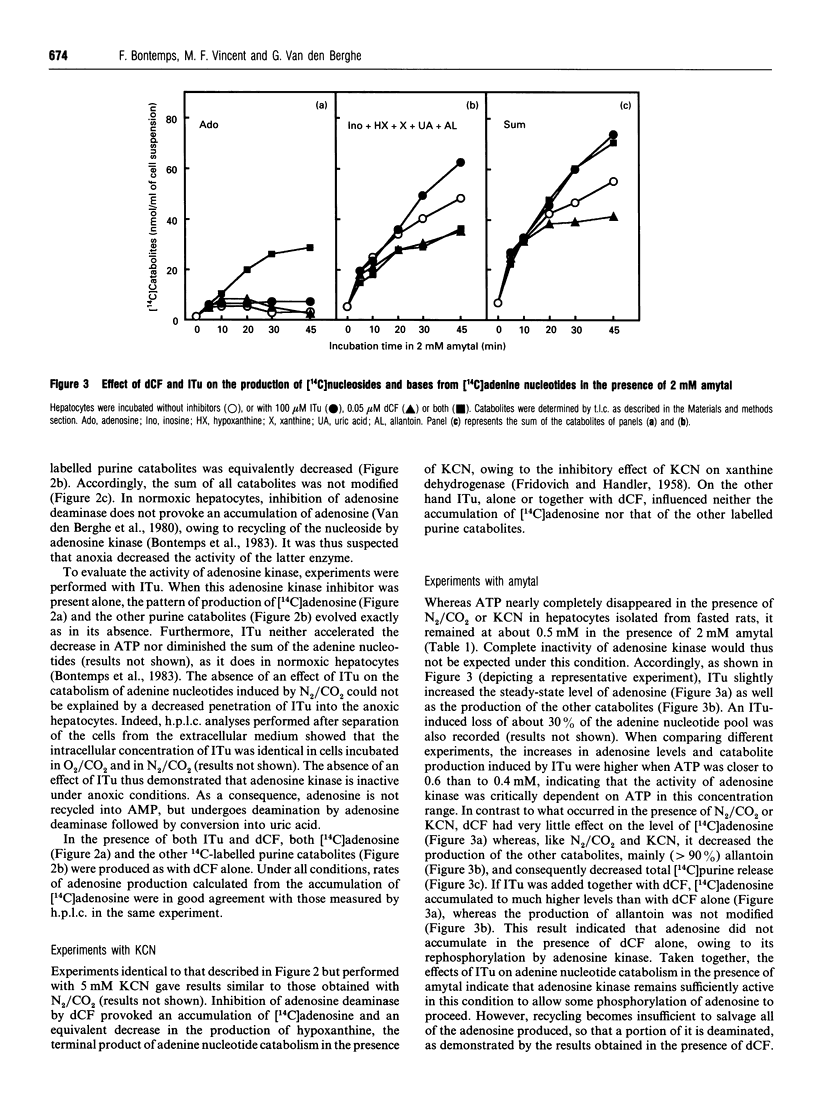
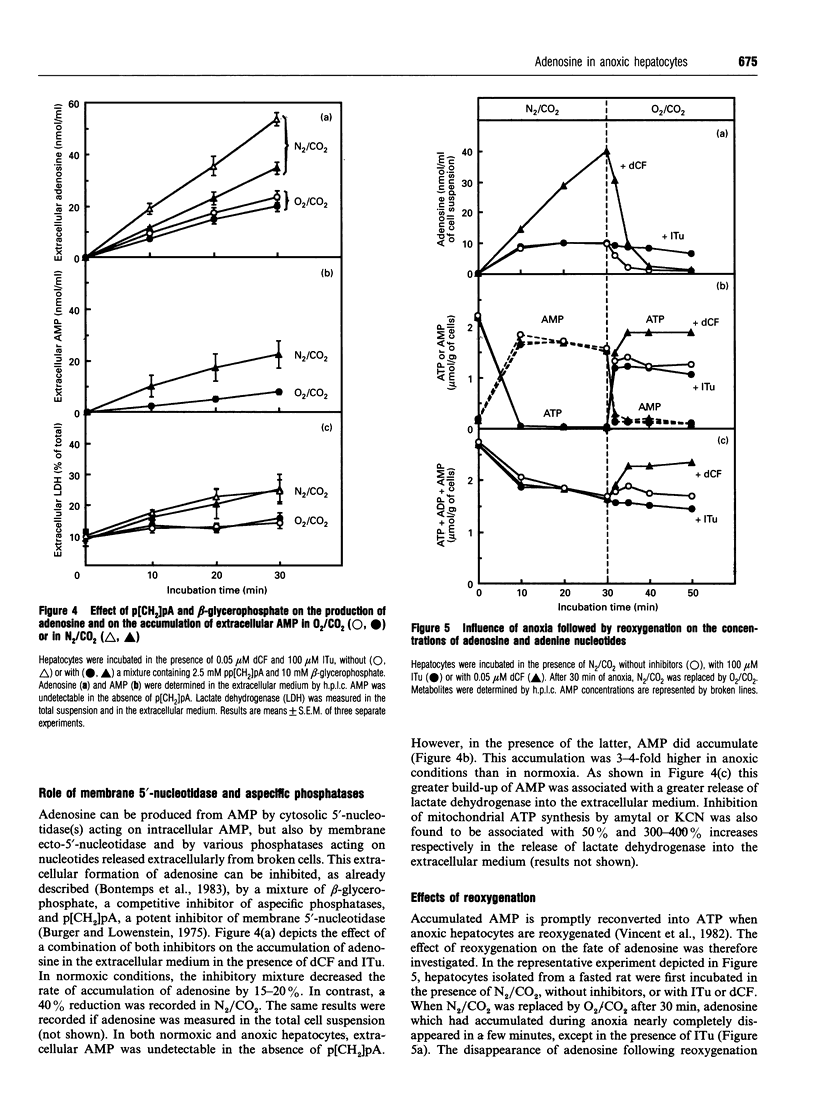
Previous work has shown that normoxic isolated rat hepatocytes continuously produce adenosine from AMP and that the nucleoside is not catabolized further but immediately rephosphorylated by adenosine kinase [Bontemps, Van den Berghe and Hers (1983) Proc. Natl. Acad. Sci. U.S.A. 80, 2829-2833]. We now report the effect of anoxia on adenosine production and on the AMP/adenosine substrate cycle. In cell suspensions incubated in O2/CO2, the adenosine concentration was about 0.4 microM. It increased 30-fold in cells incubated in N2/CO2 or with 5 mM KCN, and 20-fold in cells incubated with 2 mM amytal. Adenosine production, measured in hepatocytes in which adenosine kinase and adenosine deaminase were inhibited by 5-iodotubercidin and deoxycoformycin respectively, was about 18 nmol/min per g of cells in normoxia; it increased about 2-fold in anoxia, although AMP increased 8-16-fold in this condition. From studies with inhibitors of membrane 5'-nucleotidase and of S-adenosylhomocysteine hydrolase, it was deduced that adenosine is produced by the latter enzyme and by cytosolic 5'-nucleotidase in normoxia, and by cytosolic and membrane 5'-nucleotidases in anoxia. Unlike in normoxic hepatocytes, inhibition of adenosine kinase by 5-iodotubercidin neither elevated the adenosine concentration nor enhanced total purine release from adenine nucleotides in cells treated with N2/CO2 or KCN; it had only a slight effect in cells treated with amytal. This indicates that recycling of adenosine is suppressed or profoundly inhibited in anoxia. The rate of accumulation of adenosine in anoxia was several-fold lower than the rate of its rephosphorylation upon reoxygenation. It is concluded that the elevation of adenosine in anoxic hepatocytes is much more dependent on decreased recycling of adenosine by adenosine kinase than on increased production by dephosphorylation of AMP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achterberg P. W., de Tombe P. P., Harmsen E., de Jong J. W. Myocardial S-adenosylhomocysteine hydrolase is important for adenosine production during normoxia. Biochim Biophys Acta. 1985 Jul 5;840(3):393–400. doi: 10.1016/0304-4165(85)90220-x. [DOI] [PubMed] [Google Scholar]
- Arch J. R., Newsholme E. A. Activities and some properties of 5'-nucleotidase, adenosine kinase and adenosine deaminase in tissues from vertebrates and invertebrates in relation to the control of the concentration and the physiological role of adenosine. Biochem J. 1978 Sep 15;174(3):965–977. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch J. R., Newsholme E. A. The control of the metabolism and the hormonal role of adenosine. Essays Biochem. 1978;14:82–123. [PubMed] [Google Scholar]
- Arnold S. T., Cysyk R. L. Adenosine export from the liver: oxygen dependency. Am J Physiol. 1986 Jul;251(1 Pt 1):G34–G39. doi: 10.1152/ajpgi.1986.251.1.G34. [DOI] [PubMed] [Google Scholar]
- Bartel R. L., Borchardt R. T. Effects of adenosine dialdehyde on S-adenosylhomocysteine hydrolase and S-adenosylmethionine-dependent transmethylations in mouse L929 cells. Mol Pharmacol. 1984 May;25(3):418–424. [PubMed] [Google Scholar]
- Belloni F. L., Elkin P. L., Giannotto B. The mechanism of adenosine release from hypoxic rat liver cells. Br J Pharmacol. 1985 Jun;85(2):441–446. doi: 10.1111/j.1476-5381.1985.tb08880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- Bontemps F., Mimouni M., Van den Berghe G. Phosphorylation of adenosine in anoxic hepatocytes by an exchange reaction catalysed by adenosine kinase. Biochem J. 1993 Mar 15;290(Pt 3):679–684. doi: 10.1042/bj2900679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. 5'-Nucleotidase activities in human erythrocytes. Identification of a purine 5'-nucleotidase stimulated by ATP and glycerate 2,3-bisphosphate. Biochem J. 1988 Mar 15;250(3):687–696. doi: 10.1042/bj2500687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. Evidence for a substrate cycle between AMP and adenosine in isolated hepatocytes. Proc Natl Acad Sci U S A. 1983 May;80(10):2829–2833. doi: 10.1073/pnas.80.10.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R. M., Lowenstein J. M. 5'-Nucleotidase from smooth muscle of small intestine and from brain. Inhibition of nucleotides. Biochemistry. 1975 Jun 3;14(11):2362–2366. doi: 10.1021/bi00682a014. [DOI] [PubMed] [Google Scholar]
- Cadnapaphornchai P., Kellner D., Golembieski A., McDonald F. D. Roles of adenosine and theophylline on the recovery of adenine nucleotides in postischemic cultured renal tubular cells. J Pharmacol Exp Ther. 1991 May;257(2):774–780. [PubMed] [Google Scholar]
- Deuticke B., Gerlach E., Dierkesmann R. Abbau freier Nucleotide in Herz, Skeletmuskel, Gehirn und Leber der Ratte bei Sauerstoffmangel. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;292(3):239–254. [PubMed] [Google Scholar]
- Ezzat W. R., Lautt W. W. Hepatic arterial pressure-flow autoregulation is adenosine mediated. Am J Physiol. 1987 Apr;252(4 Pt 2):H836–H845. doi: 10.1152/ajpheart.1987.252.4.H836. [DOI] [PubMed] [Google Scholar]
- FRIDOVICH I., HANDLER P. Xanthine oxidase. IV. Participation of iron in internal electron transport. J Biol Chem. 1958 Dec;233(6):1581–1585. [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. The role of adenosine and 2'-deoxyadenosine in mammalian cells. Annu Rev Biochem. 1978;47:655–686. doi: 10.1146/annurev.bi.47.070178.003255. [DOI] [PubMed] [Google Scholar]
- Hartwick R. A., Brown P. R. The performance of microparticle chemically-bonded anion-exchange resins in the analysis of nucleotides. J Chromatogr. 1975 Oct 29;112:650–662. doi: 10.1016/s0021-9673(00)99994-1. [DOI] [PubMed] [Google Scholar]
- Hawkins C. F., Bagnara A. S. Adenosine kinase from human erythrocytes: kinetic studies and characterization of adenosine binding sites. Biochemistry. 1987 Apr 7;26(7):1982–1987. doi: 10.1021/bi00381a030. [DOI] [PubMed] [Google Scholar]
- Henderson J. F., Brox L., Zombor G., Hunting D., Lomax C. A. Specificity of adenosine deaminase inhibitors. Biochem Pharmacol. 1977 Nov 1;26(21):1967–1972. doi: 10.1016/0006-2952(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Henderson J. F., Paterson A. R., Caldwell I. C., Paul B., Chan M. C., Lau K. F. Inhibitors of nucleoside and nucleotide metabolism. Cancer Chemother Rep 2. 1972 Nov;3(1):71–85. [PubMed] [Google Scholar]
- Henrichs K. J., Matsuoka H., Schaper W. Enhanced postischemic ATP repletion by pharmacological inhibition of nucleoside washout and catabolism. J Cardiovasc Pharmacol. 1988 Jun;11(6):694–700. doi: 10.1097/00005344-198806000-00010. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S. Apparent suicide inactivation of human lymphoblast S-adenosylhomocysteine hydrolase by 2'-deoxyadenosine and adenine arabinoside. A basis for direct toxic effects of analogs of adenosine. J Biol Chem. 1979 Jan 10;254(1):22–25. [PubMed] [Google Scholar]
- Hoffman J. L. The rate of transmethylation in mouse liver as measured by trapping S-adenosylhomocysteine. Arch Biochem Biophys. 1980 Nov;205(1):132–135. doi: 10.1016/0003-9861(80)90091-0. [DOI] [PubMed] [Google Scholar]
- Itoh R. Purification and some properties of cytosol 5'-nucleotidase from rat liver. Biochim Biophys Acta. 1981 Feb 13;657(2):402–410. doi: 10.1016/0005-2744(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Itoh R., Usami C., Nishino T., Tsushima K. Kinetic properties of cytosol 5'-nucleotidase from chicken liver. Biochim Biophys Acta. 1978 Sep 11;526(1):154–162. doi: 10.1016/0005-2744(78)90300-5. [DOI] [PubMed] [Google Scholar]
- Kamiński Z. W., Jezewska M. M. Effect of NADH on hypoxanthine hydroxylation by native NAD+-dependent xanthine oxidoreductase of rat liver, and the possible biological role of this effect. Biochem J. 1981 Dec 15;200(3):597–603. doi: 10.1042/bj2000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kather H. Purine accumulation in human fat cell suspensions. Evidence that human adipocytes release inosine and hypoxanthine rather than adenosine. J Biol Chem. 1988 Jun 25;263(18):8803–8809. [PubMed] [Google Scholar]
- Lautt W. W., Legare D. J., d'Almeida M. S. Adenosine as putative regulator of hepatic arterial flow (the buffer response). Am J Physiol. 1985 Mar;248(3 Pt 2):H331–H338. doi: 10.1152/ajpheart.1985.248.3.H331. [DOI] [PubMed] [Google Scholar]
- Lloyd H. G., Deussen A., Wuppermann H., Schrader J. The transmethylation pathway as a source for adenosine in the isolated guinea-pig heart. Biochem J. 1988 Jun 1;252(2):489–494. doi: 10.1042/bj2520489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghji P., Middleton K. M., Newby A. C. Absolute rates of adenosine formation during ischaemia in rat and pigeon hearts. Biochem J. 1988 Feb 1;249(3):695–703. doi: 10.1042/bj2490695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C., Holmquist C. A., Illingworth J., Pearson J. D. The control of adenosine concentration in polymorphonuclear leucocytes, cultured heart cells and isolated perfused heart from the rat. Biochem J. 1983 Aug 15;214(2):317–323. doi: 10.1042/bj2140317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N., Yamada Y., Goto H. Adenosine and deoxyadenosine kinase from rat liver. Adv Exp Med Biol. 1979;122B:151–156. doi: 10.1007/978-1-4684-8559-2_26. [DOI] [PubMed] [Google Scholar]
- Ohisalo J. J. Regulatory functions of adenosine. Med Biol. 1987;65(4):181–191. [PubMed] [Google Scholar]
- Palella T. D., Andres C. M., Fox I. H. Human placental adenosine kinase. Kinetic mechanism and inhibition. J Biol Chem. 1980 Jun 10;255(11):5264–5269. [PubMed] [Google Scholar]
- Rotllan P., Miras Portugal M. T. Adenosine kinase from bovine adrenal medulla. Eur J Biochem. 1985 Sep 2;151(2):365–371. doi: 10.1111/j.1432-1033.1985.tb09110.x. [DOI] [PubMed] [Google Scholar]
- Skladanowski A. C., Newby A. C. Partial purification and properties of an AMP-specific soluble 5'-nucleotidase from pigeon heart. Biochem J. 1990 May 15;268(1):117–122. doi: 10.1042/bj2680117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong V. L., Collinson A. R., Lowenstein J. M. 5'-Nucleotidases in rat heart. Evidence for the occurrence of two soluble enzymes with different substrate specificities. Biochem J. 1988 Jul 1;253(1):117–121. doi: 10.1042/bj2530117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland P. M., Helland S. Binding of adenosine to intracellular S-adenosylhomocysteine hydrolase in isolated rat hepatocytes. J Biol Chem. 1983 Jan 25;258(2):747–752. [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Extracellular metabolites in suspensions of isolated hepatocytes. Biochem J. 1987 Dec 1;248(2):517–521. doi: 10.1042/bj2480517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berghe G., Bontemps F., Hers H. G. Purine catabolism in isolated rat hepatocytes. Influence of coformycin. Biochem J. 1980 Jun 15;188(3):913–920. doi: 10.1042/bj1880913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M. F., Van den Berghe G., Hers H. G. The pathway of adenine nucleotide catabolism and its control in isolated rat hepatocytes subjected to anoxia. Biochem J. 1982 Jan 15;202(1):117–123. doi: 10.1042/bj2020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worku Y., Newby A. C. The mechanism of adenosine production in rat polymorphonuclear leucocytes. Biochem J. 1983 Aug 15;214(2):325–330. doi: 10.1042/bj2140325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Waeg G., Van den Berghe G. Purine catabolism in polymorphonuclear neutrophils. Phorbol myristate acetate-induced accumulation of adenosine owing to inactivation of extracellularly released adenosine deaminase. J Clin Invest. 1991 Jan;87(1):305–312. doi: 10.1172/JCI114987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe G., van Pottelsberghe C., Hers H. G. A kinetic study of the soluble 5'-nucleotidase of rat liver. Biochem J. 1977 Mar 15;162(3):611–616. doi: 10.1042/bj1620611. [DOI] [PMC free article] [PubMed] [Google Scholar]


