Abstract
Herein, a catalytic photoredox-neutral strategy for alkyne deuterocarboxylation with tetrabutylammonium oxalate as the carbonyl source and D2O as the deuteration agent was described. For the first time, the oxalic salt acted as both the reductant and carbonyl source through single electron transfer and subsequential homolysis of the C–C bond. The strongly reductive CO2 radical anion species in situ generated from oxalate played significant roles in realizing the global deuterocarboxylation of terminal and internal alkynes to access various tetra- and tri-deuterated aryl propionic acids with high yields and deuteration ratios.
Herein, a catalytic photoredox-neutral strategy for alkyne deuterocarboxylation with tetrabutylammonium oxalate as the carbonyl source and D2O as the deuteration agent was described.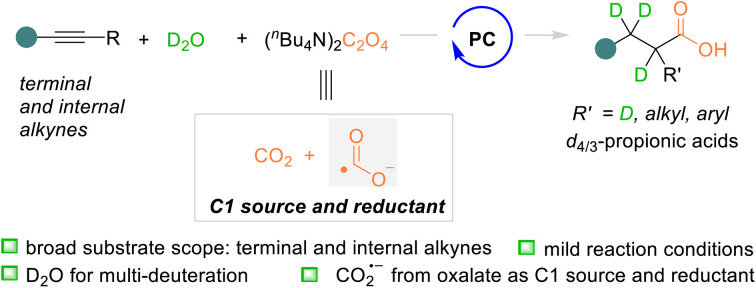
Introduction
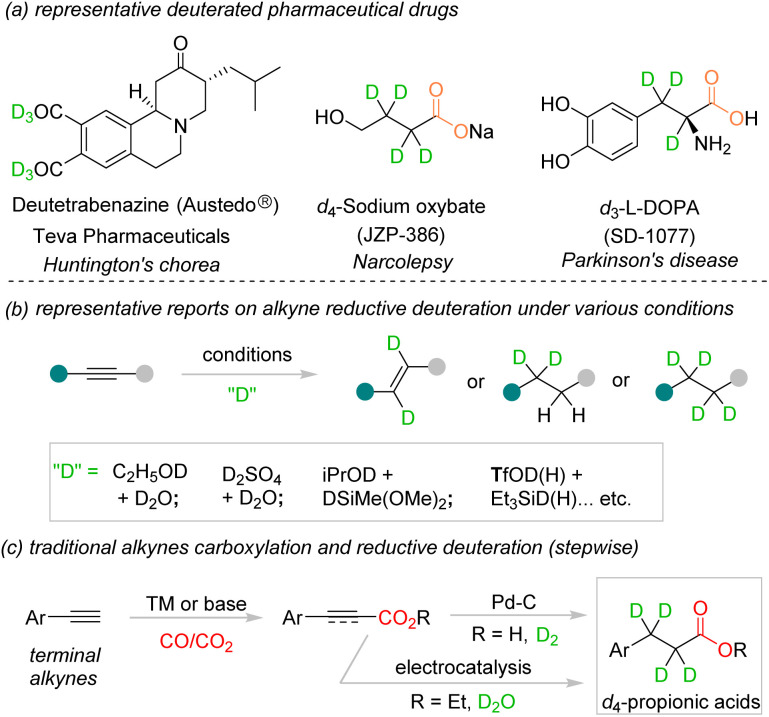
Hydrocarboxylation of unsaturated hydrocarbons, such as C–C double and triple bonds, to forge thermodynamically and kinetically stable C–C bonds and yield value-added propionic acid derivatives is an attractive research topic.1 Although the alkene carboxylation reactions were disclosed by many research groups,2 carboxylation of alkynes is rarely reported as the C–C triple bond is relatively stable and inert.3 In addition, introduction of deuterium into pharmaceutical drugs could potentially improve the metabolic stability and pharmacokinetics of the original molecules.4 For example, deutetrabenazine (Austedo®) was approved as the first deuterium-labeled drug in 2017 by the FDA for the treatment of choreas associated with Huntington's disease.5 Recently, d4-butyric acids6 and d3-l-DOPA7 were investigated in clinical trials for treatment of narcolepsy and Parkinson's disease, respectively (Fig. 1a). The development of deuterated analogues of the original drugs was proved to be the effective drug discovery process in medicinal chemistry and therefore attracted more and more attention in the synthetic chemistry community.4,8 For reductive carboxylation of alkynes, since 2018, a number of examples were reported utilizing various deuteration reagents, such as C2H5OD, D2O, TfOD, or deuterated silanes (Fig. 1b).9 In 2021, Evano and co-workers reported an elegant selective deuteration reaction with electron-poor alkynes as the substrate to access diverse di- and tetra-deuterated alkylamines.9e However, development of direct protocols for alkyne deuterocarboxylation to access multi-deuterated propionic acid derivatives is challenging yet highly desired.
Fig. 1. (a) Representative deuterated drugs on the market and in clinical trials. (b) Well developed reductive deuteration of alkynes. (c) Traditional procedures for alkyne deutero-carboxylation.
Traditional strategies to access d4-propionic acids from alkynes relied on multi-step manipulations and tedious conditions (Fig. 1c). The terminal alkynes could be carboxylated with different carbonyl sources, such as CO or CO2, in the presence of transition metals10 or basic conditions,11 respectively. Afterward, the alkynyl carboxylic acids/esters could undergo hydrogenation with D2 (ref. 12) or reductive deuteration with D2O under transition metal mediated13 or electrochemical14 conditions to access deuterated propionic acids. To date, direct conversion of either terminal or internal alkynes to the corresponding fully deuterated propionic acids in one step is still not realized. Development of a new protocol with a new carbonyl source is crucial to solve the above problem.
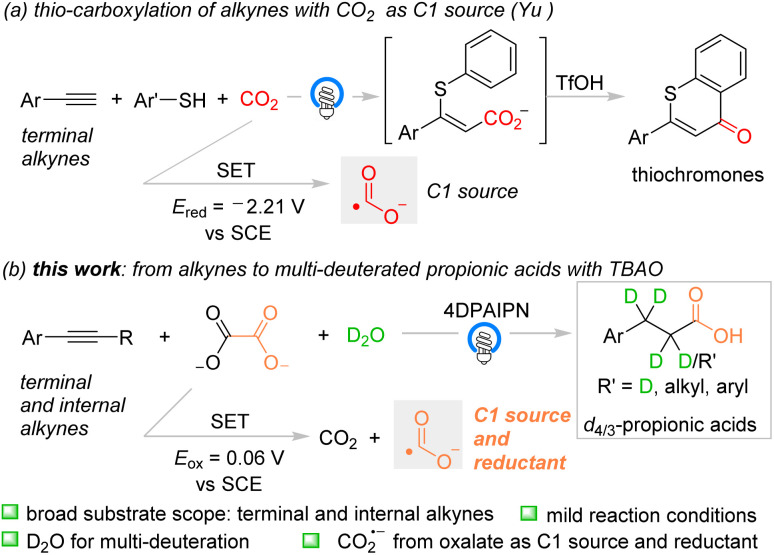
Compared with the commonly used carbon dioxide as the carbonyl source under photocatalytic conditions,15 the carbon dioxide radical anion (CO2˙−),2b,15b,c,e,16 with reversed polarity, was recently developed as a novel carbonyl source for carboxylation of alkenes via Giese radical addition in synthetic organic chemistry.17 Interestingly, in 2023, Yu and co-workers reported that CO2˙− generated from CO2 (Ered = −2.21 V vs. SCE) via single electron transfer (SET) was able to undergo radical addition to the terminal alkynes and install the carboxy group (Scheme 1a).18a In the presence of aryl thiol, the C–S bond was formed via radical–radical coupling and the subsequential cyclization afforded thiochromones as the final products. This is the first example which showcased the possibility of the carboxylation of alkynes with CO2˙− under photo-induced conditions, which encouraged us to devote efforts to alkyne carboxylation reactions under mild reaction conditions. Herein we disclose our recent discovery of deuterocarboxylation of various terminal and internal alkynes with tetrabutylammonium oxalate (TBAO) as the CO2˙− precursor under photoredox-neutral conditions to access diverse aryl d4/3-propionic acids (Scheme 1b).
Scheme 1. Reported carboxylation of terminal alkynes and the reaction design for this study.
There are a couple of obstacles to overcome for regio- and chemoselective deuterocarboxylation of alkynes with D2O as the cheapest deuteration agent. Existence of any protonic agents, solvents, or hydrogen atom transfer process could decrease the deuteration ratio. In addition, realizing the high α- or β-selectivity of the carboxylation step is also challenging.19
We noticed that TBAO, previously exploited as a co-reductant in electrogenerated chemiluminescence (ECL),20 could be used as a new CO2˙− precursor for carboxylation under mild reaction conditions.21 The reaction mechanism involves the single-electron-oxidation of the oxalic dianion (Eox = 0.06 V vs. SCE) by the oxidant or photocatalyst, followed by the subsequential homolytic C–C bond cleavage to produce CO2˙−.22 Neither the protonic agent nor HAT process is involved during the generation of CO2˙− from TBAO. Therefore, the deuterocarboxylation of terminal alkyne 1a with TBAO and D2O was used as a template reaction and initially investigated.
Results and discussion
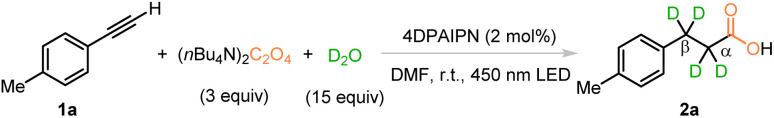
After careful screening of various reaction parameters (see the ESI† for details), the best reaction conditions are showcased in Table 1, entry 1, namely, the treatment of the terminal alkyne 1-ethynyl-4-methylbenzene (1a) with 3 equivalents of TBAO and 15 equivalents of D2O in the presence of 2 mol% 2,4,5,6-tetrakis(diphenylamino)isophthalonitrile (4DPAIPN) in DMF under 450 nm blue LED irradiation yielded d4-propionic acid 2a in 85% isolated yield. The counter cations of the ammonium salt, such as NH4+ and Na+, were tested and no conversion of the substrate was observed due to the poor solubility of these salts in organic solvent (Table 1, entries 2 and 3). Amounts of D2O were also screened and the results showed that 15 equivalents of D2O was optimal (Table 1, entries 4 and 5). Reactions conducted in DMSO provided product 2a in slightly decreased yield and deuteration ratios at both the α- and β-positions (Table 1, entry 6). When DMA was used as the solvent, the reaction yield was increased to 96%; however the deuteration ratios dropped at each position (Table 1, entry 7). Without visible-light irradiation or a photocatalyst, the reaction did not occur and only the starting material 1a was recovered (Table 1, entries 8 and 9). No reaction was observed in the absence of TBAO under a CO2 atmosphere (Table 1, entry 10).
Variation of the standard reaction conditionsa.
| |||
|---|---|---|---|
| Entry | Variations | Yield of 2ab (%) | Deuteration ratio (α/β) |
| 1 | None | 91 (85)c | 89%/94% |
| 2 | (NH4)2C2O4 | 0 | — |
| 3 | Na2C2O4 | 0 | — |
| 4 | D2O (8 equiv.) | 91 | 75%/86% |
| 5 | D2O (20 equiv.) | 94 | 85%/92% |
| 6 | Anhydrous DMSO | 85 | 82%/78% |
| 7 | Anhydrous DMA | 96 | 85%/89% |
| 8 | In dark | 0 | — |
| 9 | W/o 4DPAIPN | 0 | — |
| 10d | W/o TBAO | 0 | — |
Reaction conditions: 1a (0.2 mmol), 4DPAIPN (2 mol%), TBAO (3 equiv.), in anhydrous DMF (0.1 M), r.t., 12 h under a N2 atmosphere.
Crude 1H NMR yield.
Isolated yield.
Under a CO2 atmosphere.
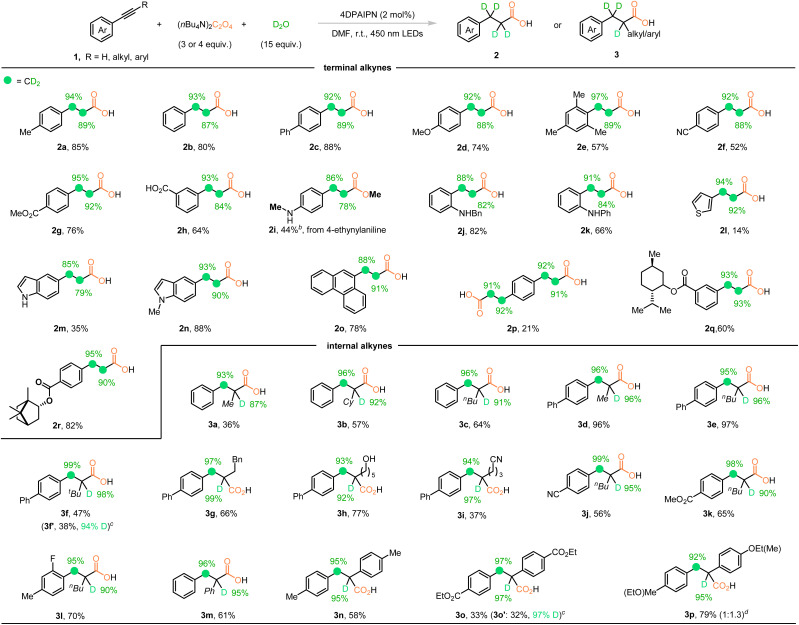
With the optimized reaction conditions in hand, the substrate scope of this novel deuterocarboxylation process of alkynes was investigated as shown in Table 2. Various terminal aryl alkynes were first investigated. Ethynylbenzene (1b) gave d4-propionic acid 2b smoothly in 80% yield. The ratios of 87% and 93% for deuteration at the α- and β-positions, respectively, were obtained. The terminal aryl alkynes tethering electron-donating groups, such as Ph and MeO groups, delivered the desired product 2c and 2d in good yields and deuteration ratios. Alkyne 1e with two methyl substitutions at the ortho position provided d4-propionic acid 2e with a decreased yield of 57% due to the steric hindrance. The CN group could also be tolerated to give product 2f in moderate yield and no decrease in the deuterium ratio at both α- and β-positions was detected. The ester moiety, which is vulnerable under reductive conditions, was also tolerated during the transformation to give product 2g in 76% yield and high deuteration ratios up to 95%. Interestingly, substrate 1h with a carboxy group on the aryl ring also worked well to give the corresponding product 2h in 64% yield. 4-ethynylaniline (1i) was converted to the corresponding methylated propionic acid product 2i in 44% yield after treatment with MeI before workup of the reaction, although the reaction yield and deuteration ratios were moderate. Ortho-N-benzyl substitution (1j) improved both the reaction yield and the deuteration ratios (2j). The more sterically hindered ortho-N-phenyl group led to better deuteration ratios with moderate yield (2k). Afterward, the sensitive heteroarene thiophene was examined. Although the reaction generated the desired product 2l in only 14% yield, an average deuteration ratio of 93% was obtained. The instability of the thiophene ring under our reaction conditions caused the low yield. The other indole substituted alkynes also worked well to give moderate to good yields and deuteration ratios (2m, 2n). The polyaromatic substrate 9-ethynylphenanthrene was converted to the corresponding aryl d4-propionic acid 2o in 78% yield and 90% average deuteration ratio. When 1,4-diethynylbenzene with two symmetric C–C triple bonds was employed under the reaction conditions, the d8-diacid 2p was isolated in high deuteration ratios, although a low yield was obtained. The alkynyl substrates derived from natural products, such as menthol and borneol, were investigated next and the corresponding d4-propionic acids were produced in good yields and high deuteration ratios over 90% (2q and 2r).
Substrate scope of various N-aryl acrylamides 1a.
|
Reaction conditions: 1 (0.2 mmol), 4CzIPN (2 mol%), TBAO (3 or 4 equiv.), anhydrous DMF (0.1 M), r.t., 12–48 h, under a N2 atmosphere.
MeI was added after completion of the reaction.
Reductive deuteration product was isolated.
Two regioisomers were isolated as an inseparable mixture.
Compared with the terminal alkynes, deuterocarboxylation of internal alkynes under photocatalysis is also challenging and has never been reported before.2h With our established protocol, various internal alkynes were next investigated. As shown in the second part in Table 2, when the hydrogen on the terminal alkyne was replaced by alkyl groups such as Me, Cy, n-Bu, and phenethyl, the corresponding d3-propionic acids 3a–3e and 3g were obtained, respectively, in up to 96% yield and over 90% deuteration ratios. Product 3a was obtained in 36% yield, along with inseparable complex mixtures. When the steric hindered t-Bu group was incorporated, the reaction yield dropped to 47%, but extremely high deuteration ratios around 99% were observed (3f). In the meantime, almost equal amounts of reduction product 3f′ were isolated with a 94% deuteration ratio because the steric hindrance of the t-Bu group interrupted the carboxylation step. To further test the functional group tolerance of this reaction, the substrates tethering a free hydroxyl or cyano group on the alkyl chain were examined to give the corresponding functionalized d3-propionic acids in moderate to good yields and high deuteration ratios (3h, 3i). The cyano, ester, and fluoro substituents on the aryl ring were also investigated and the desired products 3j–3l were obtained in good yields and deuteration ratios. Afterward, the 1,2-diaryl substituted internal alkynes were exploited and the deuterocarboxylation processes proceeded smoothly to give the desired products 3m and 3n. For an electron deficient substrate with an ester moiety as the substituent, direct reduction and deuteration was observed as a side reaction. A synthetically useful yield and high deuteration ratios were obtained for both products (3o and 3o′). The unsymmetric 1,2-diaryl alkyne substrate provided the corresponding acid 3p in 79% yield and high deuteration ratios up to 95% but poor regioselectivity was observed.
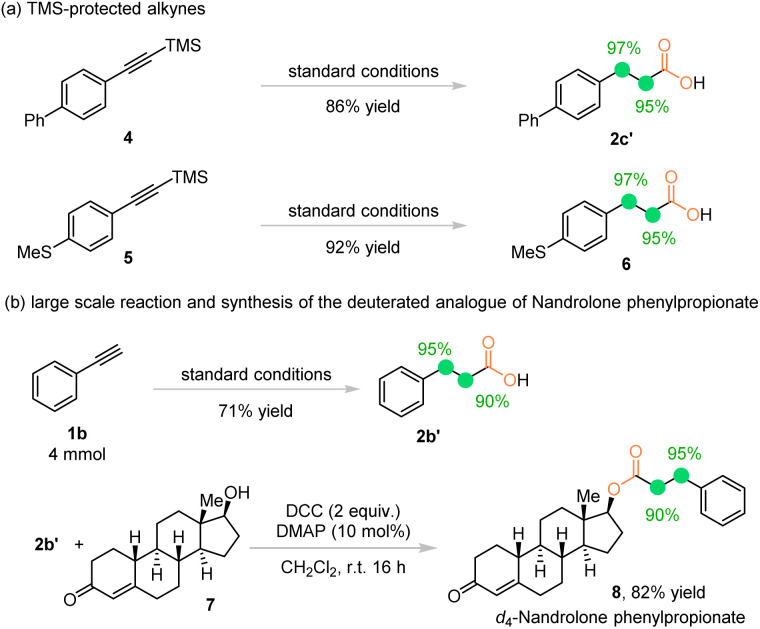
Interestingly, under our reaction conditions, the TMS protected alkyne 4 could be converted to the d4-propionic acid 2c′ in even higher yield and deuteration ratios compared with the corresponding free terminal alkyne 1c. The thiol ether substrate 5 underwent deutero-carboxylation smoothly to give acid 6 in 92% yield and 96% average deuteration ratio (Scheme 2a). Furthermore, the deuterium-labeled analogue of nandrolone phenylpropionate was synthesized in good yield and stable deuteration ratios from d4-propionic acid 2b′ synthesized from a scaled-up reaction with increased deuteration ratios, proving it to be a practical method for synthesis of the deuterated version of biologically active molecules (Scheme 2b).
Scheme 2. (a) Further application of the protocol. (b) Synthesis of the deuterated analogue of nandrolone phenylpropionate.
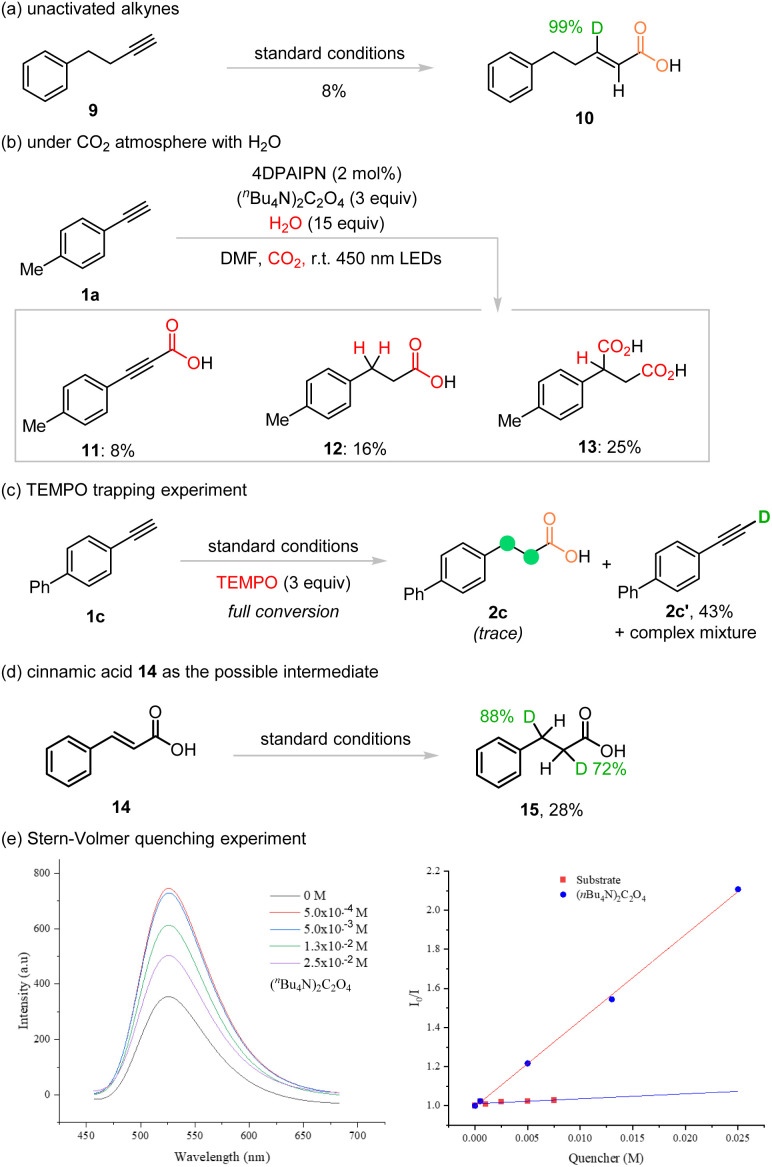
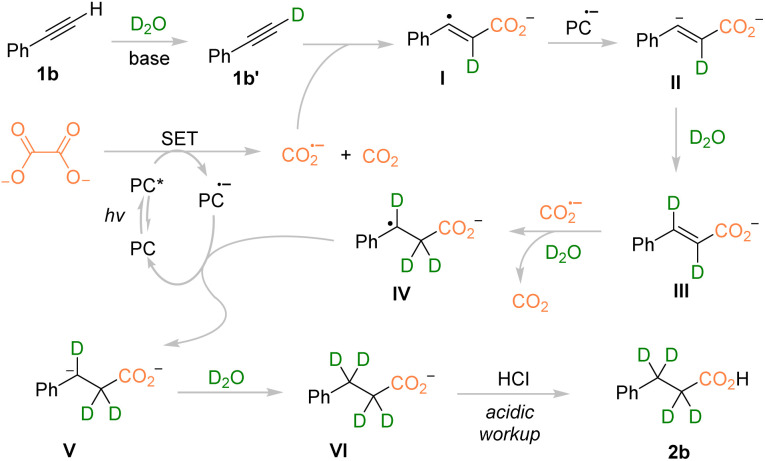
To gain further insights into the reaction mechanism, several control experiments were conducted as shown in Scheme 3. Firstly, the unactivated alkyne 9 was utilized as the substrate to probe any possible intermediate during the Giese radical addition of CO2˙− to the alkyne. When unactivated alkyne 9 was treated under the standard reaction conditions, the β-deuterated vinyl carboxylic acid 10 was observed in only 8% isolated yield with a very high deuteration ratio of 99%, along with inseparable complex mixtures (Scheme 3a). This result indicated that the alkenyl radical generated via Giese radical addition of CO2˙− was reduced to the anion form and subsequently deuterated in the presence of excess amounts of D2O. However, lack of a conjugated π-system caused low reactivity and poor mass balance. Secondly, the template reaction of 1a was investigated with normal H2O under a CO2 atmosphere (Scheme 3b). In the presence of CO2, the reaction was disturbed and a complex mixture was observed. After careful investigation, byproducts 11, 12, and 13 were isolated and each of them was characterized by using NMR spectra and HRMS analysis.
Scheme 3. Mechanistic studies.
Formation of 11 clearly showed that the alkyne 1a underwent deprotonation under basic conditions and the anion form of 1a trapped CO2 to form the carboxylic acid. Therefore, under a N2 atmosphere, proton–deuterium exchange occurred first in the presence of excess amounts of D2O in our reaction to give the deuterated 1a, which could accept the attack by CO2˙− to establish the first carboxy group. Product 12 could be derived from either 11 or the cinnamic acid intermediate. For product 13, incorporation of the second carboxy group at the benzyl position indicated formation of the benzyl anion intermediate during the transformation. When 3 equivalents of TEMPO were added to the reaction mixture, full conversion could be observed, but only trace amounts of the desired product 2c were detected, along with H–D exchanged product 2c′ in 43% isolated yield. This result indicates that the formation of radical species during the transformation and the deuterocarboxylation process was disturbed (Schemes 3c). To determine if cinnamic acid was one of the intermediates, 14 was treated under standard reaction conditions and the desired product 15 was obtained in 28% yield and good deuteration ratios (Scheme 3d). Next, the Stern–Volmer quenching analysis was conducted with substrate 1a and TBAO; the results showed that the excited state of photocatalyst 4DPAIPN was quenched by TBAO effectively (Scheme 3e, see the ESI† for more details).
As shown in Scheme 4, on the basis of the control experiments and reported literature,20–22 we proposed that the reaction was first initiated by photo-exciting 4DPAIPN to form 4DPAIPN*  that could oxidize TBAO (Eox = 0.06 V vs. SCE) and generate the oxalic radical anion (C2O4˙−), which subsequently underwent homolysis of the C–C bond to give CO2˙− and CO2. Under basic conditions, alkyne 1b was deprotonated and deuterated rapidly to give 1b′ and underwent the Giese radical addition of CO2˙− to give intermediate I. The vinyl radical I was reduced by 4DPAIPN˙− (E1/2 = −1.52 V vs. SCE) to produce anion intermediate II, which was deuterated by D2O to yield intermediate III. Afterward, the anion form of vinyl acid III could be further reduced by CO2˙− (or 4DPAIPN˙−) to generate the benzyl radical species IV, which could be reduced by 4DPAIPN˙− (or CO2˙−), producing intermediate V. Finally, deuteration of intermediate V provided d4-propionic anion VI that could be protonated upon acidic workup to give the desired product 2b.
that could oxidize TBAO (Eox = 0.06 V vs. SCE) and generate the oxalic radical anion (C2O4˙−), which subsequently underwent homolysis of the C–C bond to give CO2˙− and CO2. Under basic conditions, alkyne 1b was deprotonated and deuterated rapidly to give 1b′ and underwent the Giese radical addition of CO2˙− to give intermediate I. The vinyl radical I was reduced by 4DPAIPN˙− (E1/2 = −1.52 V vs. SCE) to produce anion intermediate II, which was deuterated by D2O to yield intermediate III. Afterward, the anion form of vinyl acid III could be further reduced by CO2˙− (or 4DPAIPN˙−) to generate the benzyl radical species IV, which could be reduced by 4DPAIPN˙− (or CO2˙−), producing intermediate V. Finally, deuteration of intermediate V provided d4-propionic anion VI that could be protonated upon acidic workup to give the desired product 2b.
Scheme 4. Proposed mechanism for deuterocarboxylation of alkyne 1b.
Conclusions
In summary, a visible-light-induced photoredox-neutral alkyne deuterocarboxylation reaction using TBAO as both the C1 source and reductant was developed. This study provides a novel approach for d4/3-propionic acid synthesis with super stoichiometric D2O as the cheapest deuteration agent. The reaction is mild, clean, and sustainable, with broad substrate scope and a novel reaction mechanism. Most of the deuteration ratios are over 90%, which is close to the requirement of pharmaceutical drugs, indicating the application potential of our synthetic method. Further applications of TBAO as the CO2˙− precursor in synthetic organic chemistry under sustainable reaction conditions are currently under investigation.
Data availability
The data supporting this article have been included as part of the ESI.†
Author contributions
Pei Xu, Hao-Qiang Jiang, and Hui Xu performed the experiments and data collections, including 1H/13C NMR data, HRMS, and Stern–Volmer quenching experiments. Sai Wang and Hui-Xian Jiang prepared all the starting materials. Song-Lei Zhu assisted in the measurements of the above experiments and helped to supervise the project. Long Yin and Dong Guo assisted in the project design and preparation of the manuscript. Xu Zhu conceived the project, supervised the research, wrote the manuscript, and provided guidance for the analysis of the results.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (22207094, Long Yin), the Jiangsu Province Shuangchuang PhD award (JSSCBS20211267, Pei Xu), and the Natural Science Research Project of Jiangsu Universities (23KJB150037, Pei Xu). This work was also sponsored by the Jiangsu Specially-Appointed Professor program (Xu Zhu) and the start-up funding provided by Xuzhou Medical University. The Public Experimental Research Center of Xuzhou Medical University was also acknowledged.
Electronic supplementary information (ESI) available: general information, experimental details, optimization of reaction conditions, mechanistic studies, characterization data for products, and NMR spectra of products. See DOI: https://doi.org/10.1039/d4sc03586k
Notes and references
- For selected reviews see: ; (a) Yeung C. S. Photoredox catalysis as a strategy for CO2 incorporation: direct access to carboxylic acids from a renewable feedstock. Angew. Chem., Int. Ed. 2019;58:5492–5502. doi: 10.1002/anie.201806285. [DOI] [PubMed] [Google Scholar]; (b) Ye J.-H. Ju T. Huang H. Liao L.-L. Yu D.-G. Radical carboxylative cyclizations and carboxylations with CO2. Acc. Chem. Res. 2021;54:2518–2531. doi: 10.1021/acs.accounts.1c00135. [DOI] [PubMed] [Google Scholar]; (c) Tortajada A. Börjesson M. Martin R. Nickel-catalyzed reductive carboxylation and amidation reactions. Acc. Chem. Res. 2021;54:3941–3952. doi: 10.1021/acs.accounts.1c00480. [DOI] [PubMed] [Google Scholar]; (d) Shi M. Yu Z. Recent advances in the electrochemically mediated chemical transformation of carbon dioxide. Chem. Commun. 2022;58:13539–13555. doi: 10.1039/D2CC05242C. [DOI] [PubMed] [Google Scholar]
- For selected examples for alkene carboxylation, see: ; (a) Seo H. Liu A. Jamison T. F. Direct β-selective hydrocarboxylation of styrenes with CO2 enabled by continuous flow photoredox catalysis. J. Am. Chem. Soc. 2017;139:13969–13972. doi: 10.1021/jacs.7b05942. [DOI] [PubMed] [Google Scholar]; (b) Huang H. Ye J.-H. Zhu L. Ran C.-K. Miao M. Wang W. Chen H. Zhou W.-J. Lan Y. Yu B. Yu D.-G. Visible-light-driven anti-markovnikov hydrocarboxylation of acrylates and styrenes with CO2. CCS Chem. 2021;3:1746–1756. doi: 10.31635/ccschem.020.202000374. [DOI] [Google Scholar]; (c) Alektiar S. N. Wickens Z. K. Photoinduced hydrocarboxylation via thiol-catalyzed delivery of formate across activated alkenes. J. Am. Chem. Soc. 2021;143:13022–13028. doi: 10.1021/jacs.1c07562. [DOI] [PubMed] [Google Scholar]; (d) Huang Y. Hou J. Zhan L.-W. Zhang Q. Tang W.-Y. Li B.-D. Photoredox activation of formate salts: hydrocarboxylation of alkenes via carboxyl group transfer. ACS Catal. 2021;11:15004–15012. doi: 10.1021/acscatal.1c04684. [DOI] [Google Scholar]; (e) Song L. Wang W. Yue J.-P. Jiang Y.-X. Wei M.-K. Zhang H.-P. Yan S.-S. Liao L.-L. Yu D.-G. Visible-light photocatalytic di- and hydro-carboxylation of unactivated alkenes with CO2. Nat. Catal. 2022;5:832–838. doi: 10.1038/s41929-022-00841-z. [DOI] [Google Scholar]; (f) Alektiar S. N. Han J. Dang Y. Rubel C. Z. Wickens Z. K. Radical hydrocarboxylation of unactivated alkenes via photocatalytic formate activation. J. Am. Chem. Soc. 2023;145:10991–10997. doi: 10.1021/jacs.3c03671. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Zhang W. Chen Z. Jiang Y.-X. Liao L.-L. Wang W. Ye J.-H. Yu D.-G. Arylcarboxylation of unactivated alkenes with CO2 via visible-light photoredox catalysis. Nat. Commun. 2023;14:3529. doi: 10.1038/s41467-023-39240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Xue T. Ma C. Liu L. Xiao C. Ni S.-F. Zeng R. Characterization of a π–π stacking cocrystal of 4-nitrophthalonitrile directed toward application in photocatalysis. Nat. Commun. 2024;15:1455. doi: 10.1038/s41467-024-45686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples for alkyne carboxylation: ; (a) Wang X. Nakajima M. Martin R. Ni-catalyzed regioselective hydrocarboxylation of alkynes with CO2 by using simple alcohols as proton sources. J. Am. Chem. Soc. 2015;137:8924–8927. doi: 10.1021/jacs.5b05513. [DOI] [PubMed] [Google Scholar]; (b) Gaydou M. Moragas T. Juliá-Hernández F. Martin R. Site-selective catalytic carboxylation of unsaturated hydrocarbons with CO2 and water. J. Am. Chem. Soc. 2017;139:12161–12164. doi: 10.1021/jacs.7b07637. [DOI] [PubMed] [Google Scholar]; (c) Hou J. Ee A. Feng W. Xu J.-H. Zhao Y. Wu J. Visible-light-driven alkyne hydro-/carbocarboxylation using CO2 via iridium/cobalt dual catalysis for divergent heterocycle synthesis. J. Am. Chem. Soc. 2018;140:5257–5263. doi: 10.1021/jacs.8b01561. [DOI] [PubMed] [Google Scholar]; (d) Börjesson M. Janssen-Müller D. Sahoo B. Duan Y. Wang X. Martin R. Remote sp2 C–H carboxylation via catalytic 1,4-Ni migration with CO2. J. Am. Chem. Soc. 2020;142:16234–16239. doi: 10.1021/jacs.0c08810. [DOI] [PubMed] [Google Scholar]
- Martino R. M. C. D. Maxwell B. D. Pirali T. Deuterium in drug discovery: progress, opportunities and challenges. Nat. Rev. Drug Discovery. 2023;22:562–584. doi: 10.1038/s41573-023-00703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C. First deuterated drug approved. Nat. Biotechnol. 2017;35:493–494. doi: 10.1038/nbt0617-493. [DOI] [PubMed] [Google Scholar]
- Malmlöf T. Rylander D. Alken R. G. Schneider F. Svensson T. H. Cenci M. A. Schilström B. Deuterium substitutions in the L-DOPA molecule improve its anti-akinetic potency without increasing dyskinesias. Exp. Neurol. 2010;225:408–415. doi: 10.1016/j.expneurol.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Pirali T. Serafini M. Cargnin S. Genazzani A. A. Applications of deuterium in medicinal chemistry. J. Med. Chem. 2019;62:5276–5297. doi: 10.1021/acs.jmedchem.8b01808. [DOI] [PubMed] [Google Scholar]
- (a) Kopf S. Bourriquen F. Li W. Neumann H. Junge K. Beller M. Recent developments for the deuterium and tritium labeling of organic molecules. Chem. Rev. 2022;122:6634–6718. doi: 10.1021/acs.chemrev.1c00795. [DOI] [PubMed] [Google Scholar]; (b) Li N. Li Y. Wu X. Zhu C. Xie J. Radical deuteration. Chem. Soc. Rev. 2022;51:6291–6306. doi: 10.1039/D1CS00907A. [DOI] [PubMed] [Google Scholar]
- (a) Han M. Ding Y. Yan Y. Li H. Luo S. Adijiang A. Ling Y. An J. Transition-metal-free, selective reductive deuteration of terminal alkynes with sodium dispersions and EtOD- d1. Org. Lett. 2018;20:3010–3013. doi: 10.1021/acs.orglett.8b01036. [DOI] [PubMed] [Google Scholar]; (b) Wu Y. Liu C. Wang C. Lu S. Zhang B. Selective transfer semihydrogenation of alkynes with H2O (D2O) as the H (D) source over a Pd-P cathode. Angew. Chem., Int. Ed. 2020;59:21170–21175. doi: 10.1002/anie.202009757. [DOI] [PubMed] [Google Scholar]; (c) Kurimoto A. Sherbo R. S. Cao Y. Loo N. W. X. Berlinguette C. P. Electrolytic deuteration of unsaturated bonds without using D2. Nat. Catal. 2020;3:719–726. doi: 10.1038/s41929-020-0488-z. [DOI] [Google Scholar]; (d) Sloane S. E. Reyes A. Vang Z. P. Li L. Behlow K. T. Clark J. R. Copper-catalyzed formal transfer hydrogenation/deuteration of aryl alkynes. Org. Lett. 2020;22:9139–9144. doi: 10.1021/acs.orglett.0c03632. [DOI] [PubMed] [Google Scholar]; (e) Lecomte M. Lahboubi M. Thilmany P. Bouzakhi A. E. Evano G. A general, versatile and divergent synthesis of selectively deuterated amines. Chem. Sci. 2021;12:11157–11165. doi: 10.1039/D1SC02622D. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Sloane S. E. Vang Z. P. Nelson G. Qi L. Sonstrom R. E. Alansari I. Y. Behlow K. T. Pate B. H. Neufeldt S. R. Clark J. R. Precision deuteration using Cu-catalyzed transfer hydrodeuteration to access small molecules deuterated at the benzylic position. JACS Au. 2023;3:1583–1589. doi: 10.1021/jacsau.3c00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples, see: ; (a) Fabriás G. Stereospecificity of an enzymatic monoene 1,4-dehydrogenation reaction: conversion of (Z)-11-tetradecenoic acid into (E,E)-10,12-tetradecadienoic acid. J. Org. Chem. 2002;67:2228–2233. doi: 10.1021/jo0109927. [DOI] [PubMed] [Google Scholar]; (b) Wendling T. Risto E. Krause T. Gooßen L. J. Salt-free strategy for the insertion of CO2 into C−H bonds: catalytic hydroxymethylation of alkynes. Chem.–Eur. J. 2018;24:6019–6024. doi: 10.1002/chem.201800526. [DOI] [PubMed] [Google Scholar]; (c) Yu D. Zhou F. Lim D. S. W. Su H. Zhang Y. NHC-Ag/Pd-catalyzed reductive carboxylation of terminal alkynes with CO2 and H2: a combined experimental and computational study for fine-tuned selectivity. ChemSusChem. 2017;10:836–841. doi: 10.1002/cssc.201601785. [DOI] [PubMed] [Google Scholar]; (d) Shah D. J. Sharma A. S. Shah A. P. Sharma V. S. Athar M. Soni J. Y. Fixation of CO2 as a carboxylic acid precursor by microcrystalline cellulose (MCC) supported Ag NPs: a more efficient, sustainable, biodegradable and eco-friendly catalyst. New J. Chem. 2019;43:8669–8676. doi: 10.1039/C8NJ06373G. [DOI] [Google Scholar]
- For selected examples, see: ; (a1) Yu B. Yang P. Gao X. Yang Z. Zhao Y. Zhang H. Liu Z. Sequential protocol for C(sp)–H carboxylation with CO2: KOtBu-catalyzed C(sp)–H silylation and KOtBu-mediated carboxylation. Sci. China: Chem. 2018;61:449–456. doi: 10.1007/s11426-017-9163-2. [DOI] [Google Scholar]; , and references cited therein; ; (b) Vigneron J. P. Bloy V. Preparation d'alkyl-4 γ-Lactones optiquement actives. Tetrahedron Lett. 1980;21:1735–1738. doi: 10.1016/S0040-4039(00)77823-3. [DOI] [Google Scholar]
- Kurita T. Aoki F. Mizumoto T. Maejima T. Esaki H. Maegawa T. Monguchi Y. Sajiki H. Facile and convenient method of deuterium gas generation using a Pd/C-catalyzed H2–D2 exchange reaction and its application to synthesis of deuterium-labeled compounds. Chem.–Eur. J. 2008;14:3371–3379. doi: 10.1002/chem.200701245. [DOI] [PubMed] [Google Scholar]
- For selected examples on transition metal mediated deuteration recently, see: ; (a) Vang Z. P. Hintzsche S. J. Clark J. R. Catalytic transfer deuteration and hydrodeuteration: emerging techniques to selectively transform alkenes and alkynes to deuterated alkanes. Chem.–Eur. J. 2021;27:9988–10000. doi: 10.1002/chem.202100635. [DOI] [PubMed] [Google Scholar]; (b) Concellón J. M. Rodríguez-Solla H. Concellón C. Deuteration of α,β-acetylenic esters, amides, or carboxylic acids without using deuterium gas: synthesis of 2,2,3,3-tetradeuterioesters, amides, or acids. Tetrahedron Lett. 2004;45:2129–2131. doi: 10.1016/j.tetlet.2004.01.051. [DOI] [Google Scholar]
- For selected reviews on electrochemical deuteration recently, see: ; (a) Norcott P. L. Current electrochemical approaches to selective deuteration. Chem. Commun. 2022;58:2944–2953. doi: 10.1039/D2CC00344A. [DOI] [PubMed] [Google Scholar]; (b) Ou W. Qiu C. Su C. Photo- and electro-catalytic deuteration of feedstock chemicals and pharmaceuticals: a review. Chin. J. Catal. 2022;43:956–970. doi: 10.1016/S1872-2067(21)63928-1. [DOI] [Google Scholar]
- For selected examples: ; (a) Seo H. Katcher M. H. Jamison T. F. Photoredox activation of carbon dioxide for amino acid synthesis in continuous flow. Nat. Chem. 2017;9:453–456. doi: 10.1038/nchem.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ye J.-H. Miao M. Huang H. Yan S.-S. Yin Z.-B. Zhou W.-J. Yu D.-G. Visible-light-driven iron-promoted thiocarboxylation of styrenes and acrylates with CO2. Angew. Chem., Int. Ed. 2017;56:15416–15420. doi: 10.1002/anie.201707862. [DOI] [PubMed] [Google Scholar]; (c) Yan S.-S. Liu S.-H. Chen L. Bo Z.-Y. Jing K. Gao T.-Y. Yu B. Lan Y. Luo S.-P. Yu D.-G. Visible-light photoredox-catalyzed selective carboxylation of C(sp3)−F bonds with CO2. Chem. 2021;7:3099–3113. doi: 10.1016/j.chempr.2021.08.004. [DOI] [Google Scholar]; (d) Yue J.-P. Xu J.-C. Luo H.-T. Chen X.-W. Song H.-X. Deng Y. Yuan L. Ye J.-H. Yu D.-G. Metallaphotoredox-enabled aminocarboxylation of alkenes with CO2. Nat. Catal. 2023;6:959–968. doi: 10.1038/s41929-023-01029-9. [DOI] [Google Scholar]; (e) Yu B. Liu Y. Xiao H.-Z. Zhang S.-R. Ran C.-K. Song L. Jiang Y.-X. Li C.-F. Ye J.-H. Yu D.-G. Switchable divergent di- or tricarboxylation of allylic alcohols with CO2. Chem. 2024;10:938–951. doi: 10.1016/j.chempr.2023.12.005. [DOI] [Google Scholar]; (f) Jiang Y.-X. Liao L.-L. Gao T.-Y. Xu W.-H. Zhang W. Song L. Sun G.-Q. Ye J.-H. Lan Y. Yu D.-G. Visible-light-driven synthesis of N-heteroaromatic carboxylic acids by thiolate-catalysed carboxylation of C(sp2)–H bonds using CO2. Nat. Synth. 2024;3:394–405. doi: 10.1038/s44160-023-00465-6. [DOI] [Google Scholar]; (g) Xiao H.-Z. Yu B. Yan S.-S. Zhang W. Li X.-X. Bao Y. Luo S.-P. Ye J.-H. Yu D.-G. Photocatalytic 1,3-Dicarboxylation of Unactivated Alkenes with CO2. Chin. J. Catal. 2023;50:222–228. doi: 10.1016/S1872-2067(23)64468-7. [DOI] [Google Scholar]
- For selected examples: ; (a) Wang H. Gao Y. Zhou C. Li G. Visible-light-driven reductive carboarylation of styrenes with CO2 and aryl halides. J. Am. Chem. Soc. 2020;142:8122–8129. doi: 10.1021/jacs.0c03144. [DOI] [PubMed] [Google Scholar]; (b) Hendy C. M. Smith G. C. Xu Z. Lian T. Jui N. T. Radical chain reduction via carbon dioxide radical anion (CO2˙–) J. Am. Chem. Soc. 2021;143:8987–8992. doi: 10.1021/jacs.1c04427. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Malandain A. Molins M. Hauwelle A. Talbot A. Loreau O. D'Anfray T. Goutal S. Tournier N. Taran F. Caillé F. Audisio D. Carbon dioxide radical anion by photoinduced equilibration between formate salts and [11C, 13C, 14C]CO2: application to carbon isotope radiolabeling. J. Am. Chem. Soc. 2023;145:16760–16770. doi: 10.1021/jacs.3c04679. [DOI] [PubMed] [Google Scholar]; (d) Xu P. Wang S. Xu H. Liu Y.-Q. Li R.-B. Liu W.-W. Wang X.-Y. Zou M.-L. Zhou Y. Guo D. Zhu X. Dicarboxylation of alkenes with CO2 and formate via photoredox catalysis. ACS Catal. 2023;13:2149–2155. doi: 10.1021/acscatal.2c06377. [DOI] [Google Scholar]; (e) Wang X.-Y. Xu P. Liu W.-W. Jiang H.-Q. Zhu S.-L. Guo D. Zhu X. Divergent defluorocarboxylation of α-CF3 alkenes with formate via photocatalyzed selective mono- or triple C–F bond cleavage. Sci. China: Chem. 2024;67:368–373. doi: 10.1007/s11426-023-1731-x. [DOI] [Google Scholar]; (f) Xu P. Xu H. Wang S. Hao T.-Z. Yan S.-Y. Guo D. Zhu X. Transition-metal free oxidative carbo-carboxylation of alkenes with formate in Air. Org. Chem. Front. 2023;10:2013–2017. doi: 10.1039/D3QO00126A. [DOI] [Google Scholar]; (g) Xu P. Wang X.-Y. Wang Z. Zhao J. Cao X.-D. Xiong X.-C. Yuan Y.-C. Zhu S. Guo D. Zhu X. Defluorinative alkylation of trifluoromethylbenzimidazoles enabled by spin-center shift: a synergistic photocatalysis/thiol catalysis process with CO2˙–. Org. Lett. 2022;24:4075–4080. doi: 10.1021/acs.orglett.2c01533. [DOI] [PubMed] [Google Scholar]; (h) Huang H. Ye J.-H. Zhu L. Ran C.-K. Miao M. Wang W. Chen H. Zhou W.-J. Lan Y. Yu B. Yu D.-G. Visible-light-driven anti-markovnikov hydrocarboxylation of acrylates and styrenes with CO2. CCS Chem. 2021;3:1746–1756. doi: 10.31635/ccschem.020.202000374. [DOI] [Google Scholar]; (i) Ye J.-H. Miao M. Huang H. Yan S.-S. Yin Z.-B. Zhou W.-J. Yu D.-G. Visible-light-driven iron-promoted thiocarboxylation of styrenes and acrylates with CO2. Angew. Chem., Int. Ed. 2017;56:15416–15420. doi: 10.1002/anie.201707862. [DOI] [PubMed] [Google Scholar]; (j) Yan S.-S. Liu S.-H. Chen L. Bo Z.-Y. Jing K. Gao T.-Y. Yu B. Lan Y. Luo S.-P. Yu D.-G. Visible-light photoredox-catalyzed selective carboxylation of C(sp3)−F bonds with CO2. Chem. 2021;7:3099–3113. doi: 10.1016/j.chempr.2021.08.004. [DOI] [Google Scholar]; (k) Yu B. Liu Y. Xiao H.-Z. Zhang S.-R. Ran C.-K. Song L. Jiang Y.-X. Li C.-F. Ye J.-H. Yu D.-G. Switchable divergent di- or tricarboxylation of allylic alcohols with CO2. Chem. 2024;10:938–951. doi: 10.1016/j.chempr.2023.12.005. [DOI] [Google Scholar]
- For selected reviews see: ; (a) Gui Y. Y. Yan S.-S. Wang W. Chen L. Zhang W. Ye J.-H. Yu D.-G. Exploring the applications of carbon dioxide radical anion in organic synthesis. Sci. Bull. 2023;68:3124–3128. doi: 10.1016/j.scib.2023.11.018. [DOI] [PubMed] [Google Scholar]; (b) Majhi J. Molander G. A. Recent discovery, development, and synthetic applications of formic acid salts in photochemistry. Angew. Chem., Int. Ed. 2023;62:e202311853. doi: 10.1002/anie.202311853. [DOI] [PubMed] [Google Scholar]; (c) Xiao W. Zhang J. Wu J. Recent advances in reactions involving carbon dioxide radical anion. ACS Catal. 2023;13:15991–16011. doi: 10.1021/acscatal.3c04125. [DOI] [Google Scholar]; (d) Wang S. Xu P. Zhu X. CO2 radical anion in photochemical dicarboxylation of alkenes. ChemCatChem. 2023;15:e202300695. doi: 10.1002/cctc.202300695. [DOI] [Google Scholar]; (e) Alkayal A. Tabas V. Montanaro S. Wright I. A. Malkov A. V. Buckley B. R. Harnessing applied potential: selective β-hydrocarboxylation of substituted olefins. J. Am. Chem. Soc. 2020;142:1780–1785. doi: 10.1021/jacs.9b13305. [DOI] [PubMed] [Google Scholar]; (f) Kang G. Romo D. Photocatalyzed, β-selective hydrocarboxylation of α,β-unsaturated esters with CO2 under flow for β-lactone synthesis. ACS Catal. 2021;11:1309–1315. doi: 10.1021/acscatal.0c05050. [DOI] [Google Scholar]; (g) Mangaonkar S. R. Hayashi H. Takano H. Kanna W. Maeda S. Mita T. Photoredox/HAT-catalyzed dearomative nucleophilic addition of the CO2 radical anion to (hetero)aromatics. ACS Catal. 2023;13:2482–2488. doi: 10.1021/acscatal.2c06192. [DOI] [Google Scholar]
- (a) Miao M. Zhu L. Zhao H. Song L. Yan S.-S. Liao L.-L. Ye J.-H. Lan Y. Yu D.-G. Visible-light-driven thio-carboxylation of alkynes with CO2: facile synthesis of thiochromones. Sci. China: Chem. 2023;66:1457–1466. doi: 10.1007/s11426-022-1554-x. [DOI] [Google Scholar]; (b) Alektiar S. N. Wickens Z. K. Photoinduced Hydrocarboxylation via Thiol Catalyzed Delivery of Formate Across Activated Alkenes. J. Am. Chem. Soc. 2021;143:13022–13028. doi: 10.1021/jacs.1c07562. [DOI] [PubMed] [Google Scholar]
- For a selected example on α-carboxylation of alkynes in the presence of transition metals, see: ; (a) Yang D. Liu H. Liu L. Guo W.-D. Lu Y. Liu Y. Co-catalysis over a tri-functional ligand modified Pd-catalyst for hydroxycarbonylation of terminal alkynes towards α,β-unsaturated carboxylic acids. Green Chem. 2019;21:5336–5344. doi: 10.1039/C9GC01887E. [DOI] [Google Scholar]; (b) Liu L. Yao Y.-Q. Chen X.-C. Guo L. Lu Y. Zhao X.-L. Liu Y. Hydrocarboxylation of alkynes with formic acid over multifunctional ligand modified Pd-catalyst with co-catalytic effect. J. Catal. 2022;405:322–332. doi: 10.1016/j.jcat.2021.12.011. [DOI] [Google Scholar]
- Kai T. Zhou M. Johnson S. Ahn H. S. Bard A. J. Direct observation of C2O4˙– and CO2˙– by oxidation of oxalate within nanogap of scanning electrochemical microscope. J. Am. Chem. Soc. 2018;140:16178–16183. doi: 10.1021/jacs.8b08900. [DOI] [PubMed] [Google Scholar]
- An example for alkene carboxylation with TBAO, see: ; Wu Z. Wu M. Zhu K. Wu J. Lu Y. Photocatalytic coupling of electron-deficient alkenes using oxalic acid as a traceless linchpin. Chem. 2023;9:978–988. [Google Scholar]
- For examples using TBAO as the CO2 radical anion precursor and reductant, see: ; (a) Draper F. Doeven E. H. Adcock J. L. Francis P. S. Connell T. U. Extending photocatalyst activity through choice of electron donor. J. Org. Chem. 2023;88:6445–6453. doi: 10.1021/acs.joc.2c02460. [DOI] [PubMed] [Google Scholar]; (b) AlSalka Y. Al-Madanat O. Curti M. Hakki A. Bahnemann D. W. Photocatalytic H2 evolution from oxalic acid: effect of cocatalysts and carbon dioxide radical anion on the surface charge transfer mechanisms. ACS Appl. Energy Mater. 2020;3:6678–6691. doi: 10.1021/acsaem.0c00826. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been included as part of the ESI.†