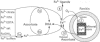Abstract
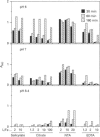
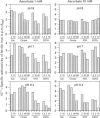
Ferritins are iron-storage proteins that accumulate in plastids during seed formation, and also in leaves during senescence or iron overload. Iron release from ferritins occurs during growth of seedlings and greening of plastids. Depending on the concentration of the reducing agent ascorbate, either an overall iron release or uptake by ferritins from iron(III) citrate may occur. We have designed methods to measure these simultaneous and independent uptake and release fluxes. Each individual step of the exchange was studied using different iron chelates and an excess of ligand. It is shown that: (i) the chelated form of iron, and not ionic Fe3+, is the substrate for iron reduction, which controls the subsequent uptake by ferritin; (ii) iron uptake by ferritins is faster at pH 8.4 than at pH 7 or 6 and is inhibited by an excess of strongly binding free ligands; and (iii) strongly binding free ligands are inhibitory during iron release by ascorbate. When reactions are allowed to proceed simultaneously, the iron chelating power is shown to be a key factor in the overall exchange. The interactions of iron chelating power, reducing capacity and pH are discussed with regard to their influence on the biochemical mobilization of iron.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S. C., Arosio P., Bottke W., Briat J. F., von Darl M., Harrison P. M., Laulhère J. P., Levi S., Lobreaux S., Yewdall S. J. Structure, function, and evolution of ferritins. J Inorg Biochem. 1992 Aug 15;47(3-4):161–174. doi: 10.1016/0162-0134(92)84062-r. [DOI] [PubMed] [Google Scholar]
- Biemond P., Swaak A. J., Beindorff C. M., Koster J. F. Superoxide-dependent and -independent mechanisms of iron mobilization from ferritin by xanthine oxidase. Implications for oxygen-free-radical-induced tissue destruction during ischaemia and inflammation. Biochem J. 1986 Oct 1;239(1):169–173. doi: 10.1042/bj2390169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer R. F., Clark H. M., LaRoche A. P. Reduction and release of ferritin iron by plant phenolics. J Inorg Biochem. 1988 Mar;32(3):171–181. doi: 10.1016/0162-0134(88)80025-4. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Chaney R. L. Effect of iron on the transport of citrate into the xylem of soybeans and tomatoes. Plant Physiol. 1971 Jun;47(6):836–840. doi: 10.1104/pp.47.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo D. A., McFadden K. M., Garland T. R., Wildung R. E. Organic Constituents and Complexation of Nickel(II), Iron(III), Cadmium(II), and plutonium(IV) in Soybean Xylem Exudates. Plant Physiol. 1988 Mar;86(3):734–739. doi: 10.1104/pp.86.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B., Tiffin L. O., Brown J. C. Organic acids and iron translocation in maize genotypes. Plant Physiol. 1973 Aug;52(2):147–150. doi: 10.1104/pp.52.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk F., Lenders J. P., Crichton R. R., Schneider W. Reductive mobilisation of ferritin iron. Eur J Biochem. 1985 Oct 1;152(1):167–172. doi: 10.1111/j.1432-1033.1985.tb09177.x. [DOI] [PubMed] [Google Scholar]
- Grady J. K., Chen Y., Chasteen N. D., Harris D. C. Hydroxyl radical production during oxidative deposition of iron in ferritin. J Biol Chem. 1989 Dec 5;264(34):20224–20229. [PubMed] [Google Scholar]
- Laulhere J. P., Laboure A. M., Briat J. F. Mechanism of the transition from plant ferritin to phytosiderin. J Biol Chem. 1989 Feb 25;264(6):3629–3635. [PubMed] [Google Scholar]
- Laulhère J. P., Labouré A. M., Briat J. F. Photoreduction and incorporation of iron into ferritins. Biochem J. 1990 Jul 1;269(1):79–84. doi: 10.1042/bj2690079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. Y., Charles S. A., Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem J. 1983 Mar 15;210(3):899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. M., Treffry A., Artymiuk P. J., Harrison P. M., Yewdall S. J., Luzzago A., Cesareni G., Levi S., Arosio P. Identification of the ferroxidase centre in ferritin. FEBS Lett. 1989 Aug 28;254(1-2):207–210. doi: 10.1016/0014-5793(89)81040-3. [DOI] [PubMed] [Google Scholar]
- Lobreaux S., Briat J. F. Ferritin accumulation and degradation in different organs of pea (Pisum sativum) during development. Biochem J. 1991 Mar 1;274(Pt 2):601–606. doi: 10.1042/bj2740601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas F. M., van de Wetering D. A., van Beusichem M. L., Bienfait H. F. Characterization of Phloem iron and its possible role in the regulation of fe-efficiency reactions. Plant Physiol. 1988 May;87(1):167–171. doi: 10.1104/pp.87.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell M. J., Ward R. J., Baum H., Peters T. J. The role of iron in ferritin- and haemosiderin-mediated lipid peroxidation in liposomes. Biochem J. 1985 Jul 1;229(1):135–139. doi: 10.1042/bj2290135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sczekan S. R., Joshi J. G. Isolation and characterization of ferritin from soyabeans (Glycine max). J Biol Chem. 1987 Oct 5;262(28):13780–13788. [PubMed] [Google Scholar]
- Thomas C. E., Morehouse L. A., Aust S. D. Ferritin and superoxide-dependent lipid peroxidation. J Biol Chem. 1985 Mar 25;260(6):3275–3280. [PubMed] [Google Scholar]
- Tiffin L. O., Chaney R. L. Translocation of iron from soybean cotyledons. Plant Physiol. 1973 Nov;52(5):393–396. doi: 10.1104/pp.52.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]