Abstract
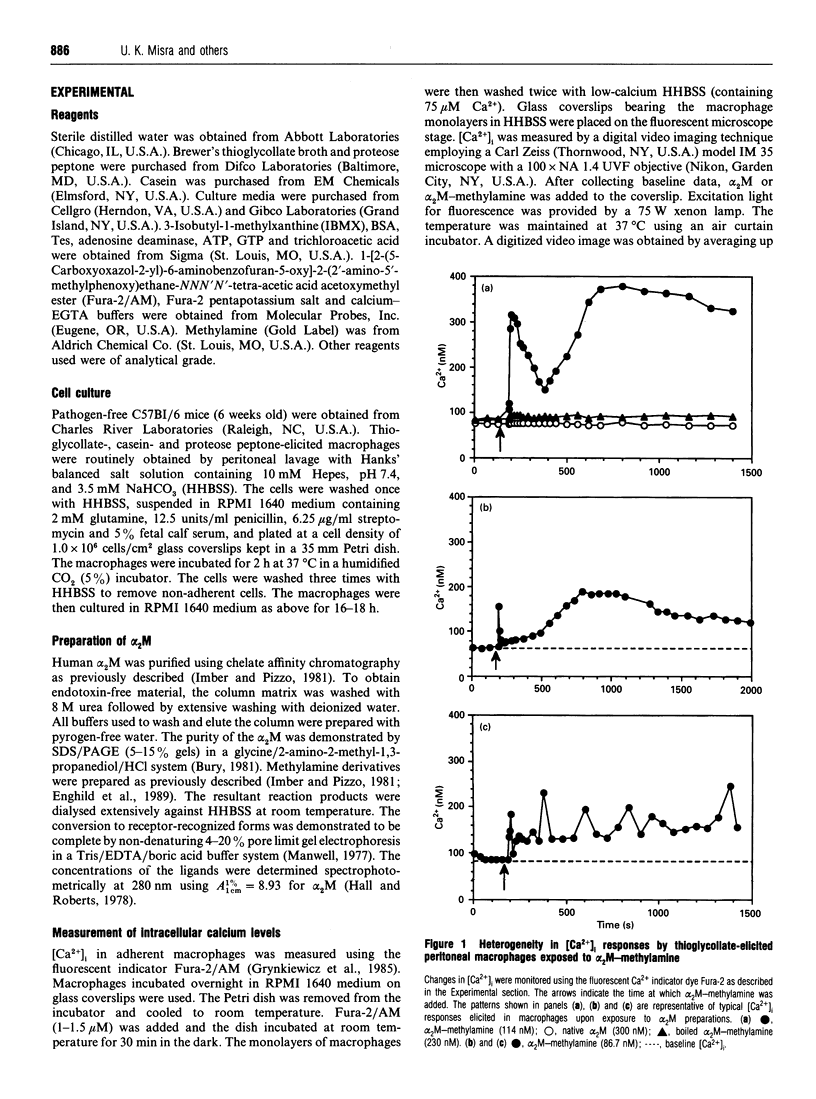
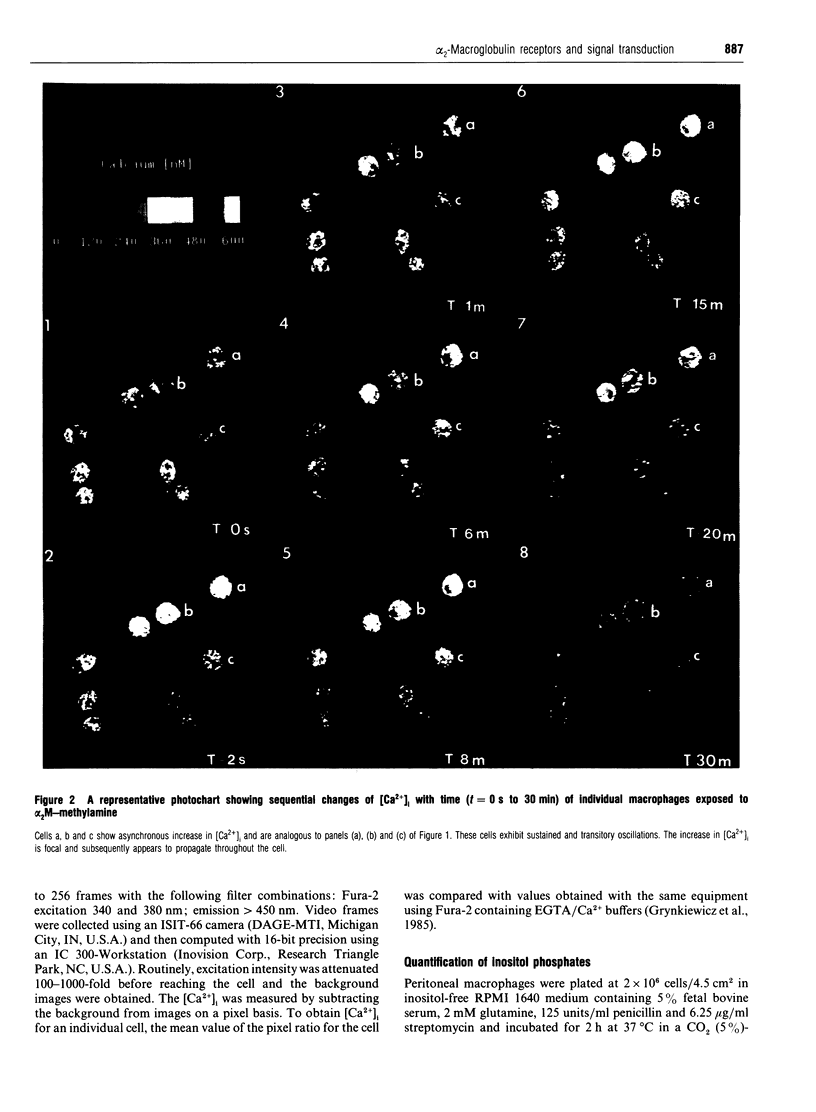
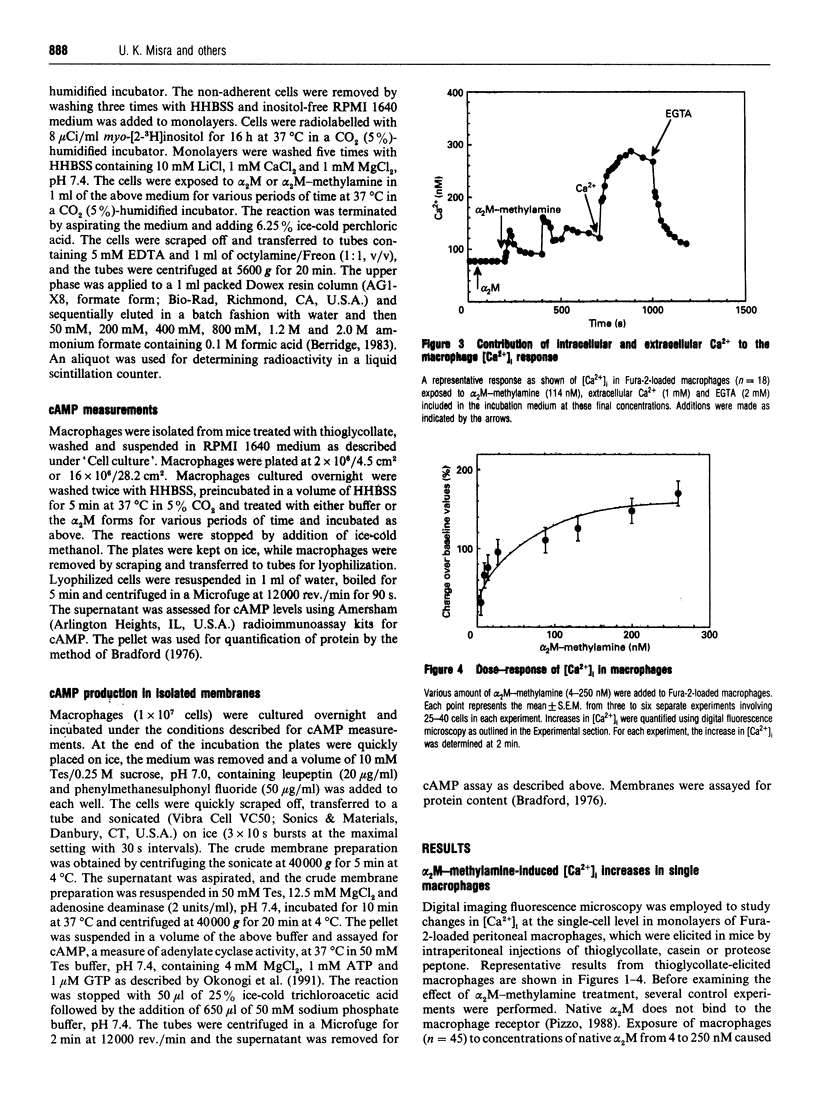
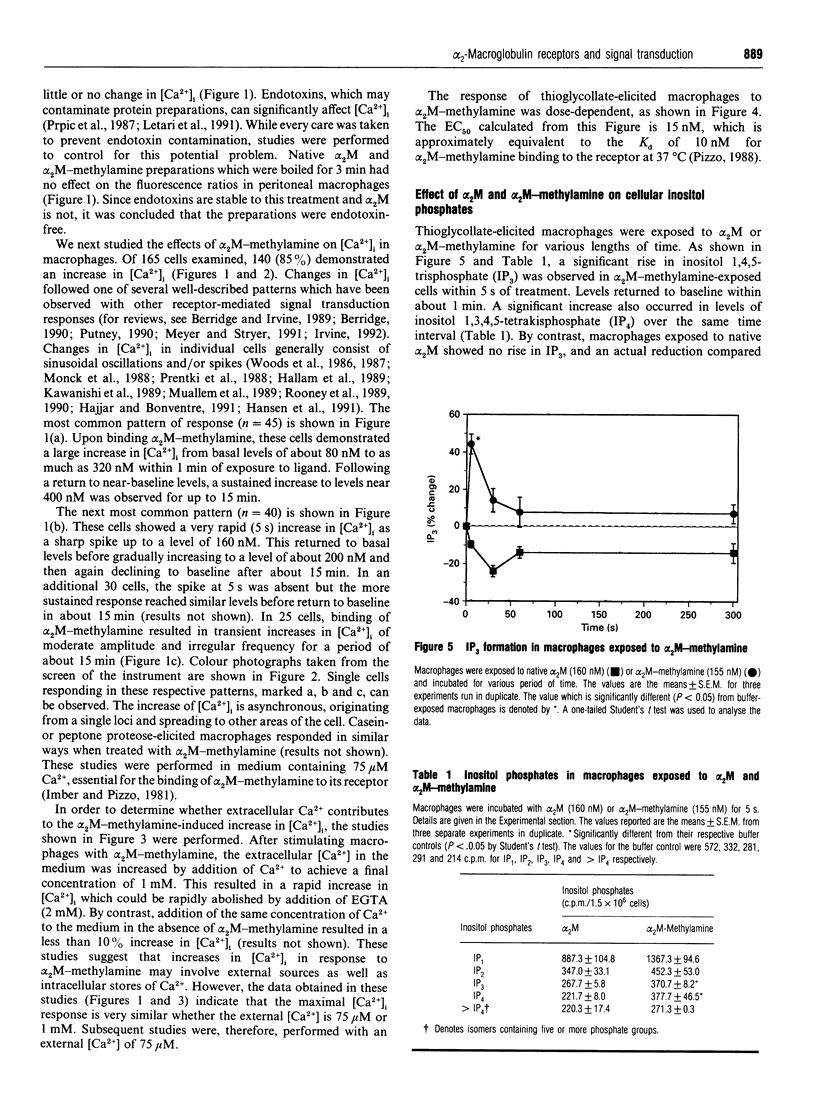
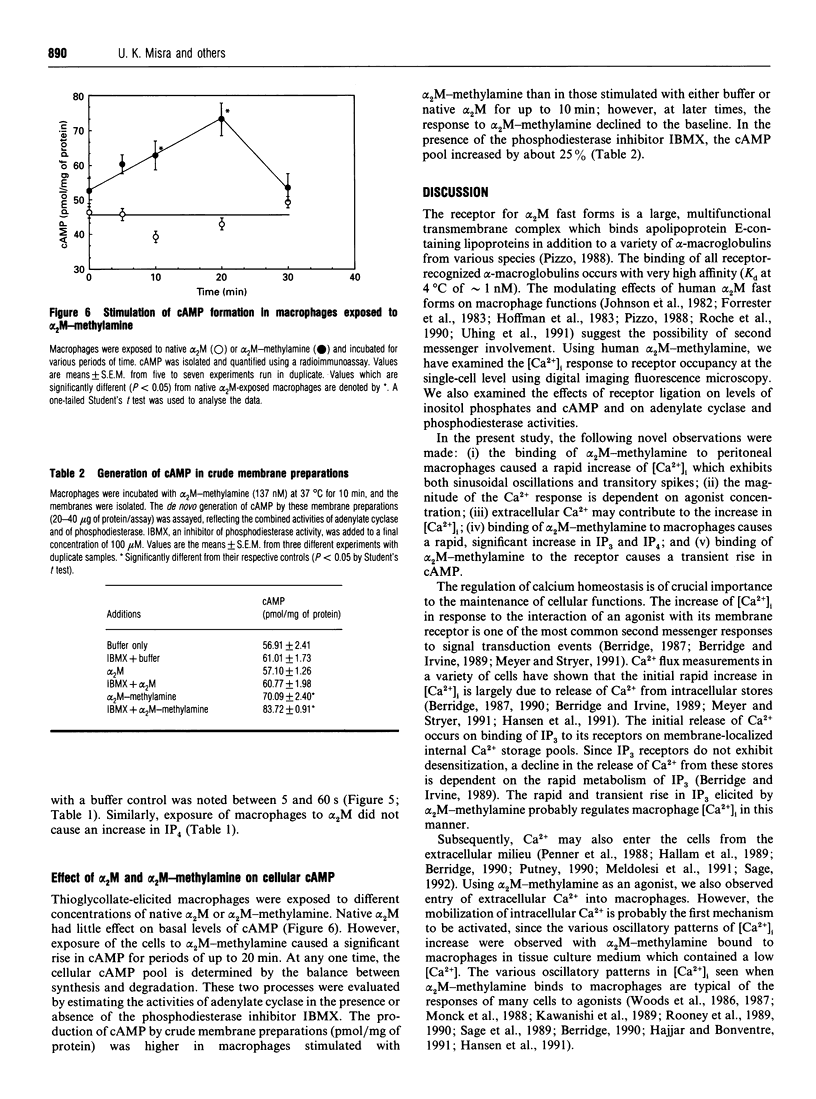
Human plasma alpha 2-macroglobulin (alpha 2M) is a tetrameric proteinase inhibitor, which undergoes a conformational change upon reaction with either a proteinase or methylamine. As a result, a receptor recognition site is exposed on each subunit of the molecule enabling it to bind to its receptors on macrophages. We have used Fura-2-loaded murine peritoneal macrophages and digital video fluorescence microscopy to examine the effects of receptor binding on second messenger levels. alpha 2M-methylamine caused a rapid 2-4-fold increase in intracellular Ca2+ concentration ([Ca2+]i) within 5 s of binding to receptors. The agonists induced a focal increase in [Ca2+]i that spread out to other areas of the cell. The increase in [Ca2+]i was dependent on the alpha 2M-methylamine concentration and on the extracellular [Ca2+]. Both sinusoidal and transitory oscillations were observed, which varied from cell to cell. Neither alpha 2M nor boiled alpha 2M-methylamine, forms that are not recognized by the receptor, affected [Ca2+]i in peritoneal macrophages under identical conditions of incubation. The alpha 2M-methylamine-induced rise in [Ca2+]i was accompanied by a rapid and transient increase in macrophage inositol phosphates, including inositol tris- and tetrakis-phosphates. Native alpha 2M did not stimulate a rise in inositol phosphates. Finally, binding of alpha 2M-methylamine to macrophages increased cyclic AMP transiently. Thus receptor-recognized alpha-macroglobulins behave as agonists whose receptor binding causes stimulation of signal transduction pathways.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Robb R. J., Welte K., Dupont B. Analysis of prostaglandin E2 effect on T lymphocyte activation. Abrogation of prostaglandin E2 inhibitory effect by the tumor promotor 12.0 tetradecanoyl phorbol-13 acetate. J Clin Invest. 1987 Aug;80(2):333–340. doi: 10.1172/JCI113077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Enghild J. J., Salvesen G., Thøgersen I. B., Pizzo S. V. Proteinase binding and inhibition by the monomeric alpha-macroglobulin rat alpha 1-inhibitor-3. J Biol Chem. 1989 Jul 5;264(19):11428–11435. [PubMed] [Google Scholar]
- Figueíredo F., Uhing R. J., Okonogi K., Gettys T. W., Johnson S. P., Adams D. O., Prpic V. Activation of the cAMP cascade inhibits an early event involved in murine macrophage Ia expression. J Biol Chem. 1990 Jul 25;265(21):12317–12323. [PubMed] [Google Scholar]
- Forrester J. V., Wilkinson P. C., Lackie J. M. Effect of modified alpha 2macroglobulin on leucocyte locomotion and chemotaxis. Immunology. 1983 Oct;50(2):251–259. [PMC free article] [PubMed] [Google Scholar]
- Hajjar R. J., Bonventre J. V. Oscillations of intracellular calcium induced by vasopressin in individual fura-2-loaded mesangial cells. Frequency dependence on basal calcium concentration, agonist concentration, and temperature. J Biol Chem. 1991 Nov 15;266(32):21589–21594. [PubMed] [Google Scholar]
- Hall P. K., Roberts R. C. Physical and chemical properties of human plasma alpha2-macroglobulin. Biochem J. 1978 Jul 1;173(1):27–38. doi: 10.1042/bj1730027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Influx of bivalent cations can be independent of receptor stimulation in human endothelial cells. Biochem J. 1989 Apr 1;259(1):125–129. doi: 10.1042/bj2590125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C. A., Yang L. J., Williamson J. R. Mechanisms of receptor-mediated Ca2+ signaling in rat hepatocytes. J Biol Chem. 1991 Oct 5;266(28):18573–18579. [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Hoffman M. R., Pizzo S. V., Weinberg J. B. Modulation of mouse peritoneal macrophage Ia and human peritoneal macrophage HLA-DR expression by alpha 2-macroglobulin "fast" forms. J Immunol. 1987 Sep 15;139(6):1885–1890. [PubMed] [Google Scholar]
- Hoffman M., Feldman S. R., Pizzo S. V. Alpha 2-macroglobulin 'fast' forms inhibit superoxide production by activated macrophages. Biochim Biophys Acta. 1983 Nov 8;760(3):421–423. doi: 10.1016/0304-4165(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Hoffman M., Pizzo S. V., Weinberg J. B. Alpha 2 macroglobulin-proteinase complexes stimulate prostaglandin E2 synthesis by peritoneal macrophages. Agents Actions. 1988 Dec;25(3-4):360–367. doi: 10.1007/BF01965043. [DOI] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V. Clearance and binding of two electrophoretic "fast" forms of human alpha 2-macroglobulin. J Biol Chem. 1981 Aug 10;256(15):8134–8139. [PubMed] [Google Scholar]
- Irvine R. F. Inositol lipids in cell signalling. Curr Opin Cell Biol. 1992 Apr;4(2):212–219. doi: 10.1016/0955-0674(92)90035-b. [DOI] [PubMed] [Google Scholar]
- Johnson W. J., Pizzo S. V., Imber M. J., Adams D. O. Receptors for maleylated proteins regulate secretion of neutral proteases by murine macrophages. Science. 1982 Nov 5;218(4572):574–576. doi: 10.1126/science.6289443. [DOI] [PubMed] [Google Scholar]
- Kammer G. M. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988 Jul-Aug;9(7-8):222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- Kawanishi T., Blank L. M., Harootunian A. T., Smith M. T., Tsien R. Y. Ca2+ oscillations induced by hormonal stimulation of individual fura-2-loaded hepatocytes. J Biol Chem. 1989 Aug 5;264(22):12859–12866. [PubMed] [Google Scholar]
- Lerner A., Jacobson B., Miller R. A. Cyclic AMP concentrations modulate both calcium flux and hydrolysis of phosphatidylinositol phosphates in mouse T lymphocytes. J Immunol. 1988 Feb 1;140(3):936–940. [PubMed] [Google Scholar]
- Letari O., Nicosia S., Chiavaroli C., Vacher P., Schlegel W. Activation by bacterial lipopolysaccharide causes changes in the cytosolic free calcium concentration in single peritoneal macrophages. J Immunol. 1991 Aug 1;147(3):980–983. [PubMed] [Google Scholar]
- Manwell C. A simplified electrophoretic system for determining molecular weights of proteins. Biochem J. 1977 Sep 1;165(3):487–495. doi: 10.1042/bj1650487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J., Clementi E., Fasolato C., Zacchetti D., Pozzan T. Ca2+ influx following receptor activation. Trends Pharmacol Sci. 1991 Aug;12(8):289–292. doi: 10.1016/0165-6147(91)90577-f. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Calcium spiking. Annu Rev Biophys Biophys Chem. 1991;20:153–174. doi: 10.1146/annurev.bb.20.060191.001101. [DOI] [PubMed] [Google Scholar]
- Monck J. R., Reynolds E. E., Thomas A. P., Williamson J. R. Novel kinetics of single cell Ca2+ transients in stimulated hepatocytes and A10 cells measured using fura-2 and fluorescent videomicroscopy. J Biol Chem. 1988 Apr 5;263(10):4569–4575. [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Hormone-evoked calcium release from intracellular stores is a quantal process. J Biol Chem. 1989 Jan 5;264(1):205–212. [PubMed] [Google Scholar]
- O'Shea J. J., Urdahl K. B., Luong H. T., Chused T. M., Samelson L. E., Klausner R. D. Aluminum fluoride induces phosphatidylinositol turnover, elevation of cytoplasmic free calcium, and phosphorylation of the T cell antigen receptor in murine T cells. J Immunol. 1987 Nov 15;139(10):3463–3469. [PubMed] [Google Scholar]
- Okonogi K., Gettys T. W., Uhing R. J., Tarry W. C., Adams D. O., Prpic V. Inhibition of prostaglandin E2-stimulated cAMP accumulation by lipopolysaccharide in murine peritoneal macrophages. J Biol Chem. 1991 Jun 5;266(16):10305–10312. [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Plaut M. Lymphocyte hormone receptors. Annu Rev Immunol. 1987;5:621–669. doi: 10.1146/annurev.iy.05.040187.003201. [DOI] [PubMed] [Google Scholar]
- Prentki M., Glennon M. C., Thomas A. P., Morris R. L., Matschinsky F. M., Corkey B. E. Cell-specific patterns of oscillating free Ca2+ in carbamylcholine-stimulated insulinoma cells. J Biol Chem. 1988 Aug 15;263(23):11044–11047. [PubMed] [Google Scholar]
- Prpic V., Weiel J. E., Somers S. D., DiGuiseppi J., Gonias S. L., Pizzo S. V., Hamilton T. A., Herman B., Adams D. O. Effects of bacterial lipopolysaccharide on the hydrolysis of phosphatidylinositol-4,5-bisphosphate in murine peritoneal macrophages. J Immunol. 1987 Jul 15;139(2):526–533. [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Hoffman M. R., Pizzo S. V. Effect of interferon-gamma and human alpha 2-macroglobulin on peritoneal macrophage morphology and Ia antigen expression. Biochim Biophys Acta. 1990 Feb 19;1051(2):166–173. doi: 10.1016/0167-4889(90)90189-k. [DOI] [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. Agonist-induced cytosolic calcium oscillations originate from a specific locus in single hepatocytes. J Biol Chem. 1990 Jun 25;265(18):10792–10796. [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J Biol Chem. 1989 Oct 15;264(29):17131–17141. [PubMed] [Google Scholar]
- Sage S. O., Adams D. J., van Breemen C. Synchronized oscillations in cytoplasmic free calcium concentration in confluent bradykinin-stimulated bovine pulmonary artery endothelial cell monolayers. J Biol Chem. 1989 Jan 5;264(1):6–9. [PubMed] [Google Scholar]
- Sage S. O. Three routes for receptor-mediated Ca2+ entry. Curr Biol. 1992 Jun;2(6):312–314. doi: 10.1016/0960-9822(92)90885-e. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Stepanik T. M., Kristensen T., Lønblad P. B., Jones C. M., Wierzbicki D. M., Magnusson S., Domdey H., Wetsel R. A., Lundwall A. Common evolutionary origin of alpha 2-macroglobulin and complement components C3 and C4. Proc Natl Acad Sci U S A. 1985 Jan;82(1):9–13. doi: 10.1073/pnas.82.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H., Putney J. W., Jr Capacitative calcium entry in parotid acinar cells. Biochem J. 1989 Mar 1;258(2):409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhing R. J., Martenson C. H., Rubenstein D. S., Hollenbach P. W., Pizzo S. V. The exposure of murine macrophages to alpha 2-macroglobulin 'fast' forms results in the rapid secretion of eicosanoids. Biochim Biophys Acta. 1991 Jul 10;1093(2-3):115–120. doi: 10.1016/0167-4889(91)90111-a. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Phorbol-ester-induced alterations of free calcium ion transients in single rat hepatocytes. Biochem J. 1987 Sep 15;246(3):619–623. doi: 10.1042/bj2460619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Suzuki T. Prostaglandin-E2-induced activation of adenosine 3'-5' cyclic monophosphate-dependent protein kinases of a murine macrophage-like cell line (P388D1). J Immunol. 1987 Nov 15;139(10):3416–3421. [PubMed] [Google Scholar]



