Abstract
Background
The COVID-19 pandemic brought greater focus to the rural mortality penalty in the U.S., which describes the greater mortality rate in rural compared to urban areas. Although it is widely thought that issues such as access to care, age structure of the population, and differences in behavior are likely drivers of the rural mortality penalty, few studies have attempted to tie delayed access to care in rural populations to healthcare outcomes quantitatively. Therefore, it is critical to try and understand these factors to enable more effective public health policy.
Methods
We performed a cross-sectional analysis of a population of patients with COVID-19 who were admitted to hospitals in the United States between 3/1/2020 and 2/26/2023 to better understand factors leading to outcome disparities amongst groups that all had some level of access to hospital care. Nevertheless, it is widely thought that rural populations often experience delayed access to care, due to transportation and other constraints. Therefore, we hypothesized that deteriorated patient condition at admission likely explained some of the observed difference in mortality between rural and urban populations.
Results
Our results supported our hypothesis, showing that the rural mortality penalty persists in this population and that by multiple measures, rural patients were likely to be admitted in worse condition, had worse overall health, and were older.
Conclusions
Although the pandemic threw the rural mortality penalty into sharp relief, it is important to remember that it existed prior to the pandemic and will continue to exist until effective interventions are implemented. This study demonstrates the critical need to address the underlying factors that resulted in rural-dwelling patients being admitted to the hospital in worse condition than their urban-dwelling counterparts during the COVID-19 pandemic, which likely affected other healthcare outcomes as well.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12963-024-00340-2.
Keywords: COVID-19, Pandemic, Rural mortality penalty, Disparities
Background
In the early 20th century, dense population in cities with poor sanitation led to a phenomenon known as the urban mortality penalty. However, improvements in sanitation and increased effectiveness and accessibility to healthcare eventually eliminated this disparity [1]. Indeed, mortality rates declined throughout the United States in the 20th century [2]. However, in 2008, Cosby et al. identified a new trend, beginning in the 1980s, in which a new rural mortality penalty became evident [3], leading to a host of research on location-based health disparities. Since its initial discovery, the rural mortality penalty has only increased [4]. Such population health disparities can have acute impacts during a public health crisis, such as the COVID-19 pandemic that began in 2019. Indeed, many have noted that rural populations in the United States suffered increased mortality due to COVID-19 compared to their urban counterparts [5–7]. When it comes to healthcare, a common assumption with rural disparities is that access to care plays a major role [8, 9]. Indeed, a few studies have shown that rural residents are more likely to delay seeking medical care or have delays in obtaining emergency medical care [10–13]. However, there has been little research that directly links delays in care for rural U.S. residents to healthcare outcomes. To that end, we focus is on the persistence of the rural mortality penalty even in those patients with access to, means for, and willingness to go to a hospital, under the hypothesis that delayed access to care nevertheless impacts outcomes for this population. Therefore, in this work, we studied mortality in a population of patients with COVID-19 following their admission to a hospital, using a large national dataset of approximately a million COVID-19 patients who were admitted to hospitals to determine its drivers.
When viewing rural dwelling as a social determinant of health, it is important to note that other social determinants are associated with excess mortality, such as education, poverty, race, and more [14]. In fact, Black Americans experience larger mortality disparities, irrespective of location, than rural populations [15], particularly with respect to the COVID-19 pandemic. Also, it is worth noting that the emergence of the rural mortality penalty has not, for most population groups, been due to increased mortality rates. In many areas, it has been driven by smaller declines in mortality in rural compared to urban populations [3, 4, 16]. Nevertheless, although COVID-19 has made the trend harder to analyze, there is evidence to suggest that mortality is increasing in younger and middle-aged adults for certain causes of death (causes related to drugs and alcohol, as well as suicide) and that the group has overall seen smaller declines in mortality due to cardiovascular disease [17].
Others have written about rural/urban mortality disparities from COVID-19. Grome et al. studied county-level mortality data in Tennessee throughout most of 2020 and found an incidence rate ratio of 1.13 for mortality in rural vs. urban counties after controlling for county-level demographics, access to care, and comorbidities [5]. Interestingly, they found that the disparity was greater for COVID-19 than for influenza or pneumonia. Iyanda et al. found similar results for a nationwide county-level analysis with a mortality incident rate ratio between 1.24 and 1.26 depending on the county classification [7], which also adjusted for county-level demographic and other factors. However, these studies did not examine COVID-19 mortality in the context of patients who were admitted to a hospital.
Although several studies on hospital outcomes for COVID-19 patients have been done [18–22], most have not evaluated location-based risk. One large national study conducted by Anzalone et al. examined whether the rural mortality penalty is present in COVID-19 patients following hospital admission, using data on approximately one million patients obtained from the National COVID Cohort Collaborative (N3C) [23]. A strength of the study is that it analyzed in-hospital risk at the individual level. Indeed, they found an increased risk of in-hospital mortality for rural patients, with an adjusted odds ratio of 1.36. However, the study did not examine patient condition at admission or vaccination status. Deteriorated condition at admission and lack of vaccination can be signs of access to care issues, or sociocultural factors that may nevertheless influence healthcare outcomes. Either may contribute to the rural mortality penalty. Therefore, the study was limited in its ability to identify drivers of the rural mortality penalty with respect to COVID-19 hospital patients.
Several studies have examined factors associated with hospital outcomes for COVID-19 patients without considering rurality. Factors associated with risk of COVID-19 mortality noted by multiple studies were age [18, 21, 25], sex [21, 25], and vital signs at admission [18, 25].
It is important to remember that the rural mortality penalty existed prior to the COVID-19 pandemic. The dramatic increase in mortality due to COVID-19 brought these existing disparities in health outcomes into sharp focus. However, the underlying drivers will remain. Furthermore, rurality, race, and most other social determinants of health are typically not causes of health outcomes. Instead, issues such as access to care, differences in demographics, differences in behavior, lower rates of belief in the efficacy of public health intervention [26–28], and many other factors are the true drivers. Therefore, it is critical to gain understanding of these drivers so we can work to improve the health of the entire population. We hypothesized that the rural mortality penalty in patients hospitalized with COVID-19 is driven by deteriorated patient health in the rural population prior to admission, a sign of delayed access to care, in combination with increased risk factors in the rural population overall.
Methods
Data
We performed our analysis using Epic’s Cosmos database using the 2/26/2023 snapshot. Epic supplies the most widely used electronic health record software in the United States, and Cosmos data are contributed from organizations that use Epic across the country, including all 50 states. We used the deidentified version of the database provided with Cosmos Data Science. At the time of this snapshot, the database contained data from 196 organizations across the United States comprising data on 186,082,759 patients. We identified COVID-19 admissions using the COVID patient data mart provided by Cosmos. For this data mart, COVID-19 admissions were identified as follows:
COVID diagnosis linked to an encounter for an admitted patient that occurred before discharge and within 42 days of admission.
Respiratory diagnosis linked to an encounter for an admitted patient that occurred before discharge and within 42 days of admission and is COVID positive between 14 days before admission and the discharge date and discharge is 42 days or less from the positive date.
Patient became COVID positive during an admission and discharge date is 42 days or less from COVID positive date.
The outcome was all cause mortality within 42 days of a COVID related admission. We collected the following demographic data: sex, race, residence in rural zip code by Rural Urban Commuting Area (RUCA), social vulnerability index of zip code, and age at admission. We also collected the following patient condition variables (closest measure to admission): oxygen saturation, respiration rate, pulse rate, mean arterial pressure, and admitted to ICU within first day. Additionally, we collected the following comorbidities data: congestive heart failure, high blood pressure, pneumonia, and cancer. Finally, we also collected data on whether a patient had at least one of any type of COVID-19 vaccination two weeks or more prior to admission. Presumably due to the urgent nature of most COVID-19 admissions, BMI was not available for most patients.
We included patients with a first COVID admission between 3/1/2020 and 2/26/2023. Patients from sources with less than 50 COVID-19 patients were excluded to ensure that the results were not biased by hospitals who did not admit many COVID patients (17 such sources which included 142 patients). Sources are not quite equivalent to hospitals, because we could get duplicated data from multiple hospitals in some cases, in which case they are combined into a single source for that patient. The study population included 1,119,199 patients in the database who met the definition of having a COVID-19 admission.
However, many patients were missing information on vital signs or vaccination. While we included all eligible patients regardless of missing data, there were 648,595 patients that had no missing vitals and only 165,534 patients with COVID vaccination records in the hospitals they were admitted to. As COVID vaccination data were missing for most patients, we reserved those data for a subset analysis. Furthermore, COVID vaccination status may be from either hospital administered vaccination or patient report.
Statistics
The main variable of interest for this analysis was rural zip code residence as defined by RUCA codes, maintained by the U.S. Department of Agriculture. Patients were categorized in Cosmos as simply rural (codes 7–10) or urban (codes 1–6) dwelling. Patient characteristics were individually tested for differences by rural or urban dwelling using a Wilcoxon rank sum test for continuous variables or a 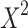 for categorical variables. The unadjusted difference in risk of mortality for urban/rural differences was tested by log rank test and visualized using a Kaplan-Meier survival curve. The adjusted difference in risk was assessed using a Cox proportional hazards model using complete case analysis. The proportional hazards assumption was assessed visually, using the scaled Schoenfeld residuals plotted against the log time, because hypothesis tests of non-proportional hazards are suspect with such a large sample size. The assumption appeared warranted for all variables. Likewise, non-linearity between the log-hazard and predictors was assessed using the Martingale residuals. This assumption appeared warranted for all variables except respiration rate, which was discretized into values representing low, normal, and high respiration. We also compared a gamma frailty model accounting for heterogeneity by data source (a proxy for hospital) to one without to select a final model.
for categorical variables. The unadjusted difference in risk of mortality for urban/rural differences was tested by log rank test and visualized using a Kaplan-Meier survival curve. The adjusted difference in risk was assessed using a Cox proportional hazards model using complete case analysis. The proportional hazards assumption was assessed visually, using the scaled Schoenfeld residuals plotted against the log time, because hypothesis tests of non-proportional hazards are suspect with such a large sample size. The assumption appeared warranted for all variables. Likewise, non-linearity between the log-hazard and predictors was assessed using the Martingale residuals. This assumption appeared warranted for all variables except respiration rate, which was discretized into values representing low, normal, and high respiration. We also compared a gamma frailty model accounting for heterogeneity by data source (a proxy for hospital) to one without to select a final model.
Results
Table 1 shows all the putative risk factors for COVID mortality we included in this study, with summary statistics broken down by whether the patient lived in a rural or urban designated zip code. Hypothesis tests were done for each variable to test if they differed by rurality. For most demographic, patient condition, and comorbidity variables, there was a statistically significant difference between rural and urban patients.
Table 1.
Characteristics of the study population
| Variable | N | Rural, N = 77,807 | Urban, N = 1,041,392 | p-value |
|---|---|---|---|---|
| Comorbidities | ||||
| Congestive Heart Failure | 1,119,199 | 8,130 (10.4%) | 105,195 (10.1%) | 0.002 |
| High Blood Pressure | 1,119,199 | 28,885 (37.1%) | 386,886 (37.2%) | 0.9 |
| Pneumonia | 1,119,199 | 4,610 (5.9%) | 5,754 (5.6%) | < 0.001 |
| Cancer | 1,119,199 | 8,059 (10.4%) | 99,552 (9.6%) | < 0.001 |
| Patient Condition | ||||
| Mean Arterial Pressure | 807,220 | < 0.001 | ||
| (60,100) | 39,309 (71.1%) | 520,490 (69.2%) | ||
| (0,60) | 756 (1.4%) | 8,772 (1.2%) | ||
| (100,300) | 15,191 (27.5%) | 222,702 (29.6%) | ||
| Unknown | 22,551 | 289,428 | ||
| ICU Admission | 1,119,199 | 8,732 (11.2%) | 62,906 (6.0%) | < 0.001 |
| SpO2 | 911,943 | 95 (93, 97) | 96 (93, 98) | < 0.001 |
| Unknown | 14,137 | 193,119 | ||
| Respiration Rate | 808,396 | < 0.001 | ||
| (12, 25) | 42,427 (78.0%) | 606,119 (80%) | ||
| (0,12) | 524 (1.0%) | 6,300 (0.8%) | ||
| (25,inf) | 11,379 (21%) | 141,547 (19%) | ||
| Unknown | 23,377 | 287,426 | ||
| Pulse Rate | 906,257 | 86 (74, 99) | 87 (75, 101) | < 0.001 |
| Unknown | 16,632 | 196,310 | ||
| Demographics | ||||
| SVI | 1,113,692 | 0.66 (0.46, 0.82) | 0.53 (0.26, 0.79) | < 0.001 |
| Unknown | 1,335 | 4,172 | ||
| Sex | 1,119,199 | < 0.001 | ||
| Female | 36,992 (47.5%) | 519,128 (49.8%) | ||
| Male | 40,815 (52.5%) | 522,264 (50.2%) | ||
| Race | 1,119,199 | < 0.001 | ||
| White | 65,276 (83.9%) | 712,506 (68.4%) | ||
| American Indian or Alaska Native | 1,809 (2.3%) | 9,350 (0.9%) | ||
| Asian | 252 (0.3%) | 24,755 (2.4%) | ||
| Black or African American | 7,128 (9.2%) | 214,787 (20.6%) | ||
| Native Hawaiian or Other Pacific Islander | 141 (0.2%) | 3,962 (0.4%) | ||
| Other | 1,639 (2.1%) | 49,573 (4.8%) | ||
| Unspecified | 1,562 (2.0%) | 26,459 (2.5%) | ||
| Age at Diagnosis | 1,119,199 | 67 (54, 78) | 65 (49, 77) | < 0.001 |
| Days from 3/1/2020 to Admission | 1,119,199 | 573 (313, 710) | 559 (309, 705) | < 0.001 |
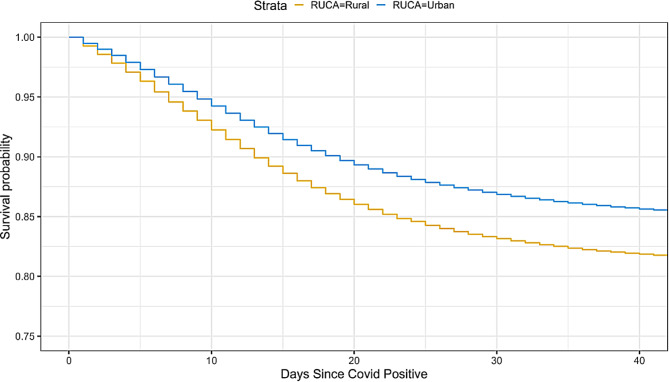
A Kaplan-Meier survival plot showing the unadjusted difference in survival between patients from rural or urban designated zip codes is shown in Fig. 1. It illustrates that patients from rural areas are at higher risk of mortality following admission for COVID-19. The unadjusted hazard ratio for this difference is 1.300 (95% CI: [1.276, 1.323]). The difference is significant at p < 0.001.
Fig. 1.
Kaplan-Meier survival curves over the days since a patient was covid positive for rural vs. urban residing patients
Univariable Cox proportional hazards models were used to estimate the individual effect on risk for each putative risk variable. The Hazard ratios for each of these variables are shown in Table 2. Some notable results are the association of increased blood oxygen level with lower risk of mortality (1% change in SpO2 - HR: 0.967, p = 0.00), low blood pressure with increased risk of mortality (HR: 2.141, p = 6.42e-309), admission of ICU with increased risk of mortality (HR: 2.270, p = 0.00), and age at diagnosis with increased risk of mortality (1 year increase in age - HR: 1.039, p = 0.00).
Table 2.
Results of univariable Cox regression (results of each variable unadjusted for the others)
| Variable | Ln HR | HR | p-value |
|---|---|---|---|
| Residence | |||
| Urban | 0.000 | 1.000 | - |
| Rural | 0.262 | 1.300 | 1.59e-178 |
| Comorbidities | |||
| Congestive Heart Failure | 0.532 | 1.702 | 0.00 |
| High Blood Pressure | 0.324 | 1.383 | 0.00 |
| Pneumonia | 0.398 | 1.488 | 0.00 |
| Cancer | 0.458 | 1.581 | 0.00 |
| Patient Condition | |||
| Mean Arterial Pressure | |||
| (60,100) | 0.000 | 1.000 | - |
| (0,60) | 0.761 | 2.141 | 6.42e-309 |
| (100,300) | -0.229 | 0.795 | 8.34e-226 |
| ICU Admission | 0.820 | 2.270 | 0.00 |
| SpO2 | -0.034 | 0.967 | 0.00 |
| Respiration Rate | |||
| (12, 25) | 0.000 | 1.000 | - |
| (0,12) | 0.247 | 1.281 | 8.31e-15 |
| (25,inf) | 0.677 | 1.968 | 0.00 |
| Pulse Rate | 0.001 | 1.001 | 5.74e-08 |
| Demographics | |||
| SVI | -0.007 | 0.993 | 0.421 |
| Sex | |||
| Female | 0.000 | 1.000 | - |
| Male | 0.286 | 1.332 | 0.00 |
| Race | |||
| White | 0.000 | 1.000 | - |
| American Indian or Alaska Native | -0.159 | 0.853 | 4.40e-09 |
| Asian | -0.233 | 0.792 | 1.83e-33 |
| Black or African American | -0.317 | 0.728 | 0.00 |
| Native Hawaiian or Other Pacific Islander | -0.414 | 0.661 | 6.24e-16 |
| Other | -0.287 | 0.750 | 2.73e-91 |
| Unspecified | 0.194 | 1.214 | 1.08e-33 |
| Age at Diagnosis | 0.038 | 1.039 | 0.00 |
A multivariable Cox proportional hazards model with gamma frailties to account for heterogeneity by data source was used to estimate the effect of rurality on risk adjusting for demographic differences, differences in patient condition at admission, and differences in comorbidities. The results are shown in Table 3. When controlling for other putative drivers of COVID-19 mortality, the effect of residence is attenuated. Rural residence has a hazard ratio of 1.137 (95% CI: [1.109, 1.170], p = 1.51e-198) in this model. Congestive heart failure, pneumonia, and cancer comorbidities all contribute to risk, as do admission to the ICU, lowered blood oxygen, increased respiration, and increased pulse at admission. Low blood pressure was associated with higher risk; however, high blood pressure was associated with lower risk, compared to normal blood pressure. Social vulnerability was associated with increased risk, as was male sex. Black patients were at slightly lower risk compared to white patients after adjusting for other factors in this population. Finally, the hazards reduced over time, reflected in the hazard ratio of 0.999 for days since 3/1/2020.
Table 3.
Results of multivariable Cox regression with gamma frailty to account for heterogeneity by data source. The concordance index for this model was 0.740. The frailty variance was 0.854
| Variable | Ln HR | HR | p-value |
|---|---|---|---|
| Residence | |||
| Urban | 0.000 | 1.000 | - |
| Rural | 0.128 | 1.137 | 1.51e-198 |
| Comorbidities | |||
| Congestive Heart Failure | 0.217 | 1.242 | 5.67e-109 |
| High Blood Pressure | -0.060 | 0.942 | 7.63e-16 |
| Pneumonia | 0.141 | 1.151 | 1.08e-28 |
| Cancer | 0.212 | 1.236 | 3.61e-104 |
| Patient Condition | |||
| Mean Arterial Pressure | |||
| (60,100) | 0.000 | 1.000 | - |
| (0,60) | 0.705 | 2.024 | 1.01e-193 |
| (100,300) | -0.256 | 0.774 | 3.48e-223 |
| ICU Admission | 0.792 | 2.208 | 0.00 |
| SpO2 | -0.029 | 0.971 | 0.00 |
| Respiration Rate | |||
| (12, 25) | 0.000 | 1.000 | - |
| (0, 12) | 0.197 | 1.218 | 1.13e-06 |
| (25, inf) | 0.621 | 1.861 | 0.00 |
| Pulse Rate | 0.008 | 1.008 | 0.00 |
| Demographics | |||
| SVI | 0.191 | 1.210 | 1.84e-45 |
| Sex | |||
| Female | 0.000 | 1.000 | - |
| Male | 0.262 | 1.300 | 1.75e-307 |
| Race | |||
| White | 0.000 | 1.000 | - |
| American Indian or Alaska Native | 0.090 | 1.094 | 1.73e-02 |
| Asian | 0.008 | 1.008 | 7.64e-01 |
| Black or African American | -0.075 | 0.928 | 2.04e-12 |
| Native Hawaiian or Other Pacific Islander | 0.044 | 1.045 | 5.24e-01 |
| Other | 0.069 | 1.071 | 3.66e-04 |
| Unspecified | 0.502 | 1.652 | 5.72e-119 |
| Age at Diagnosis | 0.043 | 1.044 | 0.00 |
| Days from 3/1/2020 to Admission | -0.001 | 0.999 | 0.00 |
Given that race and social vulnerability index are related, we built models without each to assess their independence. Removing SVI had a small impact on the estimated effect of race (without SVI in the model identifying as Black went from a hazard ratio of 0.928 to 0.965) and vice versa (without race in the model SVI went from a hazard ratio of 1.210 to 1.195). The results are shown in Tables S1 and S2. The removal of either had a small impact on the effect of rurality as expected. Given our interest in this work in identifying factors that control the effect of rurality on survival, we decided to include both.
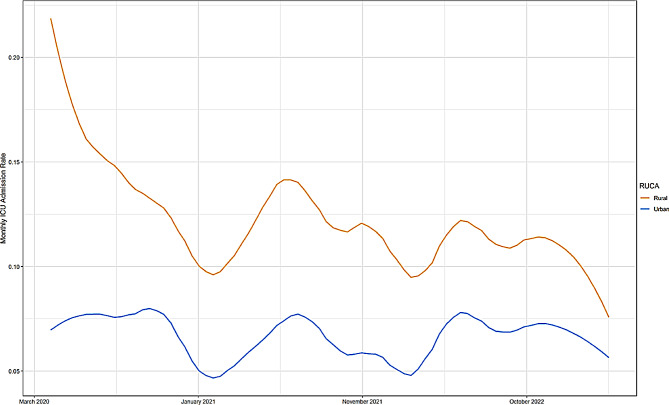
In order to examine the difference in patient condition at admission to the hospital by area of residence, we plotted the monthly rate of ICU admission for rural and urban patients over the course of the pandemic (Fig. 2). Rural patients consistently have higher rates of admission to the ICU throughout the course of the pandemic.
Fig. 2.
Monthly ICU admission rate in rural vs. urban residing patients
We built an additional multivariable Cox proportional hazards model using the subset of patients that we knew had at least one COVID-19 vaccination prior to admission with COVID-19. In this model, the hazard ratio for urban residence became 1.103 (95% CI: [1.038, 1.171], p = 1.40e-03), further reducing the hazard ratio of rural residence after accounting for vaccination.
Discussion
The rural mortality penalty is thought to be associated with numerous factors, including reduced access to care, differences in behavior, education, environmental exposure, and other factors. Access to care issues can mean that people do not have access to hospital care, have delayed access to hospital care, or do not have access to the same quality hospital care. In this analysis, we examined a population of patients that all were admitted to a hospital and then adjusted for patient condition at admission in order to address differences in access to care between rural and urban populations, although we were unable to assess differences in quality of care. We also adjusted for differences in age and sex, which can skew differently in rural vs. urban populations. We adjusted for differences in race and social vulnerability in an attempt to account for other known drivers of disparities. However, race in this context should not be thought of as a biological variable; rather, it is a proxy variable that captures other access to care and risk factor differences resulting from structural inequalities.
After these adjustments, we did manage to reduce the effect of rural residence on COVID-19 mortality from a HR of 1.300 to 1.139, indicating that we had identified some but not all of the drivers of mortality disparities in these patients. Patient condition variables and age were the strongest contributors to this result, which likely point in part to access to care and differences in demographics as some of the main factors underlying the rural mortality penalty in COVID-19. However, patient condition at admission is also driven by differences in behavior, including diet, exercise, and vaccination.
Although hospitals do not have complete vaccination records for patients, some were available. Therefore, we built a multiple Cox proportional hazards model on the subset of 165,534 patients for whom we had a record of COVID-19 vaccination at least two weeks prior to hospital admission. Of those, 9,645 were from rural areas. Although the overall results were similar for this subset analysis, we did find that the increased hazards associated with rural residence shrank even more (compared to the adjusted model) to 1.109, suggesting that differences in vaccination may further contribute to the rural mortality penalty.
Limitations of this study include the lack of information about the individual hospitals included within our Cosmos dataset, which is an inevitable consequence of using a deidentified dataset, as well as the lack of complete vaccination records for patients, which would have enabled us to better assess the impact of vaccination on the rural mortality penalty in this context. Also, due the nature of COVID-19 admissions, we lacked information on BMI for most patients, although BMI is an important risk factor for COVID-19 mortality. Finally, the analysis was limited to hospitals that use the Epic electronic health record system, which does not tend to be used by smaller organizations. Furthermore, as is common with real world data, the completeness of comorbidity data is impossible to ascertain, either due to incomplete documentation or lack of awareness of a condition. These limitations are counterbalanced to some degree by being able to perform the analysis in a nationwide dataset of patients admitted to hospital, with information on vitals, and ICU admission. Given the substantial cross-section of COVID-19 hospital admitted patients covered in this resource, these results are likely generalizable at least to relatively well-resourced hospitals.
Overall, we found that there are differences in COVID-19 mortality between rural and urban populations among hospital admitted patients. Furthermore, we found that even after adjusting for patient condition, comorbidities, and demographics differences remained, which implies that the variables we identified that might potentially explain differences between rural and urban residents’ outcomes are not complete. Likely, the residual effect of rurality is related to other comorbidities we did not account for, in addition to differences in behaviors such as diet, exercise, and vaccination. However, we did show that rural residents were more likely than their urban counterparts to be admitted to the ICU and to have worse vital signs, strong indicators that they appeared at the hospital in more deteriorated condition, indicative of delayed access to care. Furthermore, we showed that these factors are associated with COVID-19 mortality. Likewise, we showed that age and overall worse health (as reflected in the greater number of comorbidities) were associated with mortality and that the rural population is both older and in worse health overall. Furthermore, we showed that mortality differences are diminished among those who say they were vaccinated.
Conclusions
Despite the focus in this work on COVID-19 mortality in patients admitted to hospitals, we believe it adds to the body of evidence about the rural mortality penalty in general. Previous studies have shown that rural residents are more likely to delay seeking medical care or have delays in obtaining emergency medical care. Some studies have identified transportation issues as one source of delays that affect rural residents [29–31]. However, there has been little research that directly links delays in care for rural residents to healthcare outcomes, particularly in the United States. Our results strongly suggest that delayed care during the COVID-19 pandemic led to higher mortality, as rural residents were much more likely to be admitted to an intensive care unit immediately after hospital admission and tended to have lower blood oxygen levels and more abnormal respiration. Thus, our results show the direct impact on mortality that such delays can have, when admission might come too late. Vaccination was one of the biggest differentiators of mortality during the pandemic [32] and rural residents were far less likely to be vaccinated and therefore this issue correctly receives a great deal of focus. Nevertheless, our results suggest that delayed access to care is still a large contributor to mortality, even among those who say they were vaccinated. Therefore, our results help to underscore the need to invest in transportation solutions for rural residents, supporting community hospitals with emergency and inpatient care, and more complete research to understand the full range of factors associated with delayed healthcare for rural residents.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
These data come from Cosmos, a HIPAA de-identified data set of more than 186,082,759 patients from 196 health systems that use Epic software.
Abbreviations
- N3C
National COVID Cohort Collaborative
- RUCA
Rural Urban Commuting Area
Author contributions
JAT conceived of the study, acquired the data, contributed to the analysis and interpretation of the data, and helped draft the article. DPM and LCH contributed to the analysis and interpretation of the data and provided critical revisions. All authors gave approval to the manuscript.
Funding
This work was supported in part by the Kansas Institute of Precision Medicine COBRE P20GM130423, and a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (# UL1TR002366). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
Data availability
The data that support the findings of this study are available from Epic’s Cosmos environment, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available to healthcare institutions which use the Epic EHR and participate in Cosmos.
Declarations
Ethics approval and consent to participate
Cosmos is a deidentified dataset and its use does not constitute human subjects research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haines MR, National Bureau of Economic Research. The Urban Mortality Transition in the United States, 1800–1940. Cambridge, Mass.: National Bureau of Economic Research; 2001. 1 online resource p. [Google Scholar]
- 2.Guyer B, Freedman MA, Strobino DM, Sondik EJ. Annual summary of vital statistics: trends in the health of americans during the 20th century. Pediatrics. 2000;106(6):1307–17. 10.1542/peds.106.6.1307 [DOI] [PubMed] [Google Scholar]
- 3.Cosby AG, Neaves TT, Cossman RE, Cossman JS, James WL, Feierabend N, et al. Preliminary evidence for an emerging nonmetropolitan mortality penalty in the United States. Am J Public Health. 2008;98(8):1470–2. 10.2105/AJPH.2007.123778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosby AG, McDoom-Echebiri MM, James W, Khandekar H, Brown W, Hanna HL. Growth and persistence of place-based mortality in the United States: the rural mortality penalty. Am J Public Health. 2019;109(1):155–62. 10.2105/AJPH.2018.304787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grome HN, Raman R, Katz BD, Fill MM, Jones TF, Schaffner W, et al. Disparities in COVID-19 Mortality Rates: implications for Rural Health policy and preparedness. J Public Health Manag Pract. 2022;28(5):478–85. 10.1097/PHH.0000000000001507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y, Cheng KJG, Monnat SM. Rural-urban and within-rural differences in COVID-19 mortality. J Rural Social Sci. 2022;37(2).
- 7.Iyanda AE, Boakye KA, Lu Y, Oppong JR. Racial/Ethnic heterogeneity and rural-urban disparity of COVID-19 case fatality ratio in the USA: a negative binomial and GIS-Based analysis. J Racial Ethn Health Disparities. 2022;9(2):708–21. 10.1007/s40615-021-01006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loftus J, Allen EM, Call KT, Everson-Rose SA. Rural-urban differences in Access to Preventive Health Care among publicly insured minnesotans. J Rural Health. 2018;34:S48–55. 10.1111/jrh.12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirby JB, Yabroff KR. Rural-urban differences in Access to Primary Care: beyond the Usual source of Care Provider. Am J Prev Med. 2020;58(1):89–96. 10.1016/j.amepre.2019.08.026 [DOI] [PubMed] [Google Scholar]
- 10.Spleen AM, Lengerich EJ, Camacho FT, Vanderpool RC. Health Care Avoidance among Rural populations: results from a nationally Representative Survey. J Rural Health. 2014;30(1):79–88. 10.1111/jrh.12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethares KA, Sosa ME, Fisher P, Riegel B. Factors associated with delay in seeking care for acute decompensated heart failure. J Cardiovasc Nurs. 2014;29(5):429–38. 10.1097/JCN.0b013e3182a37789 [DOI] [PubMed] [Google Scholar]
- 12.Lee JC, Hasnain-Wynia R, Lau DT. Delay in seeing a Doctor due to cost: disparity between older adults with and without disabilities in the United States. Health Serv Res. 2012;47(2):698–720. 10.1111/j.1475-6773.2011.01346.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alruwaili A, Alanazy ARM. Prehospital Time Interval for Urban and Rural Emergency Medical Services: a systematic literature review. Healthc (Basel). 2022;10(12). [DOI] [PMC free article] [PubMed]
- 14.Singh GK, Daus GP, Allender M, Ramey CT, Martin EK, Perry C, et al. Social Determinants of Health in the United States: addressing Major Health Inequality trends for the Nation, 1935–2016. Int J MCH AIDS. 2017;6(2):139–64. 10.21106/ijma.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James W, Cossman JS. Long-term trends in Black and White Mortality in the Rural United States: evidence of a race-specific rural mortality penalty. J Rural Health. 2017;33(1):21–31. 10.1111/jrh.12181 [DOI] [PubMed] [Google Scholar]
- 16.James WL. All rural places are not created equal: revisiting the rural mortality penalty in the United States. Am J Public Health. 2014;104(11):2122–9. 10.2105/AJPH.2014.301989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker T, Majmundar MK, Harris KM, editors. High and Rising Mortality Rates Among Working-Age Adults. The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC)2021.
- 18.Shaw PA, Yang JB, Mowery DL, Schriver ER, Mahoney KB, Bar KJ, et al. Determinants of hospital outcomes for patients with COVID-19 in the University of Pennsylvania Health System. PLoS ONE. 2022;17(5):e0268528. 10.1371/journal.pone.0268528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors Associated with In-Hospital mortality in a US National Sample of patients with COVID-19. JAMA Netw Open. 2020;3(12):e2029058. 10.1001/jamanetworkopen.2020.29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhopalwala H, Dewaswala N, Kolagatla S, Wisnieski L, Piercy J, Bhopalwala A, et al. Predictors of mortality for patients with COVID-19 in the rural Appalachian Region. Int J Gen Med. 2022;15:2207–14. 10.2147/IJGM.S355083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asch DA, Islam MN, Sheils NE, Chen Y, Doshi JA, Buresh J, et al. Patient and Hospital Factors Associated with Differences in Mortality Rates among Black and White US Medicare beneficiaries hospitalized with COVID-19 infection. JAMA Netw Open. 2021;4(6):e2112842. 10.1001/jamanetworkopen.2021.12842 [DOI] [PubMed] [Google Scholar]
- 22.Pan AP, Khan O, Meeks JR, Boom ML, Masud FN, Andrieni JD, et al. Disparities in COVID-19 hospitalizations and mortality among black and hispanic patients: cross-sectional analysis from the greater Houston metropolitan area. BMC Public Health. 2021;21(1):1330. 10.1186/s12889-021-11431-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anzalone AJ, Horswell R, Hendricks BM, Chu S, Hillegass WB, Beasley WH, et al. Higher hospitalization and mortality rates among SARS-CoV-2-infected persons in rural America. J Rural Health. 2023;39(1):39–54. 10.1111/jrh.12689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogedegbe G, Ravenell J, Adhikari S, Butler M, Cook T, Francois F et al. Assessment of Racial/Ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. Jama Netw Open. 2020;3(12). [DOI] [PMC free article] [PubMed]
- 25.Rechtman E, Curtin P, Navarro E, Nirenberg S, Horton MK. Vital signs assessed in initial clinical encounters predict COVID-19 mortality in an NYC hospital system. Sci Rep. 2020;10(1):21545. 10.1038/s41598-020-78392-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLamore Q, Syropoulos S, Leidner B, Hirschberger G, Young K, Zein RA, et al. Trust in scientific information mediates associations between conservatism and coronavirus responses in the U.S., but few other nations. Sci Rep. 2022;12(1):3724. 10.1038/s41598-022-07508-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lister JJ, Joudrey PJ. Rural mistrust of public health interventions in the United States: a call for taking the long view to improve adoption. J Rural Health. 2023;39(1):18–20. 10.1111/jrh.12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kricorian K, Civen R, Equils O. COVID-19 vaccine hesitancy: misinformation and perceptions of vaccine safety. Hum Vaccin Immunother. 2022;18(1):1950504. 10.1080/21645515.2021.1950504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health. 2006;22(2):140–6. 10.1111/j.1748-0361.2006.00022.x [DOI] [PubMed] [Google Scholar]
- 30.Goins RT, Williams KA, Carter MW, Spencer SM, Solovieva T. Perceived barriers to health care access among rural older adults: a qualitative study. J Rural Health. 2005;21(3):206–13. 10.1111/j.1748-0361.2005.tb00084.x [DOI] [PubMed] [Google Scholar]
- 31.Smith ML, Towne SD, Bergeron CD, Zhang DL, McCord C, Mier N et al. Geographic disparities associated with travel to medical care and attendance programs to prevent/manage chronic illness among middle-aged and older adults in Texas. Rural Remote Health. 2019;19(4). [DOI] [PubMed]
- 32.Ikeokwu AE, Lawrence R, Osieme ED, Gidado KM, Guy C, Dolapo O. Unveiling the impact of COVID-19 vaccines: a Meta-analysis of Survival Rates among patients in the United States based on Vaccination Status. Cureus. 2023;15(8):e43282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Epic’s Cosmos environment, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available to healthcare institutions which use the Epic EHR and participate in Cosmos.