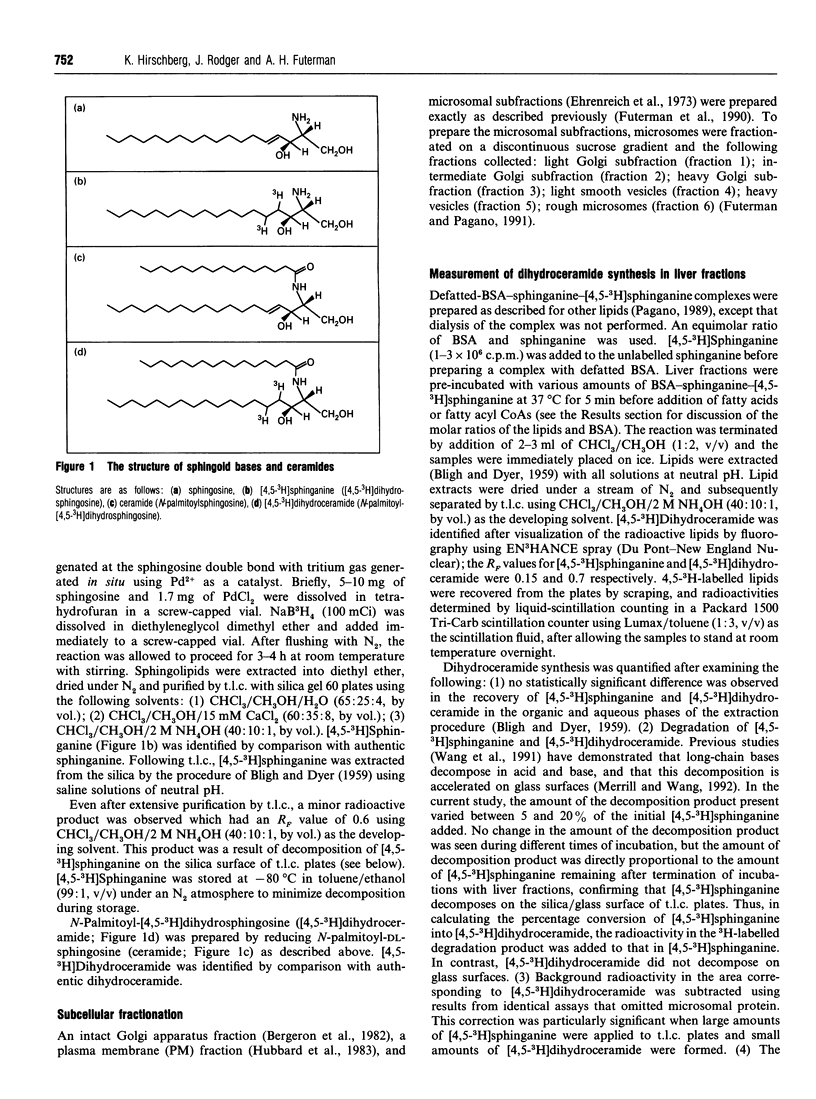
Abstract
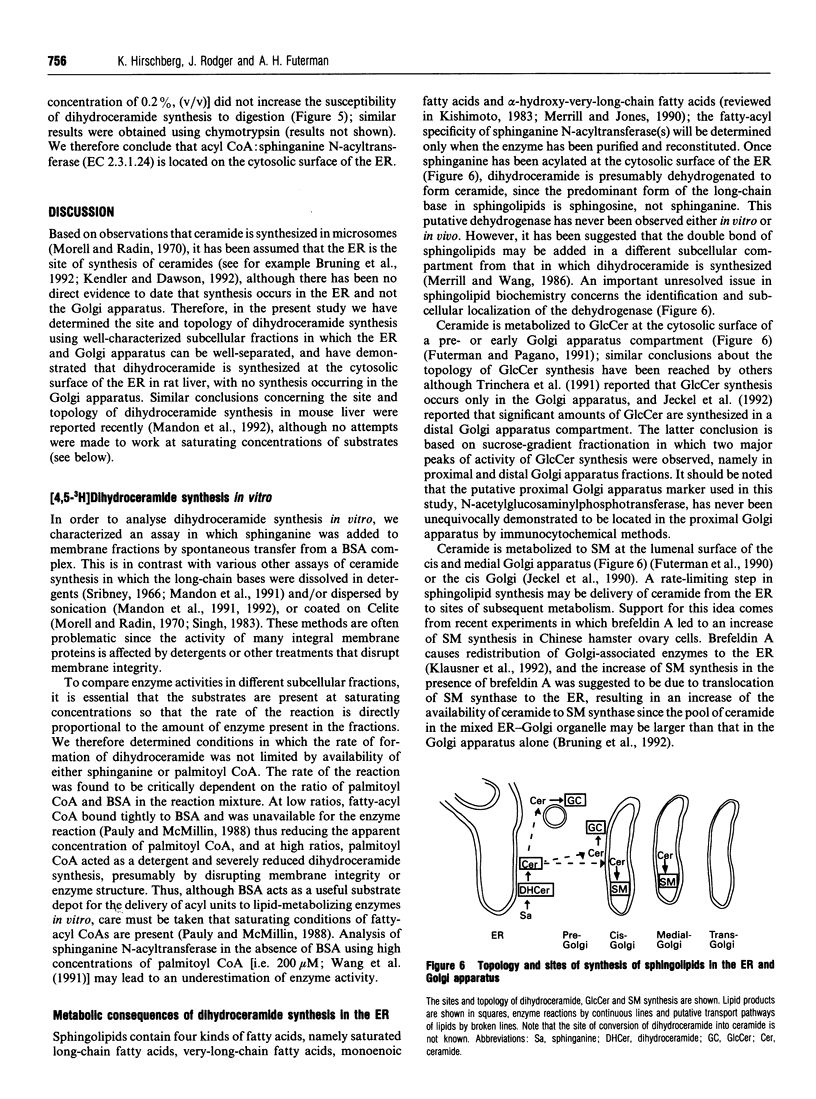
Ceramide, a key intermediate in sphingolipid metabolism, is synthesized by acylation of sphinganine followed by dehydrogenation of dihydroceramide to ceramide. Using radioactive sphinganine, we have examined the site and topology of dihydroceramide synthesis in well-characterized subcellular fractions from rat liver. [4,5-3H]Sphinganine was introduced as a complex with BSA and was metabolized to [4,5-3H]dihydroceramide upon incubation of rat liver homogenates or microsomes with fatty acyl CoA. Conditions were established in a detergent-free system in which dihydroceramide synthesis was not limited by either substrate availability or by amounts of microsomal protein or reaction time. The distribution of dihydroceramide synthesis was found to exactly parallel that of an endoplasmic reticulum (ER) marker upon subfractionation of microsomes, and no endogenous activity was detected in either purified Golgi apparatus or plasma membrane fractions. Limited protease digestion demonstrated that sphinganine N-acyltransferase is localized at the cytosolic surface of intact ER-derived vesicles. These results are discussed with regard to the subsequent transport of (dihydro)-ceramide from the ER to sites of further metabolism in a pre-Golgi apparatus compartment and in the cis and medial cisternae of the Golgi apparatus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Rachubinski R. A., Sikstrom R. A., Posner B. I., Paiement J. Galactose transfer to endogenous acceptors within Golgi fractions of rat liver. J Cell Biol. 1982 Jan;92(1):139–146. doi: 10.1083/jcb.92.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüning A., Karrenbauer A., Schnabel E., Wieland F. T. Brefeldin A-induced increase of sphingomyelin synthesis. Assay for the action of the antibiotic in mammalian cells. J Biol Chem. 1992 Mar 15;267(8):5052–5055. [PubMed] [Google Scholar]
- Das A. K., Horie S., Hajra A. K. Biosynthesis of glycerolipid precursors in rat liver peroxisomes and their transport and conversion to phosphatidate in the endoplasmic reticulum. J Biol Chem. 1992 May 15;267(14):9724–9730. [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A. H., Pagano R. E. Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem J. 1991 Dec 1;280(Pt 2):295–302. doi: 10.1042/bj2800295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A. H., Stieger B., Hubbard A. L., Pagano R. E. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Chem. 1990 May 25;265(15):8650–8657. [PubMed] [Google Scholar]
- Hubbard A. L., Wall D. A., Ma A. Isolation of rat hepatocyte plasma membranes. I. Presence of the three major domains. J Cell Biol. 1983 Jan;96(1):217–229. doi: 10.1083/jcb.96.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeckel D., Karrenbauer A., Birk R., Schmidt R. R., Wieland F. Sphingomyelin is synthesized in the cis Golgi. FEBS Lett. 1990 Feb 12;261(1):155–157. doi: 10.1016/0014-5793(90)80659-7. [DOI] [PubMed] [Google Scholar]
- Jeckel D., Karrenbauer A., Burger K. N., van Meer G., Wieland F. Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions. J Cell Biol. 1992 Apr;117(2):259–267. doi: 10.1083/jcb.117.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler A., Dawson G. Hypoxic injury to oligodendrocytes: reversible inhibition of ATP-dependent transport of ceramide from the endoplasmic reticulum to the Golgi. J Neurosci Res. 1992 Feb;31(2):205–211. doi: 10.1002/jnr.490310202. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandon E. C., Ehses I., Rother J., van Echten G., Sandhoff K. Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase, and sphinganine N-acyltransferase in mouse liver. J Biol Chem. 1992 Jun 5;267(16):11144–11148. [PubMed] [Google Scholar]
- Mandon E. C., van Echten G., Birk R., Schmidt R. R., Sandhoff K. Sphingolipid biosynthesis in cultured neurons. Down-regulation of serine palmitoyltransferase by sphingoid bases. Eur J Biochem. 1991 Jun 15;198(3):667–674. doi: 10.1111/j.1432-1033.1991.tb16065.x. [DOI] [PubMed] [Google Scholar]
- Merrill A. H., Jr, Jones D. D. An update of the enzymology and regulation of sphingomyelin metabolism. Biochim Biophys Acta. 1990 May 1;1044(1):1–12. doi: 10.1016/0005-2760(90)90211-f. [DOI] [PubMed] [Google Scholar]
- Merrill A. H., Jr, Wang E. Biosynthesis of long-chain (sphingoid) bases from serine by LM cells. Evidence for introduction of the 4-trans-double bond after de novo biosynthesis of N-acylsphinganine(s). J Biol Chem. 1986 Mar 15;261(8):3764–3769. [PubMed] [Google Scholar]
- Merrill A. H., Jr, Wang E. Enzymes of ceramide biosynthesis. Methods Enzymol. 1992;209:427–437. doi: 10.1016/0076-6879(92)09053-6. [DOI] [PubMed] [Google Scholar]
- Moreau P., Rodriguez M., Cassagne C., Morré D. M., Morré D. J. Trafficking of lipids from the endoplasmic reticulum to the Golgi apparatus in a cell-free system from rat liver. J Biol Chem. 1991 Mar 5;266(7):4322–4328. [PubMed] [Google Scholar]
- Morell P., Radin N. S. Specificity in ceramide biosynthesis from long chain bases and various fatty acyl coenzyme A's by brain microsomes. J Biol Chem. 1970 Jan 25;245(2):342–350. [PubMed] [Google Scholar]
- Ong D. E., Brady R. N. In vivo studies on the introduction of the 4-t-double bond of the sphingenine moiety of rat brain ceramides. J Biol Chem. 1973 Jun 10;248(11):3884–3888. [PubMed] [Google Scholar]
- Pagano R. E. A fluorescent derivative of ceramide: physical properties and use in studying the Golgi apparatus of animal cells. Methods Cell Biol. 1989;29:75–85. doi: 10.1016/s0091-679x(08)60188-0. [DOI] [PubMed] [Google Scholar]
- Pagano R. E. Lipid traffic in eukaryotic cells: mechanisms for intracellular transport and organelle-specific enrichment of lipids. Curr Opin Cell Biol. 1990 Aug;2(4):652–663. doi: 10.1016/0955-0674(90)90107-p. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Martin O. C. A series of fluorescent N-acylsphingosines: synthesis, physical properties, and studies in cultured cells. Biochemistry. 1988 Jun 14;27(12):4439–4445. doi: 10.1021/bi00412a034. [DOI] [PubMed] [Google Scholar]
- Pauly D. F., McMillin J. B. Importance of acyl-CoA availability in interpretation of carnitine palmitoyltransferase I kinetics. J Biol Chem. 1988 Dec 5;263(34):18160–18167. [PubMed] [Google Scholar]
- Puoti A., Desponds C., Conzelmann A. Biosynthesis of mannosylinositolphosphoceramide in Saccharomyces cerevisiae is dependent on genes controlling the flow of secretory vesicles from the endoplasmic reticulum to the Golgi. J Cell Biol. 1991 May;113(3):515–525. doi: 10.1083/jcb.113.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards E. W., Hamm M. W., Fletcher J. E., Otto D. A. The binding of palmitoyl-CoA to bovine serum albumin. Biochim Biophys Acta. 1990 Jun 14;1044(3):361–367. doi: 10.1016/0005-2760(90)90081-8. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Schwarzmann G. A simple and novel method for tritium labeling of gangliosides and other sphingolipids. Biochim Biophys Acta. 1978 Apr 28;529(1):106–114. doi: 10.1016/0005-2760(78)90108-x. [DOI] [PubMed] [Google Scholar]
- Singh I. Ceramide synthesis from free fatty acids in rat brain: function of NADPH and substrate specificity. J Neurochem. 1983 Jun;40(6):1565–1570. doi: 10.1111/j.1471-4159.1983.tb08127.x. [DOI] [PubMed] [Google Scholar]
- Slife C. W., Wang E., Hunter R., Wang S., Burgess C., Liotta D. C., Merrill A. H., Jr Free sphingosine formation from endogenous substrates by a liver plasma membrane system with a divalent cation dependence and a neutral pH optimum. J Biol Chem. 1989 Jun 25;264(18):10371–10377. [PubMed] [Google Scholar]
- Spence M. W., Beed S., Cook H. W. Acid and alkaline ceramidases of rat tissues. Biochem Cell Biol. 1986 May;64(5):400–404. doi: 10.1139/o86-056. [DOI] [PubMed] [Google Scholar]
- Sribney M. Enzymatic synthesis of ceramide. Biochim Biophys Acta. 1966 Dec 7;125(3):542–547. doi: 10.1016/0005-2760(66)90042-7. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Bister K. Stereospecificities in the metabolic reactions of the four isomeric sphinganines (dihydrosphingosines) in rat liver. Hoppe Seylers Z Physiol Chem. 1973 Feb;354(2):169–181. doi: 10.1515/bchm2.1973.354.1.169. [DOI] [PubMed] [Google Scholar]
- Trinchera M., Fabbri M., Ghidoni R. Topography of glycosyltransferases involved in the initial glycosylations of gangliosides. J Biol Chem. 1991 Nov 5;266(31):20907–20912. [PubMed] [Google Scholar]
- Van Veldhoven P. P., Mannaerts G. P. Subcellular localization and membrane topology of sphingosine-1-phosphate lyase in rat liver. J Biol Chem. 1991 Jul 5;266(19):12502–12507. [PubMed] [Google Scholar]
- Walter V. P., Sweeney K., Morré D. J. Neutral lipid precursors for gangliosides are not formed by rat liver homogenates or by purified cell fractions. Biochim Biophys Acta. 1983 Feb 7;750(2):346–352. doi: 10.1016/0005-2760(83)90039-5. [DOI] [PubMed] [Google Scholar]
- Wang E., Norred W. P., Bacon C. W., Riley R. T., Merrill A. H., Jr Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J Biol Chem. 1991 Aug 5;266(22):14486–14490. [PubMed] [Google Scholar]
- Wattenberg B. W. Glycolipid and glycoprotein transport through the Golgi complex are similar biochemically and kinetically. Reconstitution of glycolipid transport in a cell free system. J Cell Biol. 1990 Aug;111(2):421–428. doi: 10.1083/jcb.111.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]