Abstract
Background
The post-insertion clinical course of esophageal self-expandable metal stents (SEMS) in initially frail patients with esophageal carcinoma (EC) with dysphagia remains unclear. This study aimed to assess dysphagia improvement and evaluate prognosis in initially frail patients with advanced EC following SEMS insertion.
Methods
We retrospectively reviewed EC patients with EC who underwent esophageal SEMS insertion at our institution between January 2014 and March 2023. Inclusion criteria comprised Eastern Cooperative Oncology Group Performance Status (ECOG PS) ≥ 3 or ECOG PS 2 for individuals aged ≥ 75 years and recommendation for best supportive care by a multidisciplinary team.
Results
Forty-six patients met the inclusion criteria. Among them, 37 patients (80.4%) were ≥ 75 years old, and 21 patients (45.7%) exhibited ECOG PS 3 or 4. Dysphagia score (DS) ≥ 3 was observed in 27 patients (58.7%). All esophageal SEMS insertions were successfully completed. Post-procedure, there were two fatal cases of aspiration pneumonia and one perforation incident. DS improved to ≤ 1 in 25 patients (54.3%), with multivariate analysis indicating DS 3–4 and Glasgow Prognostic Score (GPS) 1–2 as negative predictive factors. The median overall survival was 4.1 months (95% confidence interval 1.8–6.5).
Conclusions
Esophageal SEMS insertion effectively alleviated dysphagia in initially frail EC patients, yet prognosis remained poor, with occurrences of some fatal adverse events. Careful selection of candidates for esophageal SEMS insertions is crucial in this demographic, particularly considering the challenges in improving dysphagia for patients with DS 3–4 and GPS 1–2.
Keywords: Esophageal cancer, Frail, Metallic stent
Background
Esophageal cancer (EC) stands as the ninth most common cancer globally and the sixth leading cause of cancer-related fatalities [1]. Pathologically, squamous cell carcinoma (SCC) and adenocarcinoma are the common types [2]. While SCC is predominant in East Asia, both types occur frequently in North America and Europe [2]. Prognosis for patients with EC is poor, with reported 5-year survival rates of 10–30% [3].
Advanced EC causes esophageal stenosis, dysphagia, and cancer cachexia, contributing to poor nutrition and frailty [4]. Moreover, EC disproportionately affects elderly patients, with the highest incidence rate among individuals in their 80s, and the largest population of it is those in their 60s and 70s [5]. Nationwide registry studies have shown that 29.3–36.9% of EC diagnoses occur in individuals in their 70s, with 10.0–21.1% diagnosed at 80 years or older [6, 7]. Treatments for EC, such as esophagectomy, combination chemotherapy, and definitive (chemo)radiotherapy, can be intensive [8–10]. However, some patients with advanced EC may prove intolerant to these treatments at diagnosis, necessitating a recommendation for best supportive care (BSC).
Esophageal self-expandable metal stent (SEMS) insertion emerges as a palliative option for patients with EC experiencing dysphagia [8–10]. This minimally invasive approach becomes particularly relevant for frail patients with EC unable to tolerate aggressive cancer treatments. While esophageal SEMS insertion swiftly ameliorates dysphagia, it does present potential adverse events (AEs), such as perforation, hemorrhage, and aspiration pneumonia [11–14]. Previous reports have included patients who received chemotherapy or (chemo)radiotherapy before or after esophageal SEMS insertion, leaving the clinical course among initially frail EC patients poorly understood [11–14]. This study aimed to examine dysphagia improvement, evaluate prognosis, and investigate safety consideration following SEMS insertion in frail patients with advanced EC initially intolerant to active anticancer treatments.
Materials and methods
Patients
We retrospectively analyzed patients with primary EC experiencing dysphagia who underwent palliative esophageal SEMS insertion at Kanagawa Cancer Center Hospital between January 2014 and March 2023. Within this cohort, we identified patients initially recommended BSC alone by a multidisciplinary team owing to their frailty. Frailty was defined as an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≥ 3 [15] or age ≥ 75 years with an ECOG PS ≥ 2, based on previous reports [16, 17]. Additional inclusion criteria were as follows: dysphagia score (DS) of ≥ 2 [18] and no history of esophagectomy, definitive chemo(radiotherapy), or chemotherapy for EC during their treatment course.
Esophageal SEMS insertion
All patients received local pharyngeal anesthesia using lidocaine spray and intravenous sedation with midazolam or diazepam prior to the procedure. Before esophageal SEMS insertion, we conducted upper gastrointestinal endoscopy and fluoroscopy to examine the location and extent of esophageal stenosis. The length of the esophageal SEMS was selected to ensure 1–2-cm proximal and distal margins. In cases where the lower edge of the esophageal SEMS resided at the gastro-esophageal junction (GEJ) or stomach, a partially covered stent equipped with an antireflux valve (HANAROSTENT® Esophagus Valve, MI Tech, Seoul, Korea or Niti-S™ Esophageal Stent [Antireflux], Taewoong Medical, Seoul, Korea) was selected. Conversely, if the lower edge of the esophageal SEMS lay above the GEJ, a standard fully covered stent (HANAROSTENT® Esophagus, MI Tech, Seoul, Korea or Niti-S™ Esophageal Stent, Taewoong Medical, Seoul, Korea) was utilized. We inserted a stent delivery system into the esophagus using a guidewire, subsequently placing the esophageal SEMS under fluoroscopic guidance.
Assessment
Cancer staging followed the Union for International Cancer Control 8th edition guidelines [19]. Dysphagia assessment utilized DS outlined by Mellow and Pinkas, categorized as follows: 0 = able to consume a normal diet/no dysphagia; 1 = able to ingest some solid foods; 2 = able to swallow only semi-solid foods; 3 = able to swallow only liquids; and 4 = unable to swallow anything/total dysphagia [18]. DS was evaluated 1 week post-esophageal SEMS insertion. When patients experienced transient inability to swallow due to AEs after esophageal SEMS insertion, DS assessment was conducted after resolution of these AEs. AEs were assessed based on the Common Terminology Criteria for Adverse Events version 5.0 [20]. Additionally, the Glasgow Prognostic Score (GPS), which is known as a prognostic factor, was evaluated as follows: GPS 0 = baseline C-reactive protein (CRP) ≤ 1.0 mg/dL and serum albumin levels (Alb) ≥ 3.5 g/dL; GPS 1 = either CRP > 1.0 mg/dL or Alb < 3.5 g/dL; GPS 2 = CRP > 1.0 mg/dL and Alb < 3.5 g/dL [21, 22].
Statistical analysis
Continuous variables are expressed as medians and ranges, while categorical variables are presented as numbers and percentages. Overall survival (OS) was defined as the duration from the date of esophageal SEMS insertion to death from any cause, with a data cutoff date of July 5, 2023. Kaplan–Meier curves were constructed to estimate OS. Univariate logistic regression analysis was employed to evaluate predictive factors for achieving DS improvement to ≤ 1 and the incidence of adverse events. Variables with a p-value < 0.10 in the univariate analysis were subjected to multivariate logistic regression analysis to determine their independent effects. Cox regression analyses were conducted for OS. Univariate factors with a p-value < 0.10 were included in the multivariate Cox regression analysis to assess their independent impact on OS. Statistical significance was set at a two-sided p-value < 0.05. All statistical analyses were performed using EZR software, version 1.32 (Saitama Medical Center, Jichii Medical University, Saitama, Japan) [23].
Results
Patient characteristics
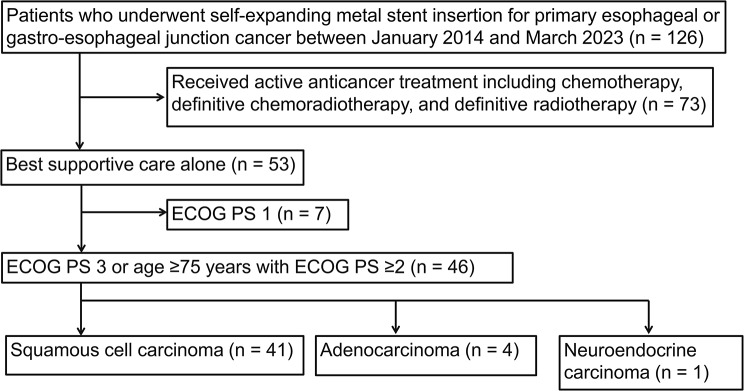
A total of 126 patients with EC underwent palliative esophageal SEMS insertion at our institution between January 2014 and March 2023. Among these, 46 patients met the inclusion criteria, while 80 patients were excluded from the present study (Fig. 1).
Fig. 1.
Patient flow diagram
ECOG PS: Eastern Cooperative Oncology Group Performance status
Table 1 displays patient characteristics. SCC emerged as the predominant histological type, observed in 41 patients (89.1%). Adenocarcinoma and neuroendocrine carcinoma were found in 4 patients (8.7%) and 1 patient (2.2%), respectively. Among the cohort, 37 patients (80.4%) were ≥ 75 years old, and 21 patients (45.7%) exhibited an ECOG PS of 3 or 4. Severe dysphagia (DS ≥ 3) was observed in 27 patients (58.7%). All patients presented with advanced EC, characterized by a clinical T stage of T3 or T4, and a clinical stage of III or IV. The GPS was elevated to 1 or 2 in 20 patients (43.5%).
Table 1.
Patient characteristics
| All (n = 46) |
SCC (n = 41) |
Adenocarcinoma (n = 4) |
NEC (n = 1) |
||
|---|---|---|---|---|---|
| Age (years) |
Median (range) ≥ 75 |
81 (57–93) 37 (80.4%) |
81 (57–93) 33 (80.5%) |
86 (72–88) 3 (75%) |
86 1 |
| Sex | Male | 39 (84.8%) | 35 (85.4%) | 3 (75%) | 1 |
| BMI, kg/m2 | Median (range) | 18.5 (12.8–26.8) | 18.5 (12.8–26.8) | 19.3 (14.7–24.2) | 25.0 |
| ECOG PS |
2 3 4 |
25 (54.3%) 16 (34.8%) 5 (10.9%) |
24 (58.5%) 12 (29.3%) 5 (12.2%) |
1 (25%) 3 (75%) 0 |
0 1 0 |
| Comorbidity index |
0 1 ≥ 2 |
20 (43.5%) 9 (19.6%) 17 (37.0%) |
17 (41.5%) 8 (19.5%) 16 (39.0%) |
3 (75%) 0 1 (25%) |
0 1 0 |
| Clinical T stage* |
cT3 cT4a cT4b |
21 (45.7%) 9 (19.6%) 16 (34.8%) |
20 (48.8%) 6 (14.6%) 15 (36.6%) |
1 (25%) 3 (75%) 0 |
0 0 1 |
| Clinical N stage* |
N0 N1 N2 N3 |
10 (21.7%) 22 (47.8%) 10 (21.7%) 4 (8.7%) |
8 (19.5%) 20 (48.8%) 9 (22.0%) 4 (9.8%) |
2 (50%) 1 (25%) 1 (25%) 0 |
0 1 0 0 |
| Clinical M stage* |
M0 M1 |
23 (50.0%) 23 (50.0%) |
23 (56.1%) 18 (43.9%) |
0 4 (100%) |
0 1 |
| Clinical stage* |
III IVA IVB |
11 (23.9%) 12 (26.1%) 23 (50.0%) |
11 (26.8%) 12 (29.3%) 18 (43.9%) |
0 0 4 (100%) |
0 0 1 |
| Dysphagia score |
2 3 4 |
19 (41.3%) 14 (30.4%) 13 (28.3%) |
17 (41.5%) 12 (29.3%) 12 (29.3%) |
1 (25%) 2 (50%) 1 (25%) |
1 0 0 |
| Borrmann classification |
1 2 3 4 |
4 (8.7%) 25 (54.3%) 13 (28.3%) 4 (8.7%) |
4 (9.8%) 24 (58.5%) 12 (29.3%) 1 (2.4%) |
0 0 1 (25%) 3 (75%) |
0 1 0 0 |
| Primary tumor length, cm | Median (range) | 7.0 (3.0–19.0) | 7.0 (3.0–19.0) | 5.5 (3.0–8.0) | 10.0 |
| Metastatic site |
Lymph node Liver Lung Peritoneal |
38 (82.6%) 1 (2.2%) 7 (15.2%) 3 (6.5%) |
35 (85.4%) 1 (2.4%) 6 (14.6%) 1 (2.4%) |
2 (50%) 0 1 (25%) 2 (50%) |
1 0 0 0 |
| Number of metastatic sites |
0 1 ≥ 2 |
5 (10.9%) 30 (65.2%) 11 (23.9%) |
5 (12.2%) 27 (65.9%) 9 (22.0%) |
0 2 (50%) 2 (50%) |
0 1 0 |
| Primary tumor location |
Upper thoracic Middle thoracic Lower thoracic GEJ |
7 (15.2%) 22 (47.8%) 12 (26.1%) 5 (10.9%) |
7 (17.1%) 21 (51.2%) 12 (29.3%) 1 (2.4%) |
0 0 0 4 (100%) |
0 1 0 0 |
| Alb (g/dL) | median (range) | 3.7 (1.5–4.3) | 3.7 (1.7–4.3) | 3.4 (1.5–4.3) | 3.7 |
| CRP (mg/dL) | median (range) | 0.72 (0.1–19.6) | 0.72 (0.1–19.6) | 1.36 (0.1–9.3) | 0.2 |
| GPS |
0 1 2 |
26 (56.5%) 7 (15.2%) 13 (28.3%) |
23 (56.1%) 7 (17.1%) 11 (26.8%) |
2 (50%) 0 2 (50%) |
1 0 0 |
*Cancer staging followed the Union for International Cancer Control 8th edition guidelines. SCC: squamous cell carcinoma; NEC: neuroendocrine carcinoma; BMI: body mass index; ECOG PS: Eastern Cooperative Oncology Group performance status; GEJ: gastro-esophageal junction; Alb: serum albumin; CRP: serum C-reactive protein; GPS: Glasgow Prognostic Score
Esophageal SEMS materials
One patient required the insertion of two esophageal SEMS: a HANAROSTENT® Esophagus Valve and a HANAROSTENT® Esophagus (without an antireflux valve) due to an extensive esophageal stricture. The remaining patients underwent a single esophageal SEMS insertion. Among these, 13 received antireflux valve stents (11 HANAROSTENT® Esophagus Valve and 2 Niti-S™ Esophageal Stent [Antireflux]), while 32 received non-antireflux valve stents (31 HANAROSTENT® Esophagus and one Niti-S™ Esophageal Stent).
Esophageal SEMS insertions
All esophageal SEMS insertions were successfully completed, and the stents were appropriately positioned as planned. Two patients required re-stenting procedures. One patient underwent re-stenting 12 days after the initial insertion due to inadequate coverage of the esophageal stricture upon stent expansion caused by stent shortening. The other patient underwent re-stenting 6 months after the initial stent insertion due to tumor progression.
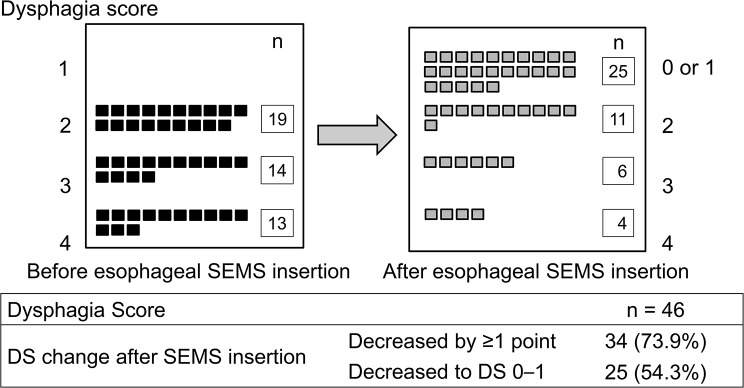
Improvement of DS after esophageal SEMS insertions
Figure 2 illustrates the DS before and after esophageal SEMS insertion. A decrease of at least 1 point in DS was observed in 34 patients (73.9%), with 25 patients (54.3%) achieving an improved DS of ≤ 1. The DS decreased by at least 1 point from the baseline score in 14 of 19 patients with DS 2 (73.7%), 9 of 14 patients with DS 3 (64.3%), and 11 of 13 patients with DS 4 (84.6%). Lowering DS to ≤ 1 was achieved in 14 of 19 patients with DS 2 (73.7%), 7 of 14 patients with DS 3 (50.0%), and 4 of 13 patients with DS 4 (30.8%).
Fig. 2.
Improvement of dysphagia score after esophageal SEMS insertion
SEMS: self-expandable metal stent; DS: dysphagia score
Univariate and multivariate logistic regression analyses were performed to identify predictive factors for DS improvement to ≤ 1 (Table 2). Univariate logistic regression analysis revealed four factors with p < 0.10. Subsequently, multivariate logistic regression analysis identified two significant risk factors: (i) DS 3–4 and (ii) GPS 1–2. Based on the number of risk factors, DS improvement to ≤ 1 was observed in 10 of 11 patients without any risk factors (90.9%), 14 of 23 patients with 1 risk factor (60.9%), and 1 of 12 patients with 2 risk factors (8.3%).
Table 2.
Predictive factors for improvement of dysphagia score to ≤ 1 on univariate and multivariate logistic regression analyses
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | ||
| Age (years) |
< 75 ≥ 75 |
1 5.75 |
- 1.04–31.70 |
- 0.04 |
1 0.85 |
- 0.08–8.73 |
- 0.89 |
| Sex |
Male Female |
1 0.28 |
- 0.05–1.62 |
- 0.15 |
|||
| BMI, kg/m2 |
≥ 18.5 < 18.4 |
1 0.69 |
- 0.22–2.22 |
- 0.54 |
|||
| ECOG PS |
2 3–4 |
1 0.42 |
- 0.13–1.39 |
- 0.16 |
|||
| Pathology |
SCC Others |
1 1.30 |
- 0.20–8.59 |
- 0.79 |
|||
| Comorbidity index |
0 ≥ 1 |
1 0.96 |
- 0.30–3.08 |
- 0.94 |
|||
| Clinical T stage* |
cT3 cT4 |
1 0.27 |
- 0.08–0.92 |
- 0.04 |
1 0.36 |
- 0.07–1.94 |
- 0.24 |
| Clinical N stage* |
N0 N1-3 |
1 1.25 |
- 0.31–5.08 |
- 0.76 |
|||
| Clinical M stage* |
M0 M1 |
1 0.59 |
- 0.18–1.90 |
- 0.38 |
|||
| Dysphagia score |
2 3–4 |
1 0.25 |
- 0.07–0.88 |
- 0.03 |
1 0.16 |
- 0.03–0.94 |
- 0.04 |
| Primary tumor length, cm |
< 7.0 ≥ 7.0 |
1 0.37 |
- 0.11–1.26 |
- 0.11 |
|||
| Number of metastatic sites |
0–1 ≥ 2 |
1 0.63 |
- 0.16–2.44 |
- 0.50 |
|||
| Primary tumor location |
Thoracic GEJ |
1 0.52 |
- 0.08–3.46 |
- 0.50 |
|||
| GPS |
0 1–2 |
1 0.10 |
- 0.03–0.39 |
- < 0.01 |
1 0.07 |
- 0.01–0.40 |
- < 0.01 |
*Cancer staging followed the Union for International Cancer Control 8th edition guidelines. OR: odds ratio; CI: confidence interval; BMI: body mass index; ECOG PS: Eastern Cooperative Oncology Group performance status; SCC: squamous cell carcinoma; GEJ: gastro-esophageal junction; GPS: Glasgow Prognostic Score
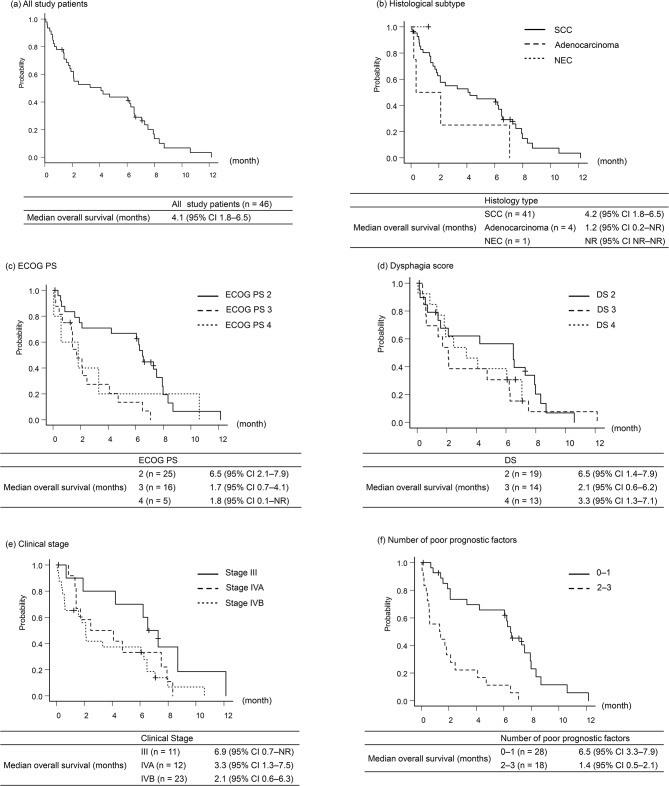
Overall survival
The median OS for all study participants was 4.1 months (95% confidence interval [CI] 1.8–6.5) in all study patients (Fig. 3a). According to histological type, the median OS was 4.2 months (95% CI 1.8–6.5) in SCC and 1.2 months (95% CI 0.2–not reached) in adenocarcinoma (Fig. 3b). Subgroup analyses based on ECOG PS, DS, and clinical stage are shown in Fig. 3c–e.
Fig. 3.
Overall survival from esophageal SEMS insertion in all study patients and subgroup analyses. (a) All study patients. (b) Histological subtype. (c) Eastern Cooperative Oncology Group Performance status. (d) Dysphagia score. (e) Clinical stage. (f) Number of poor prognostic factors. Poor prognostic factors were defined as (i) clinical stage IV, (ii) primary tumor location GEJ, and (iii) GPS 1–2
GEJ: gastro-esophageal junction; GPS: Glasgow prognostic score; SCC: squamous cell carcinoma; NEC: neuroendocrine carcinoma; CI: confidence interval; NR: not reached; ECOG PS: Eastern Cooperative Oncology Group Performance status; DS: dysphagia score
Univariate and multivariate Cox regression analyses were performed to identify prognostic factors for OS (Table 3). Univariate Cox regression analysis revealed five factors with p < 0.10. In the multivariate Cox regression analysis, three significantly poor prognostic factors were identified: (i) clinical stage IV, (ii) primary tumor location in the GEJ, and (iii) GPS 1–2. Patients with 0–1 poor prognostic factor had a median OS of 6.5 months (95% CI; 3.3–7.9), while those with 2–3 poor prognostic factors had a median OS of 1.4 months (95% CI; 0.5–2.1) (Fig. 3f).
Table 3.
Overall survival on univariate and multivariate Cox regression analyses
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | ||
| Age (years) |
< 75 ≥ 75 |
1 0.26 |
- 0.11–6.2 |
- < 0.01 |
1 0.45 |
- 0.16–1.31 |
- 0.14 |
| Sex |
Male Female |
1 0.55 |
- 0.21–1.41 |
- 0.22 |
|||
| BMI, kg/m2 |
≥ 18.5 < 18.4 |
1 0.70 |
- 0.37–1.34 |
- 0.28 |
|||
| ECOG PS |
2 3–4 |
1 2.51 |
- 1.31–4.81 |
- < 0.01 |
1 1.02 |
- 0.40–2.60 |
- 0.97 |
| Pathology |
SCC Others |
1 1.51 |
- 0.60–3.81 |
- 0.38 |
|||
| Comorbidity index |
0 ≥ 1 |
1 1.45 |
- 0.75–2.80 |
- 0.27 |
|||
| Clinical stage* |
III IV |
1 2.39 |
- 1.04–5.47 |
- 0.04 |
1 2.93 |
- 1.02–8.45 |
- 0.046 |
| Dysphagia score |
2 3–4 |
1 1.43 |
- 0.73–2.77 |
- 0.28 |
|||
| Primary tumor length, cm |
< 7.0 ≥ 7.0 |
1 1.42 |
- 0.74–2.72 |
- 0.29 |
|||
| Number of metastatic sites |
0–1 ≥ 2 |
1 1.68 |
- 0.82–3.42 |
- 0.16 |
|||
| Primary tumor location |
Thoracic GEJ |
1 2.71 |
- 1.04–7.07 |
- 0.04 |
1 3.34 |
- 1.08–10.6 |
- 0.04 |
| GPS |
0 1–2 |
1 2.21 |
- 1.17–4.18 |
- 0.02 |
1 3.12 |
- 1.41–6.89 |
- < 0.01 |
*Cancer staging followed the Union for International Cancer Control 8th edition guidelines. HR: hazard ratio; CI: confidence interval; BMI: body mass index; ECOG PS: Eastern Cooperative Oncology Group performance status; SCC: squamous cell carcinoma; GEJ: gastro-esophageal junction; GPS: Glasgow Prognostic Score
Adverse events
Any grade AEs occurred in 8 patients (17.4%). Among these, aspiration pneumonia was predominant (n = 7), comprising grade 2 in 3 patients, grade 3 in 2 patients, and grade 5 in 2 patients. The primary tumor location for the two patients experiencing grade 5 aspiration pneumonia was at the GEJ. Additionally, grade 5 perforation was observed in 1 patient with middle thoracic esophageal cancer. These grade 5 AEs occurred within 1 week following SEMS insertion. No other fatal adverse events were observed.
Univariate logistic regression analysis was performed to identify predictive factors for the incidence of adverse events (Table 4). Only GPS 1–2 was identified as a significant factor. Multivariate logistic regression analysis was not performed due to the limited number of adverse events.
Table 4.
Predictive factors for incidence of adverse events on univariate logistic regression analysis
| Univariate analysis | ||||
|---|---|---|---|---|
| OR | 95% CI | P Value | ||
| Age (years) |
< 75 ≥ 75 |
1 0.31 |
- 0.06–1.67 |
- 0.17 |
| Sex |
Male Female |
1 0.76 |
- 0.08–7.37 |
- 0.81 |
| BMI, kg/m2 |
≥ 18.5 < 18.4 |
1 3.33 |
- 0.60–18.70 |
- 0.17 |
| ECOG PS |
2 3–4 |
1 4.60 |
- 0.82–25.90 |
- 0.08 |
| Pathology |
SCC Others |
1 1.21 |
- 0.12–12.60 |
- 0.87 |
| Comorbidity index |
0 ≥ 1 |
1 0.39 |
- 0.08–1.89 |
- 0.24 |
| Clinical T stage* |
cT3 cT4 |
1 7.78 |
- 0.87–69.50 |
- 0.07 |
| Clinical N stage* |
N0 N1-3 |
1 0.92 |
- 0.38–2.25 |
- 0.86 |
| Clinical M stage* |
M0 M1 |
1 1.00 |
- 0.22–4.59 |
- 1.00 |
| Dysphagia score |
2 3–4 |
1 6.30 |
- 0.71–56.30 |
- 0.10 |
| Primary tumor length, cm |
< 7.0 ≥ 7.0 |
1 2.43 |
- 0.43–13.60 |
- 0.31 |
| Number of metastatic sites |
0–1 ≥ 2 |
1 1.07 |
- 0.18–6.28 |
- 0.94 |
| Primary tumor location |
Thoracic GEJ |
1 3.89 |
- 0.53–28.40 |
- 0.18 |
| GPS |
0 1–2 |
1 13.5 |
- 1.49–121.00 |
- 0.02 |
*Cancer staging followed the Union for International Cancer Control 8th edition guidelines. OR: odds ratio; CI: confidence interval; BMI: body mass index; ECOG PS: Eastern Cooperative Oncology Group performance status; SCC: squamous cell carcinoma; GEJ: gastro-esophageal junction; GPS: Glasgow Prognostic Score
Discussion
The present study revealed the outcomes following esophageal SEMS insertion among frail patients with EC initially intolerant to active anticancer treatments. Our findings not only elucidate predictive and prognostic factors for dysphagia improvement but also have the potential to help physicians in deciding whether to insert esophageal SEMS for patients with EC experiencing initial frailty and dysphagia.
While several studies have explored prognosis after esophageal SEMS insertion in patients with advanced EC, they often lacked a focus on initially frail patients with EC. Two previous prospective phase III studies, SIREC and ROCS, evaluated SEMS insertion for incurable EC patients with dysphagia, reporting an OS of approximately 5 months, which was longer than that observed in our study [11, 14]. These studies did not restrict participation based on frailty but rather on other criteria, such as cancer stage, medical condition, or patient preference. Patients pretreated or treated with chemotherapy after esophageal SEMS insertion were recruited. It is assumed that the inclusion of non-frail patients, who typically have better medical conditions, contributed to their extended survival in those studies. In contrast, our study strictly focused on initially frail patients with EC [11, 14]. Our criteria for intolerance to active cancer treatment align with a multicenter questionnaire survey and inclusion criteria from clinical trials [16, 17], potentially offering a widely acceptable definition for many physicians. As global populations age and more EC diagnoses occur at advanced stages, especially during the COVID-19 pandemic era, the population of initially frail EC patients is on the rise [24, 25]. Our study’s insights could significantly assist physicians in clinical practice and decision-making.
The approach to evaluating dysphagia improvement varies among studies. For instance, the SERIC study using the Mellow and Pinkas scale indicated a mean DS improvement to 1.0–1.2 points within 1 or 2 weeks [11]. Another report demonstrated a median DS improvement to 1.0–2.0 post-SEMS insertion [26], recruiting patients with DS ≥ 2 [11, 26]. In the present study, the mean or median DS was evaluated because of the difficulty in distinguishing between DS 0 and 1 retrospectively. Nevertheless, 54.3% of our patients exhibited an assumed similar improvement to DS 0–1, aligning with previous findings.
We focused on identifying predictive factors for improving DS to ≤ 1 because patients in this category do not require severe dietary restriction. DS 3–4 and GPS 1–2 emerged as risk factors associated with failure to achieve DS ≤ 1. Dysphagia in patients with EC may correlate with interrupted peristalsis [27]. In patients with severe dysphagia, peristalsis is assumed to be exacerbated by tumor invasion, which may hinder the efficacy of esophageal stenosis improvement in addressing dysphagia. GPS is a prognostic factor that reflects nutritional status and inflammation [21, 22]. It is assumed that cancer cachexia might be advanced in patients with GPS 1–2 patients, potentially accompanied by appetite loss or swallowing dysfunction [4]. Regarding prognostic factors, we identified three poor prognostic factors: clinical stage IV, primary tumor location in the GEJ, and GPS 1–2. In this study, fatal aspiration pneumonia occurred in 2 patients with GEJ cancer despite antireflux valve-inserted SEMS, hence contributing to GEJ cancer as a poor prognostic factor. The success rate for DS improvement to ≤ 1 and OS was stratified by the number of risk factors. These risk factors could be useful in efficiently selecting candidates for esophageal SEMS insertion among initially frail patients with EC.
Three fatal cases of AEs, including perforation and aspiration pneumonia, were observed in this study, consistent with reports from previous studies [11, 14, 26]. No unexpected AEs were encountered by our team. Notably, aspiration pneumonia emerged as the most frequent AE. GPS 1–2 was associated with the occurrence of AEs in the univariate analysis. Patients with poor nutrition were more likely to be bedridden, increasing the risk of aspiration pneumonia. As these three fatal AEs occurred within 1 week following SEMS insertion, they could be associated with the treatment. It is crucial to proactively manage such AEs post-esophageal SEMS insertion and carefully consider SEMS insertion for patients anticipated to have challenging improvements in dysphagia.
The present study has several limitations. First, it was retrospective and conducted at a single institution, involving a limited number of patients. Due to the limited number of adverse events, multivariate logistic regression analysis could not effectively identify predictive factors for their incidence. Second, DS and AEs were assessed only in the short-term. Typically, our patients were discharged within a week post-SEMS insertion, transitioning to palliative care at home or in a unit. Because the SERIC study indicated DS improvement within 1 or 2 weeks, 1 week might be sufficient to evaluate the best point of DS [11]. However, our follow-up period was insufficient to evaluate long-term outcomes of DS and AEs. Third, while we identified factors for predicting dysphagia relief and poor prognosis, these factors were not validated. Fourth, quality of life (QOL) metrics were not captured in the present study. Thus, the contribution of SEMS insertion to QOL improvement in initially frail patients with EC remains unclear.
Conclusions
Esophageal SEMS insertion was effective in alleviating dysphagia among initially frail patients with EC. Despite this relief, their overall prognosis remained poor, with dysphagia persisting in several cases. Furthermore, instances of fatal AEs were observed. It is imperative to cautiously identify suitable candidates for esophageal SEMS insertions, particularly considering the challenges of improving dysphagia in patients with DS 3–4 and GPS 1–2. Further studies are essential to validate and substantiate these findings comprehensively.
Acknowledgements
We would like to thank Charles McKay and Editage (www.editage.com) for the English language editing.
Abbreviations
- AEs
Adverse events
- Alb
Serum albumin levels
- BMI
Body mass index
- BSC
Best supportive care
- CI
Confidence interval
- CRP
C-reactive protein
- DS
Dysphagia score
- EC
Esophageal cancer
- ECOG
Eastern Cooperative Oncology Group
- GEJ
Gastro-esophageal junction
- GPS
The Glasgow Prognostic Score
- NEC
Neuroendocrine carcinoma
- NR
Not reached
- OR
Odds Ratio
- OS
Overall survival
- PS
Performance status
- QOL
Quality of life
- SCC
Squamous cell carcinoma
- SEMS
Self-expandable metal stent
Author contributions
MF, NM, and AN participated in literature research and drafting of the article. MF, KH, MW, TH, MO, KF, YI, and NM participated in treating patients. MF and AN participated in analyzing the study data. FJ and SM edited the final version of the article. All Authors read and approved the final manuscript.
Funding
No funding.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to the protection of personal information, but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study received approval from the Institutional Review Board of Kanagawa Cancer Center (approval number: 2022 epidemiologic study-126) and adhered strictly to the principles outlined in the Declaration of Helsinki. Written informed consent for participation in the study was waived by the institutional review board of the Kanagawa Cancer Center due to the retrospective nature of this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163:649–58.e2 e642. 10.1053/j.gastro.2022.05.054 [DOI] [PubMed] [Google Scholar]
- 3.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–75. 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anandavadivelan P, Lagergren P. Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol. 2016;13:185–98. 10.1038/nrclinonc.2015.200 [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, Lin Y, Wen Y, Fu W, Wang R, He J, et al. Global trends in the burden of esophageal cancer, 1990–2019: results from the global burden of disease study 2019. J Thorac Dis. 2023;15:348–64. 10.21037/jtd-22-856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sirviö VEJ, Räsänen JV, Kauppila JH. Time trends in mortality of oesophageal cancer in Finland over 30 years. Eur J Surg Oncol. 2023;49:106905. 10.1016/j.ejso.2023.04.004 [DOI] [PubMed] [Google Scholar]
- 7.Watanabe M, Toh Y, Ishihara R, Kono K, Matsubara H, Miyazaki T, et al. Comprehensive registry of esophageal cancer in Japan, 2015. Esophagus. 2023;20:1–28. 10.1007/s10388-022-00950-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajani JA, D’Amico TA, Bentrem DJ, Cooke D, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2023;21:393–422. 10.6004/jnccn.2023.0019 [DOI] [PubMed] [Google Scholar]
- 9.Kitagawa Y, Ishihara R, Ishikawa H, Ito Y, Oyama T, Oyama T, et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus. 2023;20:343–72. 10.1007/s10388-023-00993-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitagawa Y, Ishihara R, Ishikawa H, Ito Y, Oyama T, Oyama T, et al. Esophageal cancer practice guidelines 2022 edited by the Japan Esophageal Society: part 2. Esophagus. 2023;20:373–89. 10.1007/s10388-023-00994-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Homs MYV, Steyerberg EW, Eijkenboom WMH, Tilanus HW, Stalpers LJA, Bartelsman JFWM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet. 2004;364:1497–504. 10.1016/S0140-6736(04)17272-3 [DOI] [PubMed] [Google Scholar]
- 12.Bergquist H, Wenger U, Johnsson E, Nyman J, Ejnell H, Hammerlid E, et al. Stent insertion or endoluminal brachytherapy as palliation of patients with advanced cancer of the esophagus and gastroesophageal junction. Results of a randomized, controlled clinical trial. Dis Esophagus. 2005;18:131–9. 10.1111/j.1442-2050.2005.00467.x [DOI] [PubMed] [Google Scholar]
- 13.Shenfine J, McNamee P, Steen N, Bond J, Griffin SM. A randomized controlled clinical trial of palliative therapies for patients with inoperable esophageal cancer. Am J Gastroenterol. 2009;104:1674–85. 10.1038/ajg.2009.155 [DOI] [PubMed] [Google Scholar]
- 14.Adamson D, Byrne A, Porter C, Blazeby J, Griffiths G, Nelson A, et al. Palliative radiotherapy after oesophageal cancer stenting (ROCS): a multicentre, open-label, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6:292–303. 10.1016/S2468-1253(21)00004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 16.Hamamoto Y, Akutsu Y, Nagashima F, Hironaka S, Ito Y, Kato K, et al. Multicenter questionnaire survey on patterns of care for elderly patients with esophageal squamous cell carcinoma by the Japan Esophageal Oncology Group. Jpn J Clin Oncol. 2016;46:111–5. [DOI] [PubMed] [Google Scholar]
- 17.Saito Y, Hamamoto Y, Hirata K, Yamasaki M, Watanabe M, Abe T, et al. Real-world management and outcomes of older patients with locally advanced esophageal squamous cell carcinoma: a multicenter retrospective study. BMC Cancer. 2023;23:283. 10.1186/s12885-023-10710-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction. Analysis of technical and functional efficacy. Arch Intern Med. 1985;145:1443–6. 10.1001/archinte.1985.00360080117017 [DOI] [PubMed] [Google Scholar]
- 19.Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the esophagus and esophagogastric junction-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:304–17. 10.3322/caac.21399 [DOI] [PubMed] [Google Scholar]
- 20.Institute NC Common terminology criteria for adverse rvents (CTCAE) v5.0 quick reference. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. Accessed 5 Oct 2023.
- 21.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–30. 10.1038/sj.bjc.6601242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okuno T, Wakabayashi M, Kato K, Shinoda M, Katayama H, Igaki H, et al. Esophageal stenosis and the Glasgow prognostic score as independent factors of poor prognosis for patients with locally advanced unresectable esophageal cancer treated with chemoradiotherapy (exploratory analysis of JCOG0303). Int J Clin Oncol. 2017;22:1042–49. 10.1007/s10147-017-1154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nations U. World population prospects; 2022. Summary of Results. https://desapublications.un.org/file/1062/download. Accessed 5 Oct 2023.
- 25.Doeve BH, Bakx JAC, Siersema PD, Rosman C, van Grieken NCT, van Berge Henegouwen MI, et al. The impact of the COVID-19 pandemic on the diagnosis, stage, and treatment of esophagogastric cancer. J Gastroenterol. 2023;58:965–77. 10.1007/s00535-023-02009-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persson J, Smedh U, Johnsson Å, Ohlin B, Sundbom M, Nilsson M, et al. Fully covered stents are similar to semi-covered stents with regard to migration in palliative treatment of malignant strictures of the esophagus and gastric cardia: results of a randomized controlled trial. Surg Endosc. 2017;31:4025–33. 10.1007/s00464-017-5441-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyama T, Umeoka S, Saga T, Watanabe G, Tamai K, Kobayashi A, et al. Evaluation of esophageal peristalsis in patients with esophageal tumors: initial experience with cine MR imaging. Magn Reson Med Sci. 2005;4:109–14. 10.2463/mrms.4.109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to the protection of personal information, but are available from the corresponding author on reasonable request.