Abstract
The role of autoimmunity in large-vessel vasculitis in humans remains unclear. We have previously shown that infection of gamma interferon receptor knockout (IFN-γR−/−) mice with gammaherpesvirus 68 (γHV68) results in severe inflammation of the large elastic arteries that is pathologically similar to the lesions observed in Takayasu's arteritis, the nongranulomatous variant of temporal arteritis, and Kawasaki's disease (K. E. Weck et al., Nat. Med. 3:1346–1353, 1997). Here we define the mechanism of damage to the elastic arteries. We show that there is a persistent productive infection of the media of the large elastic vessels. In addition, we demonstrate that persistent virus replication is necessary for chronic arteritis, since antiviral therapy of mice with established disease resulted in increased survival, clearance of viral antigen from the media of the affected vessel, and dramatic amelioration of arteritic lesions. These data argue that ongoing virus replication, rather than autoimmunity, is the cause of γHV68-induced elastic arteritis.
The potential etiologies of human inflammatory vascular diseases can be grouped into three categories: (i) infection mediated, dependent on infection for initiation and maintenance of disease; (ii) infection initiated, but maintained by subsequent induction of autoimmunity; or (iii) unrelated to infection, and therefore induced by autoimmunity or some other undefined factor. A number of animal models have been used to study the relationship between infection and vascular disease (reviewed in reference 3). Of these models, murine gammaherpesvirus 68 (γHV68)-induced arteritis has several distinct advantages (30, 33). The murine system has well-defined genetics and a manipulable immune system. In addition, γHV68 is amenable to genetic analysis, allowing specific viral genes involved in pathogenic processes to be identified (2, 29). Finally, γHV68 infection consistently results in chronic elastic arteritis within 3 to 6 weeks postinfection, allowing us to accurately predict when disease will occur and affording the opportunity to focus studies over a relatively short time.
Gamma interferon receptor knockout (IFN-γR−/−) mice are very susceptible to γHV68-induced vascular disease. After infection, the elastic arteries develop intense mononuclear inflammation and thickening of the intima and adventitia, with a neutrophilic infiltrate extending into the media and necrosis of smooth muscle cells (38). Viral antigen is detectable in smooth muscle cells of the media weeks to months after visceral infection is cleared to undetectable levels (38). Inflammatory lesions surround areas of medial infection, while uninfected regions do not show pathology (38). Notably, Takayasu's arteritis, the nongranulomatous variant of temporal arteritis, and Kawasaki's disease all exhibit pathology similar to the lesions observed in γHV68-infected IFN-γR−/− mice.
Here, we determine whether ongoing virus infection is required for maintenance of chronic γHV68-induced arteritis. Of the three possible processes for induction and maintenance of arteritis enumerated above, we sought to distinguish between the two possibilities contingent on induction by an infectious agent. In particular, we determined whether the persistent viral antigen detectable in the arteritic media reflected chronic virus replication, and whether ongoing productive infection was required for the maintenance of vascular pathology.
MATERIALS AND METHODS
Viruses and tissue culture.
γHV68 (WUMS clone [32]) was passaged and assayed as previously described (37). The virus was diluted in low-endotoxin Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 100 U of penicillin/ml, 100 mg of streptomycin/ml, 10 mM HEPES, and 2 mM l-glutamine (complete DMEM) for infection.
Mouse strains.
Mice were bred and housed at the Washington University School of Medicine at biosafety level 2 in accordance with all federal government and University policies. Mice were on a pure 129Ev/Sv background. IFN-γR−/− and 129 mice were obtained from Michel Aguet (20).
Infection and analysis of infected mice.
Mice were infected intraperitoneally at the age of 5 to 7 weeks with different doses of γHV68 in 0.5 ml of complete DMEM. Organs were titered by plaque assay on NIH 3T12 cells (ATCC CCC 164) (37). Organs for pathology were collected into 10% buffered formalin and embedded in paraffin for sectioning and staining with hematoxylin and eosin (H&E) as previously described (38). Parallel sections were used for immunostaining and DNA in situ hybridization. Slides were read in a blinded fashion by A.J.D. for arteritis and viral antigen staining. Lesion scores were determined by criteria set forth in Table 1. Slides were read blinded by Eric T. Clambey, A.J.D., and H.W.V., and scores were averaged for each specimen. Microscopy pictures were taken on a Zeiss microscope equipped with a Spot camera and Spot software 1.1 (Diagnostics Instruments, Sterling Heights, Mich.) and with Northern Eclipse v2.0 software (Empix Imaging, North Tonawanda, N.Y.).
TABLE 1.
Scoring criteria for inflammatory lesions
| Score | Histology of lesions |
|---|---|
| 0 | Normal artery |
| 1 | Mild intimal/adventitial thickening with minimal infiltrates |
| 2 | Pronounced intimal/adventitial mononuclear infiltrates; no neutrophilic infiltrates |
| 3 | Pronounced intimal/adventitial mononuclear infiltrates and neutrophilic infiltrates at the adventitial/medial border |
| 4 | Pronounced intimal/adventitial mononuclear infiltrates and medial neutrophilic infiltrates |
| 5 | Severe lesions with extensive neutrophilic infiltrates and/or necrosis in the media |
Immunohistochemistry.
Immunohistochemical staining was performed as previously described (38). Briefly, a 1:8,000 dilution of an anti-γHV68 polyclonal rabbit serum, generated by infection of rabbits with γHV68 as previously described (38), was used as the primary antibody. A horseradish peroxidase (HRP)-conjugated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch) was used at 1 μg/ml. Biotin Tyramide Plus (tyramide signal amplification [TSA]; NEN Life Science Products, Boston, Mass.) was used at a 1:1,000 dilution for signal amplification, followed by HRP-conjugated streptavidin (Jackson ImmunoResearch) at 1 μg/ml. HRP activity was localized by a 5-min incubation in DAB/metal solution (Pierce, Rockford, Ill.).
DNA in situ hybridization.
In situ hybridization was performed with a biotinylated γHV68-specific probe targeted to the 40-bp BamHI repeat region within the viral genome (probe sequence, biotin-TCCGGGCCCCAGCTCGGGAGGGGGCCGGGGAGGTCGGGGA). A control murine cytomegalovirus (MCMV)-specific probe with a similar G+C content was made (probe sequence, biotin-AGTCAGCCTCCGGGCCGCGCGCCGCGTCCGCGGGAAGGCG). Tissue sections were deparaffinized in xylene, rehydrated through ethanol gradients, and fixed with fresh, chilled 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min. Sections were treated with 20 μg of proteinase K/ml in Tris-EDTA (TE) for 15 min at room temperature, then refixed with 4% paraformaldehyde for 5 min. Probes were added to a concentration of 375 fmol/μl in preheated (55°C) hybridization solution (50% formamide, 0.3 M NaCl, 20 mM Tris-HCl [pH 8.0], 5 mM EDTA, 10 mM NaPO4 [pH 8.0], 10% dextran sulfate, 1× Denhardt's solution) plus 100 μg of salmon sperm DNA/ml. The probe solution was put on siliconized coverslips and placed on aortic sections. The edges of the coverslips were sealed with rubber cement. Slides were heated to 90°C for 5 min and then incubated overnight at 42°C. The rubber cement was removed, and slides were washed at 37°C for 15 min each with 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 2× SSC, and 1× SSC, followed by two 5-min PBS washes at room temperature. Slides were then treated with 0.3% H2O2 in PBS for 5 min, washed, and blocked (1% bovine serum albumin [BSA], 0.2% powdered skim milk, 0.3% Triton in PBS) for 30 min. Specific signal was detected using HRP-streptavidin (Jackson ImmunoResearch) at 0.5 μg/ml for 1 h at room temperature, followed by amplification with Biotin Tyramide Plus (NEN Life Sciences) at a 1:1,000 dilution for 5 min. HRP-streptavidin (Jackson ImmunoResearch) at 1 μg/ml was then added for 30 min, and HRP activity was localized by tetramethylbenzidine (Moss Inc., Pasadena, Md.) deposition for 5 min.
Electron microscopy.
Tissue was fixed upon death or at sacrifice by perfusion with 3% glutaraldehyde in 0.1 M cacodylate buffer (0.1 M sodium cacodylate in water [pH 7.0], to which 0.54% [wt/vol] dextrose was added). All incubations were carried out at room temperature, unless otherwise noted. Tissue was rinsed in 0.1 M cacodylate buffer for 25 min, stained in 1% (wt/vol) OsO4 (in cacodylate buffer) for 60 min, then washed in 1.0 M cacodylate buffer. It was then placed in 50% ethanol for 25 min, immersed in 3% (wt/vol) uranyl acetate (in water) for 25 min, and then put through an alcohol gradient (75, 95, and 100% ethanol). Tissue was then placed in a BEEM embedding capsule (Electron Microscopy Sciences, Fort Washington, Pa.) and immersed first in a 50:50 mix of Spurr resin (hydrophobic methacrylate resin)–100% ethanol for 2 h and then in 100% Spurr resin for 2 h. The resin was then polymerized at 80°C for 12 h. One-micrometer-thick sections were cut and stained with toluidine blue to evaluate tissue by light microscopy. Ultrathin sections (500 to 700 Å) were cut, placed on 100/200-mesh copper grids, and stained with 0.2% (wt/vol) lead citrate (in distilled water) for 1 to 2 min. Specimens were evaluated with a Philips CM10 electron microscope.
Antiviral therapy.
Cidofovir (Vistide; Gilead Sciences, Foster City, Calif.) was diluted in low-endotoxin PBS to 6.25 mg/ml and filter sterilized. Cidofovir was administered subcutaneously in the scruff of the neck at a dose of 25 mg/kg of body weight (80 to 150 μl/mouse) (22). Individual mice were weighed each time prior to injection. To test drug efficacy, SCID mice were infected with 1,000 PFU of γHV68 and treated with cidofovir as indicated in the legend to Fig. 2. Mice were sacrificed 10 days postinfection (p.i.), and spleens were harvested for a plaque assay to determine viral titers. For short- and long-term therapy of IFN-γR−/− mice, mice were given a 2-day loading dose (25 mg/kg subcutaneously, starting on day 24 p.i.) and were then injected every 3rd day with the same dose for 3 weeks. Mice were injected twice a week for the remaining time of therapy. For short-term therapy, mice were treated for 4.5 weeks and were sacrificed 56 or 57 days p.i. For long-term therapy, mice were treated for 8.5 weeks and were sacrificed 84 or 85 days p.i.
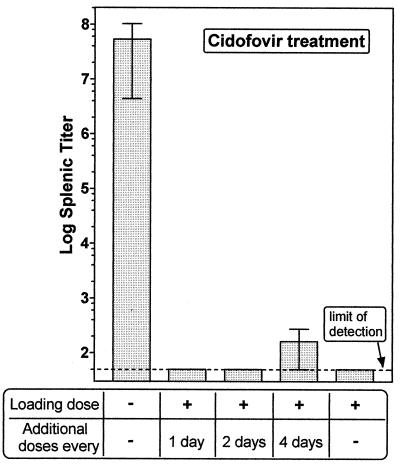
FIG. 2.
Cidofovir treatment of γHV68-infected SCID mice. Mice were infected with 1,000 PFU of γHV68. Mice were either left untreated or given subcutaneous injections of the antiviral drug cidofovir (25 mg/kg) beginning at day 1 postinfection, as indicated. All treatments consisted of a 2-day loading dose (days 1 and 2 postinfection) followed by the indicated schedules. Data are means ± standard errors of the means for 3 to 4 mice per group in a single experiment. Mice were sacrificed 10 days postinfection, and splenic viral titers were determined.
Statistical analyses.
Data were plotted and statistically analyzed using GraphPad Prism (GraphPad Software, San Diego, Calif.). Statistics on survival data were performed using the Mantel-Haenzel test. Nonparametric analysis of arteritis scores was carried out using a two-tailed Mann-Whitney test. All other results were analyzed with unpaired t tests or chi-square tests.
RESULTS
Chronic replication of γHV68 in the aortic media.
Viral antigen, surrounded by intimal and adventitial inflammation, was previously observed in the media of γHV68-induced arteritic lesions in IFN-γR−/− mice months p.i. (38). The ongoing presence of viral antigen in the media of the great elastic arteries could reflect either continued virus replication or failure to clear viral antigen from the media after viral replication had ceased. We distinguished between these possibilities using in situ hybridization to detect the presence of viral DNA and electron microscopy to detect the presence of virions.
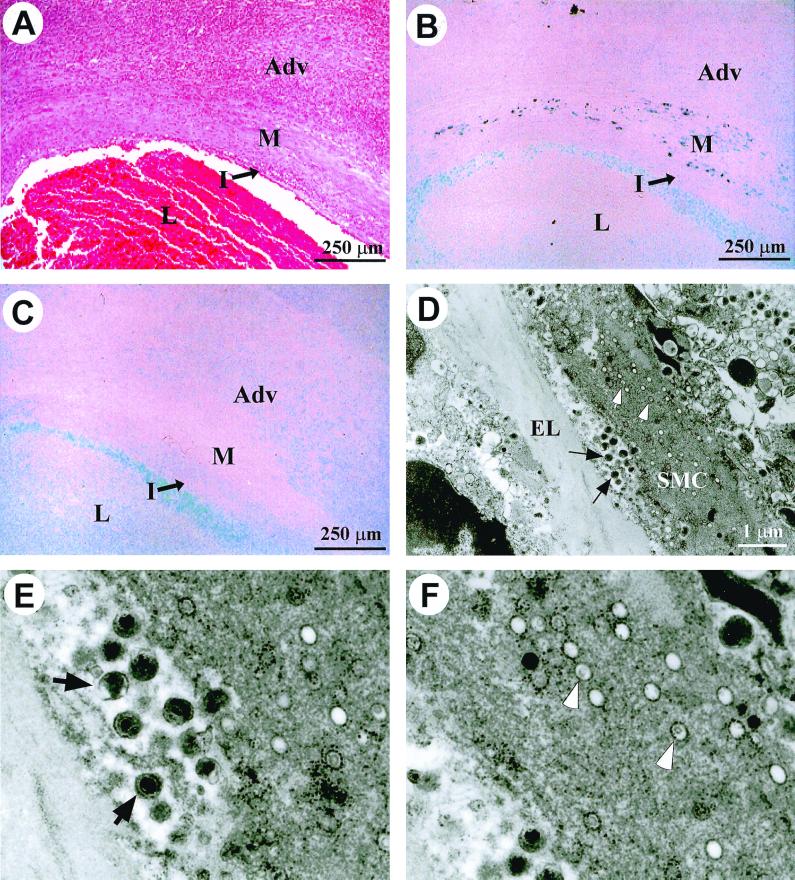
DNA in situ hybridization with a γHV68-specific probe detected viral genome in the media of an arteritic lesion from an IFN-γR−/− mouse (Fig. 1B). The area positive for viral DNA corresponded to the region containing γHV68 antigen by immunohistochemistry (data not shown). A control G+C-matched probe, specific for a region of the MCMV genome, resulted in no medial staining (Fig. 1C; note that the light blue staining in the lumen represents background staining from the glass slide and was observed with both the γHV68 and MCMV probes). The presence of γHV68 DNA and viral antigen within the media is most consistent with ongoing virus replication in the vessel wall. This was confirmed using electron microscopy of aortic lesions, which revealed abundant extracellular virions, as well as cytoplasmic viral particles, within smooth muscle cells of the arteritic media (Fig. 1D to F). Death of infected cells within the media was noted, consistent with a lytic infection. These data demonstrate ongoing productive infection within smooth muscle cells of the aortic media.
FIG. 1.
Chronic productive γHV68 infection in the arteritic media. (A through C) Serial sections from an arteritic lesion in a γHV68-infected IFN-γR−/− mouse that was sacrificed 11 weeks p.i. Adv, adventitia; M, media; I, intima; L, lumen. (A) H&E-stained section. (B) DNA in situ hybridization with a γHV68-specific DNA probe. Dark staining within the media represents specific signal. (C) DNA in situ hybridization with an MCMV-specific DNA probe. Light blue staining in the lumen with both γHV68 and MCMV DNA probes represents background staining from the glass slide. (D) Electron micrograph of the media of an arteritic lesion from an IFN-γR−/− mouse that died 6 weeks p.i. Black arrows indicate extracellular mature virions. White arrows indicate virions in the cytoplasm of a smooth muscle cell (SMC). EL, elastic lamina; SMC, smooth muscle cell. Magnification, ×11,500. (E) Enlargement of region containing extracellular virions from the electron micrograph shown in panel D. (F) Enlargement of region containing cytoplasmic virions from the electron micrograph shown in panel D.
Cidofovir inhibits γHV68 replication in SCID mice.
We considered two potential mechanisms for chronic elastic arteritis: (i) virus-induced tissue damage leading to an autoimmune response, the latter being important for maintenance of inflammation or (ii) the inflammatory response being solely dependent on continued virus replication in the vessel wall. To differentiate between these possibilities, we tested whether viral replication was necessary for the maintenance of viral antigen within arteritic lesions, and for the persistence of inflammatory pathology, by treating infected IFN-γR−/− mice with an antiviral drug.
Cidofovir, a nucleoside analog, was used for the antiviral therapy because it has been shown to be more efficacious in blocking γHV68 replication than other antiviral agents (21). To determine an effective dosing schedule, CB.17 SCID mice were infected with 1,000 PFU of γHV68 followed by treatment with different cidofovir regimens using a dose based on a previously published study (21). Mice were sacrificed 10 days postinfection, and virus titers in the spleen were determined (Fig. 2). While spleens from untreated mice had ∼107 PFU of virus, mice treated with a 2-day loading dose of cidofovir (administered on days 1 and 2 postinfection) had no detectable virus in the spleen (Fig. 2; limit of detection, 50 PFU/organ). One of four mice treated with the 2-day loading dose and then given additional doses every 4th day had detectable virus in the spleen (500 PFU/spleen), while the other three mice did not have detectable virus in the spleen (Fig. 2; mice with no detectable virus in the spleen were assigned a value of 50 PFU based on the limit of detection of the plaque assay). Overall, only 1 out of a total of 15 mice receiving cidofovir had detectable virus in the spleen compared to 4 of 4 untreated control mice.
Decreased mortality of γHV68-infected IFN-γR−/− mice treated with cidofovir.
Based on the efficacy of cidofovir treatment in SCID mice, we designed a regimen for treatment of chronically γHV68 infected IFN-γR−/− mice (see Materials and Methods). We did not initiate cidofovir treatment until 24 days postinfection, at which time acute infection has resolved in the spleen and lung (7, 26, 38). In addition, when a cohort of IFN-γR−/− mice were sacrificed 24 days postinfection, 83% (15 of 18) had arteritis. Thus, the effect of therapy on established arteritic lesions was assessed in these experiments.
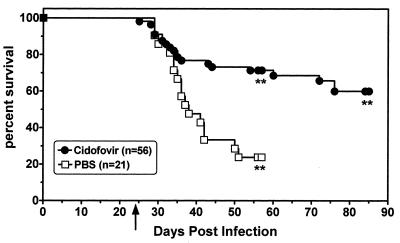
In two experiments, mice were sacrificed after 4.5 weeks of cidofovir therapy, while in two other experiments, therapy was continued for 8.5 weeks. Across four experiments using 77 mice (21 PBS-treated control mice and 56 cidofovir-treated mice), cidofovir treatment significantly improved survival (P = 0.0003). This effect was most prominent after prolonged therapy. For the first 10 days of therapy, there was no difference between the survival curves of PBS- and drug-treated mice, consistent with the fact that arterial pathology was established prior to initiating drug therapy. After 4.5 weeks of antiviral therapy, the difference in survival between PBS- and cidofovir-treated mice was not significant (P = 0.08 for data pooled from all four experiments) (Fig. 3). Thus, cidofovir protects against virus-induced mortality, but this requires prolonged therapy.
FIG. 3.
Cidofovir increases the survival of γHV68-infected IFN-γR−/− mice infected with 4 × 107 PFU of virus. Antiviral therapy was initiated 24 days postinfection, at which time a group of 18 mice were sacrificed; 15 of these (83%) had arteritis. Each point represents an event (death or sacrifice). The arrow indicates the day on which antiviral therapy was initiated. Asterisks represent the time points at which mice were sacrificed. P = 0.0003 for the survival advantage of cidofovir-treated mice over PBS-treated controls.
Antiviral therapy diminishes the severity of chronic γHV68-induced arteritis.
Cidofovir therapy resulted in significant changes in arteritic lesions. The presence of viral antigen was assessed by immunohistochemistry, and the severity of the inflammatory lesions was scored based on the nature and extent of mononuclear and neutrophilic infiltrates detected by H&E histology (Fig. 5; see Table 1 for scoring criteria and Fig. 4 for representative lesions). While 19 of 19 PBS-treated mice had viral antigen in their lesions, only 6 of 14 cidofovir-treated mice sacrificed after 4.5 weeks of therapy had detectable viral antigen in their lesions (P = 0.0002) (Fig. 5). Notably, cidofovir treatment resulted in a significant decrease in the number of lesions with viral antigen at a time when no statistically significant difference in survival between control and treated animals was seen. Similarly, after 8.5 weeks of cidofovir therapy, viral antigen was detectable in only 4 of 18 lesions from cidofovir-treated mice (P < 0.0001 compared to PBS controls) (Fig. 5). Thus, cidofovir treatment resulted in clearance of viral antigen.
FIG. 5.
Cidofovir treatment leads to clearance of γHV68 antigen and amelioration of pathology. Shown is a plot of lesion severity scores, as described in Table 1, as a function of time of death or sacrifice. Only mice with lesions are represented. The presence or absence of viral antigen (Ag) in lesions was assessed by immunohistochemistry as described in Materials and Methods (Ag staining). Weeks p.i., time postinfection of death or sacrifice. Since there were no differences in lesion severity for viral antigen-positive lesions of mice that died or were sacrificed 4 to 8 weeks postinfection, these data were pooled in columns 1 and 2. Mice in columns 3, 4, and 5 were all sacrificed. ∗, P < 0.0001 compared to scores for PBS-treated mice. ∗∗, P = 0.01 compared to scores in column 3.
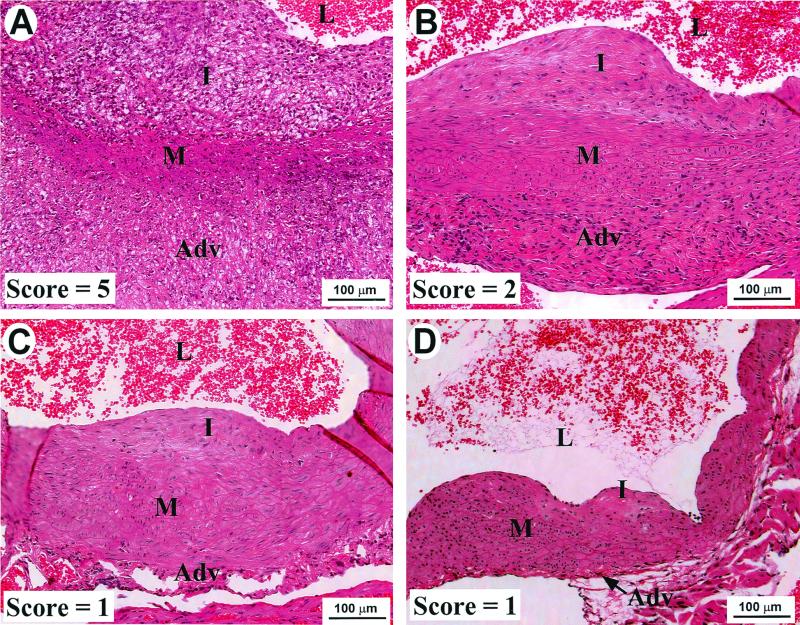
FIG. 4.
Representative aortic lesions and scores from control and cidofovir-treated mice. γHV68-infected IFN-γR−/− mice were treated with either PBS or cidofovir beginning at 24 days postinfection, as described in Materials and Methods. (A) Lesion with a score of 5 from a PBS-treated control mouse that died 6 weeks postinfection. (B) Representative lesion with a score of 2 from a cidofovir-treated mouse that was sacrificed after 8.5 weeks of therapy. (C and D) Representative lesions with scores of 1 from cidofovir-treated mice that were sacrificed after 8.5 weeks of therapy. Note the noninflammatory intimal thickening in panels B, C, and D. Adv, adventitia; M, media; I, intima; L, lumen.
Minimal differences in pathology were seen between lesions from cidofovir-treated mice that still had viral antigen (average lesion score, 4.2 ± 0.1; Fig. 5, columns 2 and 4 combined) and lesions from PBS-treated mice (average lesion score, 4.9 ± 0.1; Fig. 5, column 1). In contrast, lesions from cidofovir-treated mice that had no detectable viral antigen showed improvement. After 4.5 weeks of therapy, lesions without γHV68 antigen lacked neutrophilic infiltrates, although they still had pronounced intimal and adventitial infiltrates (average lesion score, 2.4 ± 0.4; Fig. 5, column 3; P < 0.0001 compared to PBS-treated mice). After prolonged therapy (8.5 weeks), lesions without viral antigen demonstrated dramatic improvement (average lesion score, 1.5 ± 0.1; Fig. 5, column 5; P < 0.0001 compared to PBS-treated mice, P = 0.01 compared to lesions without detectable viral antigen after 4.5 weeks of cidofovir therapy). After prolonged therapy the intimal lesions, in particular, had few inflammatory cells and there was only mild intimal and/or adventitial thickening (Fig. 4C, 4D). Thus, antiviral therapy initiated after the establishment of disease resulted in clearance of viral antigen. Subsequent to antigen clearance, the neutrophilic infiltrates resolved and, with time, the intimal and adventitial mononuclear inflammation also improved significantly.
DISCUSSION
Here we demonstrate that persistence of γHV68 replication in the walls of the large elastic arteries is required for the maintenance of arteritis. The dramatic amelioration of γHV68-induced lesions with antiviral therapy demonstrates that if autoimmunity was induced during this vascular disease, it was not sufficient for maintaining the inflammation once viral replication was inhibited. This result is consistent with the previous observation that inflammation did not extend to uninfected regions of the aorta and pulmonary artery (38). This was particularly evident in skip lesions, where there was a normal uninfected region flanked by two areas of arteritis; inflammation was restricted to regions of the vessel where viral antigen was present (38). If autoimmunity was sufficient for disease, one would expect that infiltrates would not be restricted to infected areas.
For viral infection to induce autoimmunity, the antiviral immune response would have to lead to recognition of self antigens distinct from viral antigens. This could occur via molecular mimicry or epitope spreading (reviewed in references 6 and 14). Epitope spreading has been well demonstrated in Theiler's murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD) (reviewed in reference 31). After infection of the brain and an initial antiviral immune response, T cells specific for several epitopes within myelin are activated and are thought to be responsible for chronic disease (reviewed in reference 31). These T cells have been shown to mediate cytotoxicity, since they demyelinate organotypic cultures in vitro (4). Thus, viral infection can lead to organ-specific autoimmunity, which could potentially occur in vascular disease as well. The potential for epitope spreading existed in γHV68-induced arteritis, since extensive cell death within lesions was noted by electron microscopy. Smooth muscle cell antigens could be processed and presented by local antigen-producing cells (APCs) to T cells, thus inducing an anti-self response. However, the efficacy of antiviral therapy argues against this possibility.
It could be argued that infection is necessary for effective presentation of autoantigens by APCs. The need for activating APCs to induce autoimmunity has been observed, for example, in a model of diabetes with transgenic mice expressing a lymphocytic choriomeningitis virus (LCMV) antigen in the pancreatic β-islet cells (23). Infection of these mice with LCMV activates tolerant peripheral CD8+ T cells, inducing infiltration and destruction of the islets, resulting in diabetes (23). However, when the activated cytotoxic T lymphocytes (CTLs) are transferred to uninfected transgenic mice, β cell destruction and disease occur only if costimulation and antigen presentation by APCs was induced experimentally (23, 34; reviewed in reference 35). Thus, though self-reactivity was induced in this model, it was dependent either on infection or on immune stimulation, even after the initial CTL activation. With respect to γHV68-induced arteritis, if autoimmunity contributes to the development of lesions, it clearly requires continued viral replication, since inflammation did not spread beyond the limits of infection and the inflammatory infiltrates resolved once viral replication was inhibited.
Although in most mice the arteritic lesions showed significant improvement after cidofovir therapy, several mice still had arteritic lesions with detectable viral antigen and severe disease even after 8.5 weeks of therapy. The presence of severe lesions after 8.5 weeks of antiviral therapy may have resulted from inefficient drug access, the local development of drug-resistant viral mutants, or insufficient time for control and clearance of replicating virus.
It is possible that some human vascular diseases may be caused and maintained by vascular infection. Interestingly, the strongest viral candidates for human vascular disease are those that establish long-term persistent infections, such as hepatitis B and hepatitis C viruses, human cytomegalovirus, Epstein-Barr virus, herpes simplex virus, and human immunodeficiency virus (reviewed in references 13, 16, and 22). These viruses have all developed various mechanisms for escaping the immune response, both by affecting antigen presentation and immune effector functions (reviewed in references 1, 8, 10, 24, and 27). If infectious agents can be etiologically linked to vasculitis, the data presented here suggest that antimicrobial therapy may help control or eliminate active disease. Evidence for infectious etiologies of atherosclerosis, arteritis, and coronary artery and transplant restenosis have been reported (11, 12; reviewed in references 5, 9, 15–19, 22, 28, and 36). Two randomized trials of antibiotic therapy in patients with unstable angina or myocardial infarcts were conducted with the hypothesis that Chlamydia pneumoniae played a role in the clinical manifestations of atherosclerosis (reviewed in reference 15). The results demonstrated improved clinical outcomes in antibiotic-treated patients compared to placebo-treated controls, suggesting that infection may indeed be important in chronic pathogenesis or acute thrombosis (reviewed in reference 15).
Given the efficacy of antimicrobial therapy in γHV68-induced arteritis, identification of specific pathogens that may be associated with the development of similar human diseases is critical. Takayasu's arteritis, the nongranulomatous variant of temporal arteritis, and Kawasaki's disease all exhibit pathology similar to the lesions observed in γHV68-infected IFN-γR−/− mice. Identification of infectious etiologies may well be complicated by the fact that different agents can cause similar diseases, as seen in mice infected with either γHV68 or MCMV (3, 25). Regardless, any successful antimicrobial treatment of human vasculitides will be dependent on the identification of the specific pathogen(s) involved. The data presented here demonstrate, however, that once a pathogen is identified, it is possible to reverse even severe vessel damage sustained during chronic inflammation.
ACKNOWLEDGMENTS
We thank Gilead Sciences for their generous donation of cidofovir. We thank Ramzi Cotran and Paul Swanson for help with electron microscopy. We also thank members of David Leib's laboratory and members of the Speck and Virgin laboratories for helpful discussions.
This work was supported in part by a research grant from the Monsanto-Searle Biomedical Agreement and by NIH grants CA43143, CA52004, and CA58524 to S.H.S. and CA74730, HL60090, and AI39616 to H.W.V.
REFERENCES
- 1.Brodsky F M, Lem L, Solache A, Bennett E M. Human pathogen subversion of antigen presentation. Immunol Rev. 1999;168:199–215. doi: 10.1111/j.1600-065x.1999.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 2.Clambey E T, Virgin IV H W, Speck S H. Disruption of the murine gammherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J Virol. 2000;74:1973–1984. doi: 10.1128/jvi.74.4.1973-1984.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dal Canto A J, Virgin H W., IV Animal models of infection-mediated vasculitis. Curr Opin Rheumatol. 1999;11:17–23. doi: 10.1097/00002281-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Dal Canto M C, Calenoff M A, Miller S D, Vanderlugt C L. Lymphocytes from mice chronically infected with Theiler's murine encephalomyelitis virus (TMEV) produce demyelination of organotypic cultures after stimulation with the major encephalitogenic epitope of myelin proteolipid protein (PLP). Epitope spreading in TMEV infection has functional activity. J Neuroimmunol. 2000;104:79–84. doi: 10.1016/s0165-5728(99)00230-1. [DOI] [PubMed] [Google Scholar]
- 5.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 6.Di Rosa F, Barnaba V. Persisting viruses and chronic inflammation: understanding their relation to autoimmunity. Immunol Rev. 1998;164:17–27. doi: 10.1111/j.1600-065x.1998.tb01204.x. [DOI] [PubMed] [Google Scholar]
- 7.Dutia B M, Clarke C J, Allen D J, Nash A A. Pathological changes in the spleens of gamma interferon receptor-deficient mice infected with murine gammaherpesvirus: a role for CD8 T cells. J Virol. 1997;71:4278–4283. doi: 10.1128/jvi.71.6.4278-4283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrlich R. Modulation of antigen processing and presentation by persistent virus infections and in tumors. Hum Immunol. 1997;54:104–116. doi: 10.1016/s0198-8859(97)00083-9. [DOI] [PubMed] [Google Scholar]
- 9.Ellis R W. Infection and coronary heart disease. J Med Microbiol. 1997;46:535–539. doi: 10.1099/00222615-46-7-535. [DOI] [PubMed] [Google Scholar]
- 10.Farrell H E, Davis-Poynter N J. From sabotage to camouflage: viral evasion of cytotoxic T lymphocyte and natural killer cell-mediated immunity. Semin Cell Dev Biol. 1998;9:369–378. doi: 10.1006/scdb.1998.0246. [DOI] [PubMed] [Google Scholar]
- 11.Gao S Z, Hunt S A, Schroeder J S, Alderman E L, Hill I R, Stinson E B. Early development of accelerated graft coronary artery disease: risk factors and course. J Am Coll Cardiol. 1996;28:673–679. doi: 10.1016/0735-1097(96)00201-x. [DOI] [PubMed] [Google Scholar]
- 12.Grattan M T, Moreno-Cabral C E, Starnes V A, Oyer P E, Stinson E B, Shumway N E. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989;261:3561–3566. [PubMed] [Google Scholar]
- 13.Hendrix M G R, Salimans M M M, van Boven C P A, Bruggeman C A. High prevalence of latently present cytomegalovirus in arterial walls of patients suffering from grade III atherosclerosis. Am J Pathol. 1990;136:23–28. [PMC free article] [PubMed] [Google Scholar]
- 14.Horwitz M S, Sarvetnick N. Viruses, host responses, and autoimmunity. Immunol Rev. 1999;169:241–253. doi: 10.1111/j.1600-065X.1999.tb01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juvonen T, Juvonen J, Savolainen M J. Is vasculitis a significant component of atherosclerosis? Curr Opin Rheumatol. 1999;11:3–10. doi: 10.1097/00002281-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Mandell B F, Calabrese L H. Infections and systemic vasculitis. Curr Opin Rheumatol. 1998;10:51–57. doi: 10.1097/00002281-199801000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Mehta J L, Saldeen T G, Rand K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J Am Coll Cardiol. 1998;31:1217–1225. doi: 10.1016/s0735-1097(98)00093-x. [DOI] [PubMed] [Google Scholar]
- 18.Melnick J L, Adam E, DeBakey M E. Possible role of cytomegalovirus in atherogenesis. JAMA. 1990;263:2204–2207. [PubMed] [Google Scholar]
- 19.Melnick J L, Adam E, DeBakey M E. Cytomegalovirus and atherosclerosis. Bioessays. 1995;17:899–903. doi: 10.1002/bies.950171012. [DOI] [PubMed] [Google Scholar]
- 20.Muller U, Steinhoff U, Reis L F L, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 21.Neyts J, De Clercq E. In vitro and in vivo inhibition of murine gammaherpesvirus 68 replication by selected antiviral agents. Antimicrob Agents Chemother. 1998;42:170–172. doi: 10.1128/aac.42.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nowack R, Flores-Suarez L F, van der Woude F J. New developments in pathogenesis of systemic vasculitis. Curr Opin Rheumatol. 1998;10:3–11. doi: 10.1097/00002281-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Oldstone M B, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 24.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 25.Presti R M, Pollock J L, Dal Canto A J, O'Guin A K, Virgin H W., IV Interferon-gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J Exp Med. 1998;188:577–588. doi: 10.1084/jem.188.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarawar S R, Cardin R D, Brooks J W, Mehrpooya M, Hamilton-Easton A-M, Mo X Y, Doherty P C. Gamma interferon is not essential for recovery from acute infection with murine gammaherpesvirus 68. J Virol. 1997;71:3916–3921. doi: 10.1128/jvi.71.5.3916-3921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seow H F. Pathogen interactions with cytokines and host defence: an overview. Vet Immunol Immunopathol. 1998;63:139–148. doi: 10.1016/s0165-2427(98)00090-7. [DOI] [PubMed] [Google Scholar]
- 28.Shih J C H, Keleman D W. Possible roles of viruses in atherosclerosis. Adv Exp Med Biol. 1995;369:89–98. doi: 10.1007/978-1-4615-1957-7_9. [DOI] [PubMed] [Google Scholar]
- 29.Simas J P, Bowden R J, Paige V, Efstathiou S. Four tRNA-like sequences and a serpin homologue encoded by murine gammaherpesvirus 68 are dispensable for lytic replication in vitro and latency in vivo. J Gen Virol. 1998;79:149–153. doi: 10.1099/0022-1317-79-1-149. [DOI] [PubMed] [Google Scholar]
- 30.Speck S H, Virgin H W. Host and viral genetics of chronic infection: a mouse model of gamma-herpesvirus pathogenesis. Curr Opin Microbiol. 1999;2:403–409. doi: 10.1016/s1369-5274(99)80071-x. [DOI] [PubMed] [Google Scholar]
- 31.Vanderlugt C L, Begolka W S, Neville K L, Katz-Levy Y, Howard L M, Eagar T N, Bluestone J A, Miller S D. The functional significance of epitope spreading and its regulation by co-stimulatory molecules. Immunol Rev. 1998;164:63–72. doi: 10.1111/j.1600-065x.1998.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 32.Virgin H W, IV, Latreille P, Wamsley P, Hallsworth K, Weck K E, Dal Canto A J, Speck S H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virgin H W, Speck S H. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr Opin Immunol. 1999;11:371–379. doi: 10.1016/s0952-7915(99)80063-6. [DOI] [PubMed] [Google Scholar]
- 34.von Herrath M, Holz A. Pathological changes in the islet milieu precede infiltration of islets and destruction of beta-cells by autoreactive lymphocytes in a transgenic model of virus-induced IDDM. J Autoimmun. 1997;10:231–238. doi: 10.1006/jaut.1997.0131. [DOI] [PubMed] [Google Scholar]
- 35.von Herrath M G. Selective immunotherapy of IDDM: a discussion based on new findings from the RIP-LCMV model for autoimmune diabetes. Transplant Proc. 1998;30:4115–4121. doi: 10.1016/s0041-1345(98)01362-1. [DOI] [PubMed] [Google Scholar]
- 36.Waldman W J, Adams P W, Knight D A, Sedmak D D. CMV as an exacerbating agent in transplant vascular sclerosis: potential immune-mediated mechanisms modelled in vitro. Transplant Proc. 1997;29:1545–1546. doi: 10.1016/s0041-1345(96)00670-7. [DOI] [PubMed] [Google Scholar]
- 37.Weck K E, Barkon M L, Yoo L I, Speck S H, Virgin H W., IV Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J Virol. 1996;70:6775–6780. doi: 10.1128/jvi.70.10.6775-6780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weck K E, Dal Canto A J, Gould J D, O'Guin A K, Roth K A, Saffitz J E, Speck S H, Virgin H W. Murine gammaherpesvirus 68 causes large vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus induced vascular disease. Nat Med. 1997;3:1346–1353. doi: 10.1038/nm1297-1346. [DOI] [PubMed] [Google Scholar]