Abstract
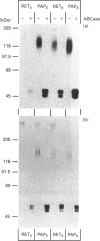
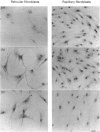
Immunostaining of adult human skin shows that the small dermatan sulphate proteoglycan decorin is abundant in the whole dermal layer but absent from the epidermis. In the papillary layer adjacent to the dermal-epidermal border, more decorin was detected than in the reticular layer of the dermis. Expression of decorin mRNA by cells in the papillary dermis could also be shown by in situ hybridization. In contrast, biglycan, another small chondroitin sulphate/dermatan sulphate proteoglycan, is found only at the dermal-epidermal border. Therefore the biosynthesis of these two proteoglycans by papillary and reticular fibroblasts from two different donors was compared in tissue culture. Papillary fibroblasts secrete up to 5.9 times more decorin than reticular fibroblasts, while the amounts of cell-associated decorin in both cell types are similar. By Northern blot analysis as well as by in situ hybridization it was shown that papillary fibroblasts contain more mRNA coding for decorin than do reticular cells. In addition, no mosaic pattern of decorin expression was found in the cultured cells. The expression and synthesis of biglycan compared with decorin was about 10 times lower and did not show any significant differences for the two cells types. The kinetics of secretion and the rate of endocytosis of decorin were similar for both types of fibroblasts. These results were found with fibroblasts between the 9th and 15th passage from a newborn subject as well as from a 78-year-old donor, indicating that the pattern of decorin synthesis is not age-dependent in the range investigated. These results further show that fibroblasts from different layers of the dermis have a specific pattern of synthesis of small chondroitin sulphate/dermatan sulphate proteoglycans, and they also maintain these patterns in cell culture.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzarone B., Macieira-Coelho A. Heterogeneity of the kinetics of proliferation within human skin fibroblastic cell populations. J Cell Sci. 1982 Oct;57:177–187. doi: 10.1242/jcs.57.1.177. [DOI] [PubMed] [Google Scholar]
- Bayreuther K., Rodemann H. P., Hommel R., Dittmann K., Albiez M., Francz P. I. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5112–5116. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyth R. J., Culp L. A. Glycosaminoglycan distribution in substratum adhesion sites of aging human skin fibroblasts, including papillary and reticular subpopulations. Mech Ageing Dev. 1985 Feb;29(2):151–169. doi: 10.1016/0047-6374(85)90015-6. [DOI] [PubMed] [Google Scholar]
- Bianco P., Fisher L. W., Young M. F., Termine J. D., Robey P. G. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990 Nov;38(11):1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- Bidanset D. J., Guidry C., Rosenberg L. C., Choi H. U., Timpl R., Hook M. Binding of the proteoglycan decorin to collagen type VI. J Biol Chem. 1992 Mar 15;267(8):5250–5256. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bosse A., Schwarz K., Vollmer E., Kresse H. Divergent and co-localization of the two small proteoglycans decorin and proteoglycan-100 in human skeletal tissues and tumors. J Histochem Cytochem. 1993 Jan;41(1):13–19. doi: 10.1177/41.1.8417108. [DOI] [PubMed] [Google Scholar]
- Breuer B., Schmidt G., Kresse H. Non-uniform influence of transforming growth factor-beta on the biosynthesis of different forms of small chondroitin sulphate/dermatan sulphate proteoglycan. Biochem J. 1990 Jul 15;269(2):551–554. doi: 10.1042/bj2690551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chopra R. K., Pearson C. H., Pringle G. A., Fackre D. S., Scott P. G. Dermatan sulphate is located on serine-4 of bovine skin proteodermatan sulphate. Demonstration that most molecules possess only one glycosaminoglycan chain and comparison of amino acid sequences around glycosylation sites in different proteoglycans. Biochem J. 1985 Nov 15;232(1):277–279. doi: 10.1042/bj2320277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day A. A., McQuillan C. I., Termine J. D., Young M. R. Molecular cloning and sequence analysis of the cDNA for small proteoglycan II of bovine bone. Biochem J. 1987 Dec 15;248(3):801–805. doi: 10.1042/bj2480801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Fleischmajer R., Fisher L. W., MacDonald E. D., Jacobs L., Jr, Perlish J. S., Termine J. D. Decorin interacts with fibrillar collagen of embryonic and adult human skin. J Struct Biol. 1991 Feb;106(1):82–90. doi: 10.1016/1047-8477(91)90065-5. [DOI] [PubMed] [Google Scholar]
- Glössl J., Beck M., Kresse H. Biosynthesis of proteodermatan sulfate in cultured human fibroblasts. J Biol Chem. 1984 Nov 25;259(22):14144–14150. [PubMed] [Google Scholar]
- Glössl J., Schubert-Prinz R., Gregory J. D., Damle S. P., von Figura K., Kresse H. Receptor-mediated endocytosis of proteoglycans by human fibroblasts involves recognition of the protein core. Biochem J. 1983 Nov 1;215(2):295–301. doi: 10.1042/bj2150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring S. R., Stephenson M. L., Downie E., Krane S. M., Korn J. H. Heterogeneity in hormone responses and patterns of collagen synthesis in cloned dermal fibroblasts. J Clin Invest. 1990 Mar;85(3):798–803. doi: 10.1172/JCI114506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve H., Blumberg P., Schmidt G., Schlumberger W., Rauterberg J., Kresse H. Influence of collagen lattice on the metabolism of small proteoglycan II by cultured fibroblasts. Biochem J. 1990 Jul 1;269(1):149–155. doi: 10.1042/bj2690149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M. RNA detection and localization in cells and tissue sections by in situ hybridization of 35S-labeled RNA probes. Methods Enzymol. 1987;151:539–551. doi: 10.1016/s0076-6879(87)51043-6. [DOI] [PubMed] [Google Scholar]
- Harper R. A., Grove G. Human skin fibroblasts derived from papillary and reticular dermis: differences in growth potential in vitro. Science. 1979 May 4;204(4392):526–527. doi: 10.1126/science.432659. [DOI] [PubMed] [Google Scholar]
- Hausser H., Ober B., Quentin-Hoffmann E., Schmidt B., Kresse H. Endocytosis of different members of the small chondroitin/dermatan sulfate proteoglycan family. J Biol Chem. 1992 Jun 5;267(16):11559–11564. [PubMed] [Google Scholar]
- Hedbom E., Heinegård D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem. 1989 Apr 25;264(12):6898–6905. [PubMed] [Google Scholar]
- Heino J., Kähäri V. M., Mauviel A., Krusius T. Human recombinant interleukin-1 regulates cellular mRNA levels of dermatan sulphate proteoglycan core protein. Biochem J. 1988 May 15;252(1):309–312. doi: 10.1042/bj2520309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent W. M., Funderburg F. M., Culp L. A. Proteoglycans in the substratum adhesion sites of human papillary or reticular dermal fibroblasts. Aging in vivo or in vitro. Mech Ageing Dev. 1986 Jan;33(2):115–137. doi: 10.1016/0047-6374(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larjava H., Heino J., Krusius T., Vuorio E., Tammi M. The small dermatan sulphate proteoglycans synthesized by fibroblasts derived from skin, synovium and gingiva show tissue-related heterogeneity. Biochem J. 1988 Nov 15;256(1):35–40. doi: 10.1042/bj2560035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowska K., Choi H. U., Rosenberg L. C., Zardi L., Culp L. A. Fibronectin-mediated adhesion of fibroblasts: inhibition by dermatan sulfate proteoglycan and evidence for a cryptic glycosaminoglycan-binding domain. J Cell Biol. 1987 Sep;105(3):1443–1454. doi: 10.1083/jcb.105.3.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride O. W., Fisher L. W., Young M. F. Localization of PGI (biglycan, BGN) and PGII (decorin, DCN, PG-40) genes on human chromosomes Xq13-qter and 12q, respectively. Genomics. 1990 Feb;6(2):219–225. doi: 10.1016/0888-7543(90)90560-h. [DOI] [PubMed] [Google Scholar]
- Moos M., Gallwitz D. Structure of two human beta-actin-related processed genes one of which is located next to a simple repetitive sequence. EMBO J. 1983;2(5):757–761. doi: 10.1002/j.1460-2075.1983.tb01496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neame P. J., Choi H. U., Rosenberg L. C. The primary structure of the core protein of the small, leucine-rich proteoglycan (PG I) from bovine articular cartilage. J Biol Chem. 1989 May 25;264(15):8653–8661. [PubMed] [Google Scholar]
- Romarís M., Heredia A., Molist A., Bassols A. Differential effect of transforming growth factor beta on proteoglycan synthesis in human embryonic lung fibroblasts. Biochim Biophys Acta. 1991 Jul 10;1093(2-3):229–233. doi: 10.1016/0167-4889(91)90127-j. [DOI] [PubMed] [Google Scholar]
- Schafer I. A., Pandy M., Ferguson R., Davis B. R. Comparative observation of fibroblasts derived from the papillary and reticular dermis of infants and adults: growth kinetics, packing density at confluence and surface morphology. Mech Ageing Dev. 1985 Sep;31(3):275–293. doi: 10.1016/0047-6374(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Hausser H., Kresse H. Interaction of the small proteoglycan decorin with fibronectin. Involvement of the sequence NKISK of the core protein. Biochem J. 1991 Dec 1;280(Pt 2):411–414. doi: 10.1042/bj2800411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Robenek H., Harrach B., Glössl J., Nolte V., Hörmann H., Richter H., Kresse H. Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol. 1987 Jun;104(6):1683–1691. doi: 10.1083/jcb.104.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz K., Breuer B., Kresse H. Biosynthesis and properties of a further member of the small chondroitin/dermatan sulfate proteoglycan family. J Biol Chem. 1990 Dec 15;265(35):22023–22028. [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel K. G., Clark P. E. Small proteoglycan synthesis by skin fibroblasts cultured from elderly donors and patients with defined defects in types I and III collagen metabolism. Eur J Cell Biol. 1989 Aug;49(2):236–243. [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergren-Thorsson G., Antonsson P., Malmström A., Heinegård D., Oldberg A. The synthesis of a family of structurally related proteoglycans in fibroblasts is differently regulated by TFG-beta. Matrix. 1991 Jun;11(3):177–183. doi: 10.1016/s0934-8832(11)80156-3. [DOI] [PubMed] [Google Scholar]
- Winnemöller M., Schmidt G., Kresse H. Influence of decorin on fibroblast adhesion to fibronectin. Eur J Cell Biol. 1991 Feb;54(1):10–17. [PubMed] [Google Scholar]
- Winnemöller M., Schön P., Vischer P., Kresse H. Interactions between thrombospondin and the small proteoglycan decorin: interference with cell attachment. Eur J Cell Biol. 1992 Oct;59(1):47–55. [PubMed] [Google Scholar]
- Witsch-Prehm P., Miehlke R., Kresse H. Presence of small proteoglycan fragments in normal and arthritic human cartilage. Arthritis Rheum. 1992 Sep;35(9):1042–1052. doi: 10.1002/art.1780350909. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Mann D. M., Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990 Jul 19;346(6281):281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]