Abstract
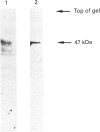
Erythropoietin (Epo)-producing hepatoma cells (HepG2) reveal, in addition to the cytochromes of the respiratory chain, a photometrically measurable haem signal with absorbance maxima at 559 nm and 427 nm, suggesting the presence of a b-type cytochrome. This activity exhibited a low midpoint potential, CO-binding spectra and reduction which was insensitive to both cyanide and antimycin. This haem possessed a 22 kDa subunit and might be part of an electron transfer chain similar to the NADPH oxidase, since the NADPH oxidase cytosolic activating factor (p47) could be identified by Western blot analysis. H2O2, which was detected inside the cells by confocal microscopy, might therefore be produced by the suggested electron transfer chain. This cyanide- and antimycin-insensitive but hypoxia-sensitive cytochrome b would be an attractive candidate for controlled Epo production in response to pO2.
Full text
PDF

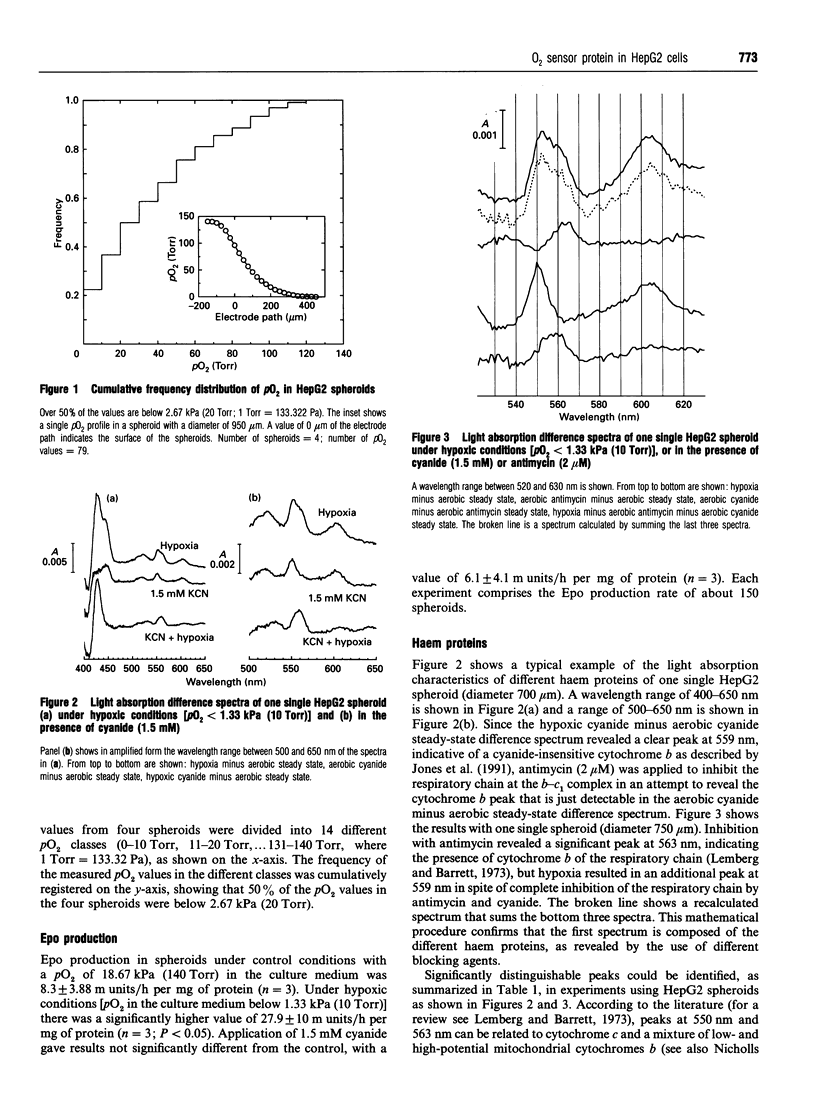
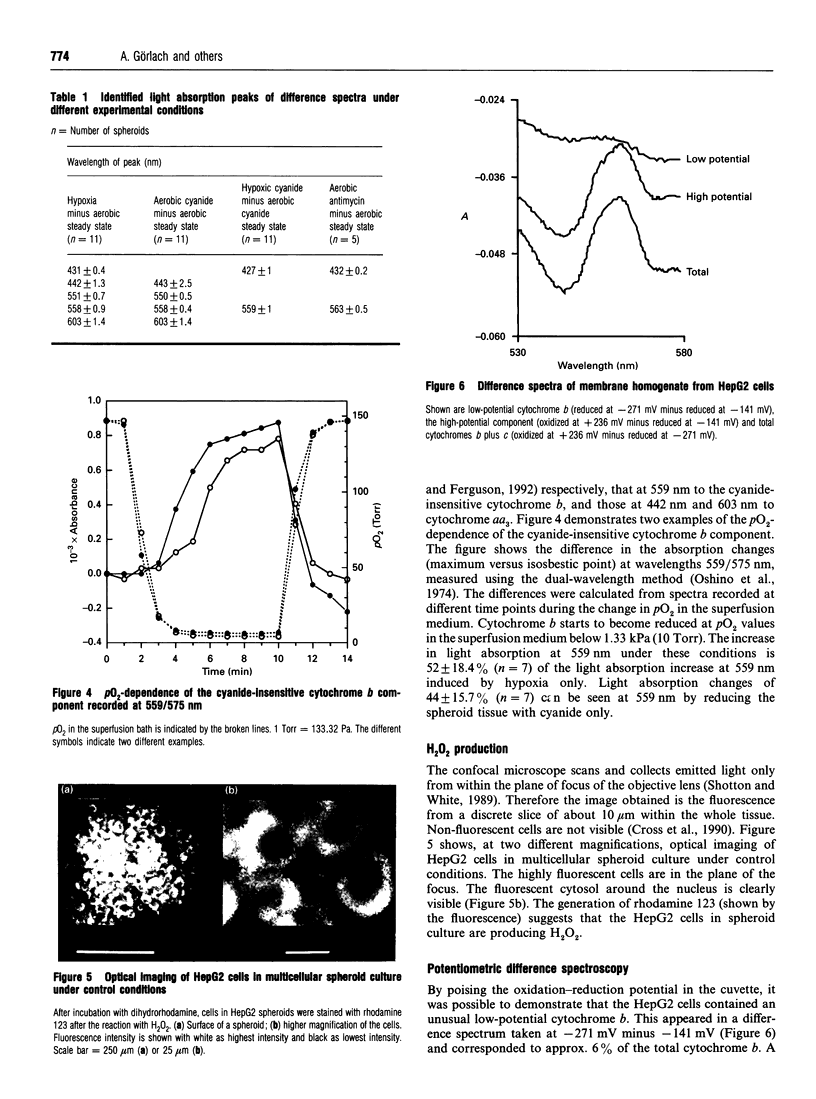


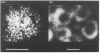
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker H., Holtermann G., Carlsson J., Nederman T. Methodological aspects of microelectrode measurements in cellular spheroids. Adv Exp Med Biol. 1983;159:445–462. doi: 10.1007/978-1-4684-7790-0_38. [DOI] [PubMed] [Google Scholar]
- Annable L., Cotes P. M., Mussett M. V. The second international reference preparation of erythropoietin, human, urinary, for bioassay. Bull World Health Organ. 1972;47(1):99–112. [PMC free article] [PubMed] [Google Scholar]
- Cherry P. D., Omar H. A., Farrell K. A., Stuart J. S., Wolin M. S. Superoxide anion inhibits cGMP-associated bovine pulmonary arterial relaxation. Am J Physiol. 1990 Oct;259(4 Pt 2):H1056–H1062. doi: 10.1152/ajpheart.1990.259.4.H1056. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Henderson L., Jones O. T., Delpiano M. A., Hentschel J., Acker H. Involvement of an NAD(P)H oxidase as a pO2 sensor protein in the rat carotid body. Biochem J. 1990 Dec 15;272(3):743–747. doi: 10.1042/bj2720743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. Enzymic mechanisms of superoxide production. Biochim Biophys Acta. 1991 May 6;1057(3):281–298. doi: 10.1016/s0005-2728(05)80140-9. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Harper A. M., Segal A. W. Oxidation-reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem J. 1981 Feb 15;194(2):599–606. doi: 10.1042/bj1940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandrey J., Seydel F. P., Siegers C. P., Jelkmann W. Role of cytochrome P450 in the control of the production of erythropoietin. Life Sci. 1990;47(2):127–134. doi: 10.1016/0024-3205(90)90225-g. [DOI] [PubMed] [Google Scholar]
- Goldberg M. A., Dunning S. P., Bunn H. F. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science. 1988 Dec 9;242(4884):1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- Goldberg M. A., Imagawa S., Strair R. K., Bunn H. F. Regulation of the erythropoietin gene in Hep 3B cells. Semin Hematol. 1991 Jul;28(3 Suppl 3):35–41. [PubMed] [Google Scholar]
- Jones O. T., Cross A. R., Hancock J. T., Henderson L. M., O'Donnell V. B. Inhibitors of NADPH oxidase as guides to its mechanism. Biochem Soc Trans. 1991 Feb;19(1):70–72. doi: 10.1042/bst0190070. [DOI] [PubMed] [Google Scholar]
- Koury S. T., Bondurant M. C., Koury M. J. Localization of erythropoietin synthesizing cells in murine kidneys by in situ hybridization. Blood. 1988 Feb;71(2):524–527. [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Greenberger J. S., Cohen H. J. Functional changes in human leukemic cell line HL-60. A model for myeloid differentiation. J Cell Biol. 1979 Aug;82(2):315–322. doi: 10.1083/jcb.82.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshino N., Sugano T., Oshino R., Chance B. Mitochondrial function under hypoxic conditions: the steady states of cytochrome alpha+alpha3 and their relation to mitochondrial energy states. Biochim Biophys Acta. 1974 Dec 19;368(3):298–310. doi: 10.1016/0005-2728(74)90176-5. [DOI] [PubMed] [Google Scholar]
- Shotton D., White N. Confocal scanning microscopy: three-dimensional biological imaging. Trends Biochem Sci. 1989 Nov;14(11):435–439. doi: 10.1016/0968-0004(89)90096-0. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Curnutte J. T. Molecular basis of chronic granulomatous disease. Blood. 1991 Feb 15;77(4):673–686. [PubMed] [Google Scholar]
- Tan C. C., Ratcliffe P. J. Effect of inhibitors of oxidative phosphorylation on erythropoietin mRNA in isolated perfused rat kidneys. Am J Physiol. 1991 Dec;261(6 Pt 2):F982–F987. doi: 10.1152/ajprenal.1991.261.6.F982. [DOI] [PubMed] [Google Scholar]
- Ueno M., Brookins J., Beckman B. S., Fisher J. W. Effects of reactive oxygen metabolites on erythropoietin production in renal carcinoma cells. Biochem Biophys Res Commun. 1988 Jul 29;154(2):773–780. doi: 10.1016/0006-291x(88)90207-0. [DOI] [PubMed] [Google Scholar]