Abstract
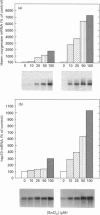
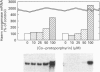
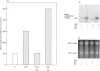
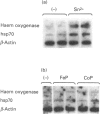
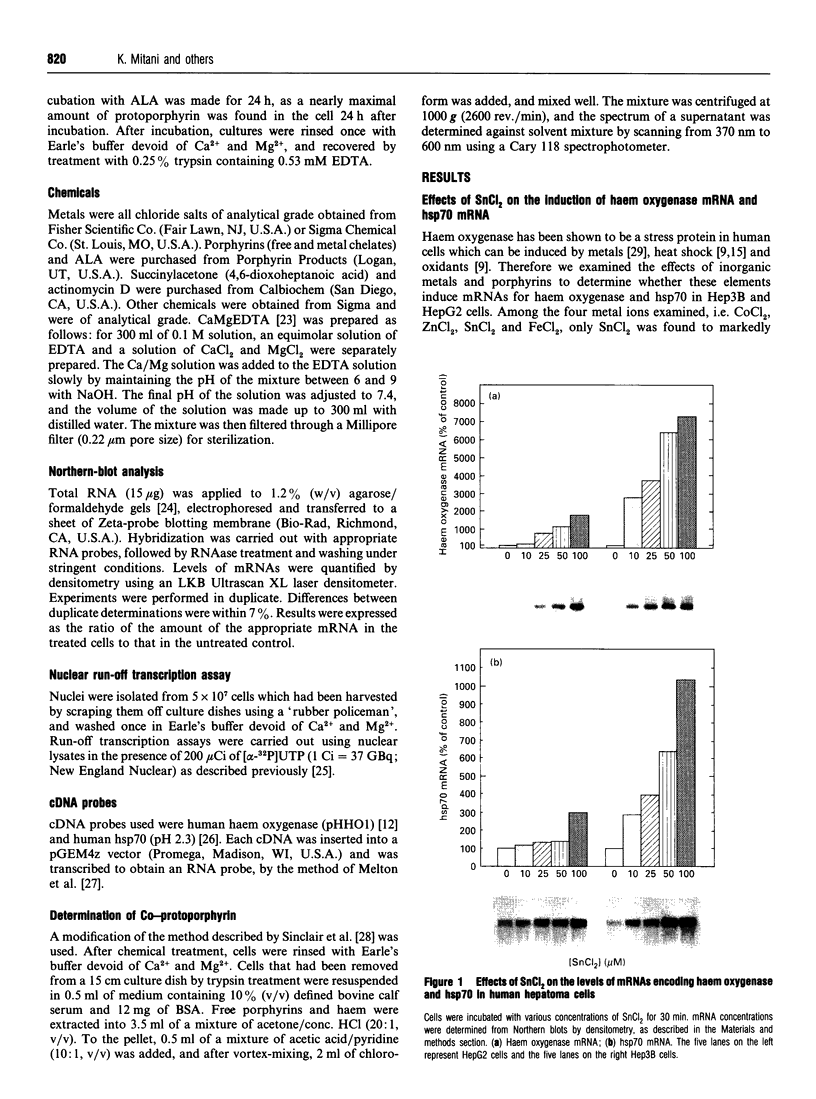
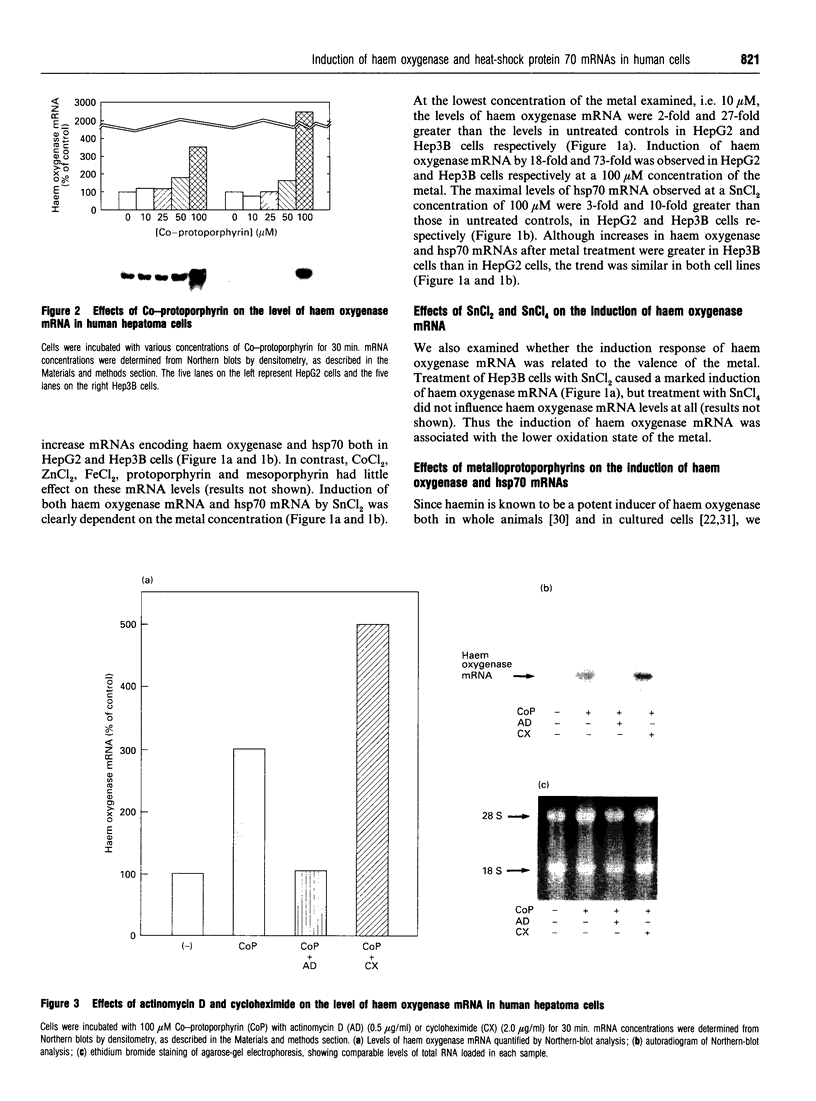
The role of inorganic metals and metalloporphyrins in the induction of mRNAs for haem oxygenase and heat-shock protein 70 (hsp70), the two heat-shock proteins, was examined in human HepG2 and Hep3B hepatoma cells. SnCl2, but not Sn-protoporphyrin, was found to be a potent inducer of both haem oxygenase and hsp70 mRNAs. In contrast, CoCl2, ZnCl2 and FeCl2 caused little induction of haem oxygenase and hsp70 mRNAs, whereas the porphyrin complexes of these metals strongly induced haem oxygenase mRNA, without influencing the level of hsp70 mRNA. The induction process was largely transcriptional, as judged by the inhibition of induction by actinomycin D, but not by cycloheximide, and by increased transcription demonstrated by nuclear run-off analysis. Since CoCl2 is a potent inducer of haem oxygenase in vivo in animals, the possibility of the biosynthesis of Co-protoporphyrin was examined in human hepatoma cells by incubating them with CoCl2 and protoporphyrin, or delta-aminolaevulinate (ALA), the precursor of protoporphyrin. Both types of treatment led to a potent induction of haem oxygenase mRNA. Co-protoporphyrin formation was also spectrally demonstrated in cells incubated with the metal and ALA. The results of this study indicate that certain metals, e.g. SnCl2, may directly induce haem oxygenase mRNA, whereas with other elements, incorporation of the metal into the porphyrin macrocycle is necessary for induction. Therefore CoCl2, like haemin, may activate the haem oxygenase gene via a haem-responsive transcription factor, whereas SnCl2 may exert its effect via a metal-responsive transcription factor.
Full text
PDF
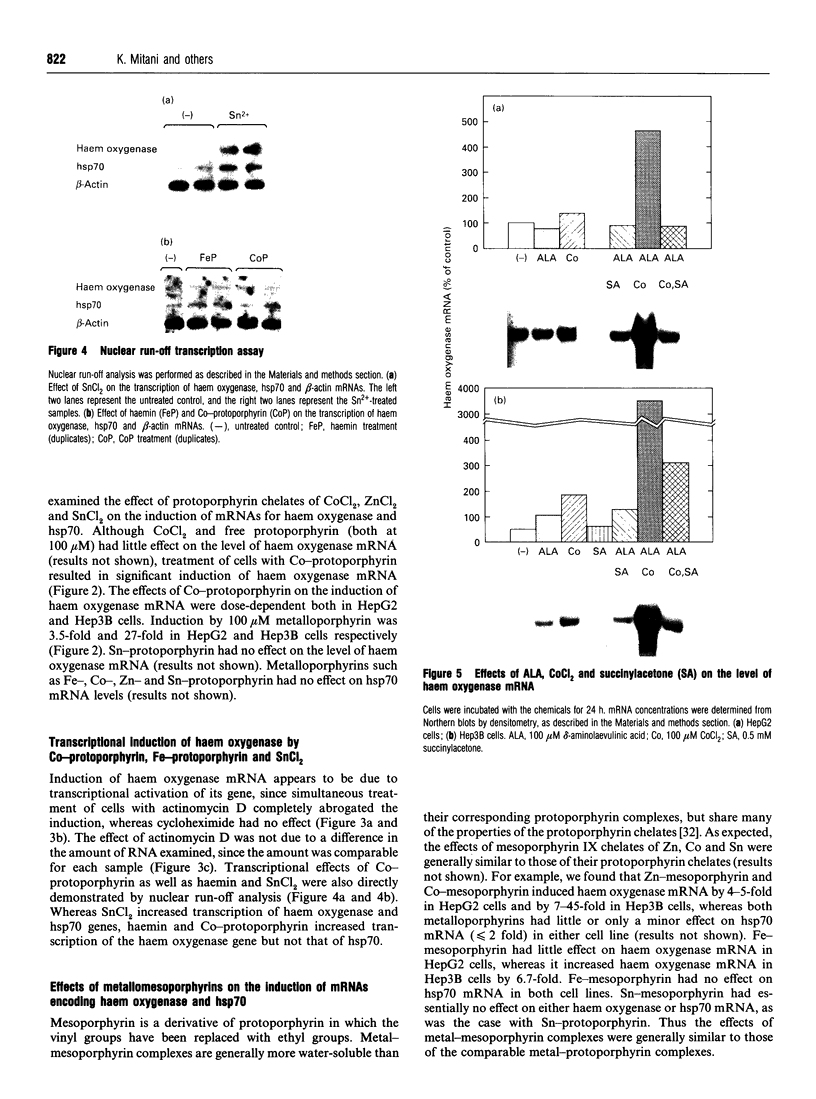
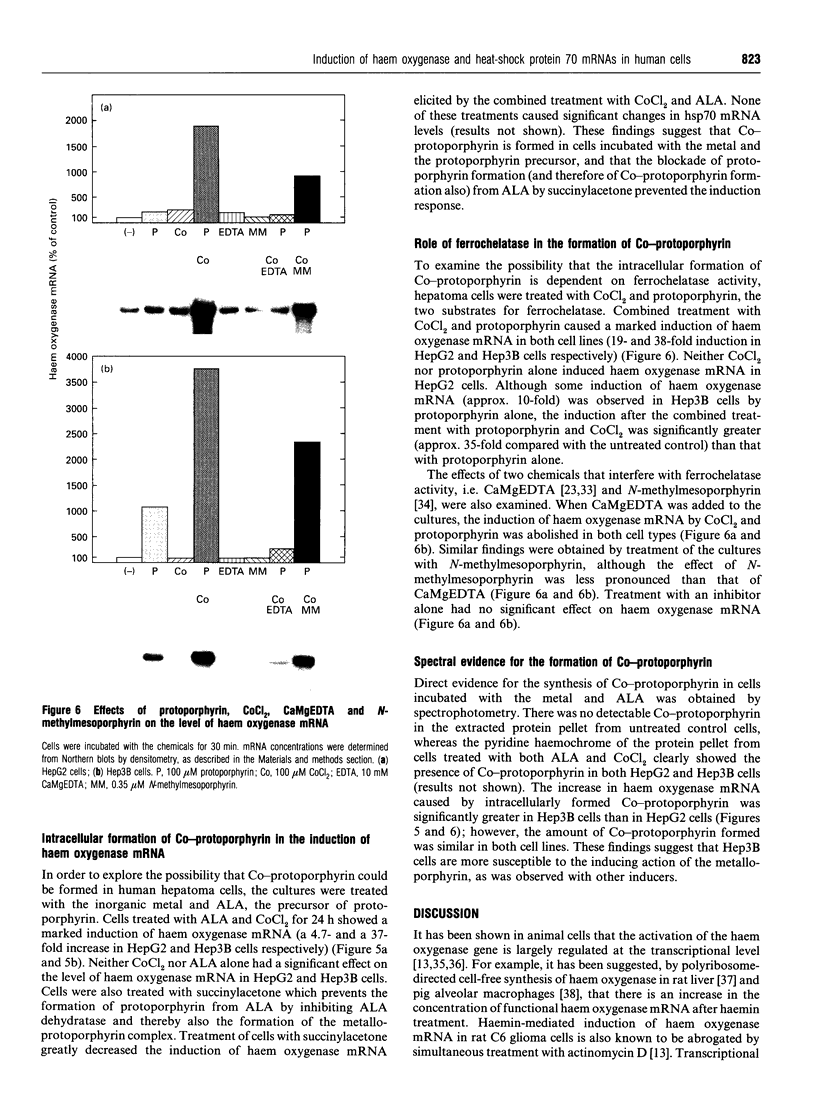


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam J., Shibahara S., Smith A. Transcriptional activation of the heme oxygenase gene by heme and cadmium in mouse hepatoma cells. J Biol Chem. 1989 Apr 15;264(11):6371–6375. [PubMed] [Google Scholar]
- Chiba M., Kikuchi M. Effect of tin compounds on activity of 5-aminolevulinate dehydratase in blood. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1057–1061. doi: 10.1016/0006-291x(78)90294-2. [DOI] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H., Smith A. G. Inhibition of protohaem ferro-lyase by N-substituted porphyrins. Structural requirements for the inhibitory effect. Biochem J. 1980 Sep 1;189(3):645–648. doi: 10.1042/bj1890645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Marks G. S. The effect of N-methylprotoporphyrin and succinyl-acetone on the regulation of heme biosynthesis in chicken hepatocytes in culture. FEBS Lett. 1983 Aug 8;159(1-2):127–131. doi: 10.1016/0014-5793(83)80430-x. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Galbraith R. A., Sardana M. K., Kappas A. Reduction of the C2 and C4 vinyl groups of Sn-protoporphyrin to form Sn-mesoporphyrin markedly enhances the ability of the metalloporphyrin to inhibit in vivo heme catabolism. Arch Biochem Biophys. 1987 May 15;255(1):64–74. doi: 10.1016/0003-9861(87)90294-3. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Potent heme-degrading action of antimony and antimony-containing parasiticidal agents. J Exp Med. 1981 Feb 1;153(2):245–256. doi: 10.1084/jem.153.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. The cytochrome P-450-depleted animal: an experimental model for in vivo studies in chemical biology. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2384–2388. doi: 10.1073/pnas.79.7.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith R. A., Sassa S., Kappas A. Induction of haem synthesis in Hep G2 human hepatoma cells by dimethyl sulphoxide. A transcriptionally activated event. Biochem J. 1986 Jul 15;237(2):597–600. doi: 10.1042/bj2370597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa S., Yoshida T., Kikuchi G. Induction of heme oxygenase in rat liver. Increase of the specific mRNA by treatment with various chemicals and immunological identity of the enzymes in various tissues as well as the induced enzymes. J Biol Chem. 1983 Apr 10;258(7):4220–4225. [PubMed] [Google Scholar]
- Iwasa F., Sassa S., Kappas A. delta-Aminolaevulinate synthase in human HepG2 hepatoma cells. Repression by haemin and induction by chemicals. Biochem J. 1989 Sep 15;262(3):807–813. doi: 10.1042/bj2620807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt N. B. Hep G2 cells as a resource for metabolic studies: lipoprotein, cholesterol, and bile acids. FASEB J. 1990 Feb 1;4(2):161–168. doi: 10.1096/fasebj.4.2.2153592. [DOI] [PubMed] [Google Scholar]
- Kappas A., Maines M. D. Tin: a potent inducer of heme oxygenase in kidney. Science. 1976 Apr 2;192(4234):60–62. doi: 10.1126/science.1257757. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi G., Yoshida T. Function and induction of the microsomal heme oxygenase. Mol Cell Biochem. 1983;53-54(1-2):163–183. doi: 10.1007/BF00225252. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Metals as regulators of heme metabolism. Science. 1977 Dec 23;198(4323):1215–1221. doi: 10.1126/science.337492. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Studies on the mechanism of induction of haem oxygenase by cobalt and other metal ions. Biochem J. 1976 Jan 15;154(1):125–131. doi: 10.1042/bj1540125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. The induction of heme oxidation in various tissues by trace metals: evidence for the catabolism of endogenous heme by hepatic heme oxygenase. Ann Clin Res. 1976;8 (Suppl 17):39–46. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani K., Fujita H., Sassa S., Kappas A. Activation of heme oxygenase and heat shock protein 70 genes by stress in human hepatoma cells. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1429–1434. doi: 10.1016/0006-291x(90)91026-o. [DOI] [PubMed] [Google Scholar]
- Mitani K., Fujita H., Sassa S., Kappas A. Heat shock induction of heme oxygenase mRNA in human Hep 3B hepatoma cells. Biochem Biophys Res Commun. 1989 Nov 30;165(1):437–441. doi: 10.1016/0006-291x(89)91089-9. [DOI] [PubMed] [Google Scholar]
- Mosser D. D., Theodorakis N. G., Morimoto R. I. Coordinate changes in heat shock element-binding activity and HSP70 gene transcription rates in human cells. Mol Cell Biol. 1988 Nov;8(11):4736–4744. doi: 10.1128/mcb.8.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardana M. K., Drummond G. S., Sassa S., Kappas A. The potent heme oxygenase inducing action of arsenic and parasiticidal arsenicals. Pharmacology. 1981;23(5):247–253. doi: 10.1159/000137557. [DOI] [PubMed] [Google Scholar]
- Sassa S., Bradlow H. L., Kappas A. Steroid induction of delta-aminolevulinic acid synthase and porphyrins in liver. Structure-activity studies and the permissive effects of hormones on the induction process. J Biol Chem. 1979 Oct 25;254(20):10011–10020. [PubMed] [Google Scholar]
- Sassa S., Kappas A. Induction of aminolevulinate synthase and porphyrins in cultured liver cells maintained in chemically defined medium. Permissive effects of hormones on induction process. J Biol Chem. 1977 Apr 10;252(7):2428–2436. [PubMed] [Google Scholar]
- Sassa S., Sugita O., Galbraith R. A., Kappas A. Drug metabolism by the human hepatoma cell, Hep G2. Biochem Biophys Res Commun. 1987 Feb 27;143(1):52–57. doi: 10.1016/0006-291x(87)90628-0. [DOI] [PubMed] [Google Scholar]
- Schoenfeld N., Wysenbeek A. J., Greenblat Y., Epstein O., Atsmon A., Tschudy D. P. The effects of metalloporphyrins, porphyrins and metals on the activity of delta-aminolevulinic acid synthase in monolayers of chick embryo liver cells. Biochem Pharmacol. 1984 Sep 1;33(17):2783–2788. doi: 10.1016/0006-2952(84)90696-8. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Müller R. M., Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem. 1987 Sep 25;262(27):12889–12892. [PubMed] [Google Scholar]
- Shibahara S., Sato M., Muller R. M., Yoshida T. Structural organization of the human heme oxygenase gene and the function of its promoter. Eur J Biochem. 1989 Feb 15;179(3):557–563. doi: 10.1111/j.1432-1033.1989.tb14583.x. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Yoshida T., Kikuchi G. Induction of heme oxygenase by hemin in cultured pig alveolar macrophages. Arch Biochem Biophys. 1978 Jun;188(2):243–250. doi: 10.1016/s0003-9861(78)80006-x. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Yoshida T., Kikuchi G. Mechanism of increase of heme oxygenase activity induced by hemin in cultured pig alveolar macrophages. Arch Biochem Biophys. 1979 Oct 15;197(2):607–617. doi: 10.1016/0003-9861(79)90285-6. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Sinclair P. R., Healey J. F., Smith E. L., Bonkowsky H. L. Decrease in hepatic cytochrome P-450 by cobalt. Evidence for a role of cobalt protoporphyrin. Biochem J. 1982 Apr 15;204(1):103–109. doi: 10.1042/bj2040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair P. R., Sinclair J. F., Bonkowsky H. L., Gibbs A. H., De Matteis F. Formation of cobalt protoporphyrin by chicken hepatocytes in culture. Relationship to decrease of 5-aminolaevulinate synthase caused by cobalt. Biochem Pharmacol. 1982 Mar 15;31(6):993–999. doi: 10.1016/0006-2952(82)90333-1. [DOI] [PubMed] [Google Scholar]
- Sinclair P., Gibbs A. H., Sinclair J. F., de Matteis F. Formation of cobalt protoporphyrin in the liver of rats. A mechanism for the inhibition of liver haem biosynthesis by inorganic cobalt. Biochem J. 1979 Mar 15;178(3):529–538. doi: 10.1042/bj1780529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J., Haque S., Drummond G. S. Induction of heme oxygenase mRNA by cobalt protoporphyrin in rat liver. Biochim Biophys Acta. 1991 Jan 23;1073(1):221–224. doi: 10.1016/0304-4165(91)90206-v. [DOI] [PubMed] [Google Scholar]
- Stuart G. W., Searle P. F., Palmiter R. D. Identification of multiple metal regulatory elements in mouse metallothionein-I promoter by assaying synthetic sequences. 1985 Oct 31-Nov 6Nature. 317(6040):828–831. doi: 10.1038/317828a0. [DOI] [PubMed] [Google Scholar]
- Taketani S., Kohno H., Yoshinaga T., Tokunaga R. Induction of heme oxygenase in rat hepatoma cells by exposure to heavy metals and hyperthermia. Biochem Int. 1988 Oct;17(4):665–672. [PubMed] [Google Scholar]
- Taketani S., Kohno H., Yoshinaga T., Tokunaga R. The human 32-kDa stress protein induced by exposure to arsenite and cadmium ions is heme oxygenase. FEBS Lett. 1989 Mar 13;245(1-2):173–176. doi: 10.1016/0014-5793(89)80215-7. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970 Mar;75(3):410–421. [PubMed] [Google Scholar]
- Theodorakis N. G., Zand D. J., Kotzbauer P. T., Williams G. T., Morimoto R. I. Hemin-induced transcriptional activation of the HSP70 gene during erythroid maturation in K562 cells is due to a heat shock factor-mediated stress response. Mol Cell Biol. 1989 Aug;9(8):3166–3173. doi: 10.1128/mcb.9.8.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaro M. L., Frydman J., Frydman R. B. Heme oxygenase induction by CoCl2, Co-protoporphyrin IX, phenylhydrazine, and diamide: evidence for oxidative stress involvement. Arch Biochem Biophys. 1991 May 1;286(2):610–617. doi: 10.1016/0003-9861(91)90088-z. [DOI] [PubMed] [Google Scholar]
- Watkins S., Baron J., Tephly T. R. Identification of cobalt protoporphyrin IX formation in vivo following cobalt administration to rats. Biochem Pharmacol. 1980 Sep 1;29(17):2319–2323. doi: 10.1016/0006-2952(80)90264-6. [DOI] [PubMed] [Google Scholar]
- Wu B., Hunt C., Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985 Feb;5(2):330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Biro P., Cohen T., Müller R. M., Shibahara S. Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur J Biochem. 1988 Feb 1;171(3):457–461. doi: 10.1111/j.1432-1033.1988.tb13811.x. [DOI] [PubMed] [Google Scholar]
- Yoshinaga T., Sassa S., Kappas A. A comparative study of heme degradation by NADPH-cytochrome c reductase alone and by the complete heme oxygenase system. Distinctive aspects of heme degradation by NADPH-cytochrome c reductase. J Biol Chem. 1982 Jul 10;257(13):7794–7802. [PubMed] [Google Scholar]
- Yoshinaga T., Sassa S., Kappas A. Purification and properties of bovine spleen heme oxygenase. Amino acid composition and sites of action of inhibitors of heme oxidation. J Biol Chem. 1982 Jul 10;257(13):7778–7785. [PubMed] [Google Scholar]