Abstract
Background:
Preventing emergence delirium is a clinical goal for pediatric anesthesia, yet there is no consensus on its prevention. This study investigated the hypothesis that a continuous infusion or a single bolus of remimazolam can reduce the incidence of emergence delirium in children.
Methods:
A total of 120 children aged 1 to 6 yr were randomly and equally allocated into three groups: group RC, which received a continuous infusion of remimazolam at 1 mg · kg−1 · h−1; group RB, which received a single bolus of remimazolam at 0.2 mg · kg−1 at the beginning of wound closure; and group C, which received a continuous infusion of saline at 1 ml · kg−1 · h−1 and a single bolus of saline at 0.2 ml · kg−1 at the beginning of sutures. The primary outcome was the incidence of emergence delirium assessed by the Pediatric Anesthesia Emergence Delirium scale. Secondary outcomes included the number of rescue propofol administrations in the postanesthesia care unit, recovery time, and adverse events.
Results:
Emergence delirium was observed in 14 of 40 (35%) patients in group C, 2 of 40 (5%) patients in group RC (vs. group C, P = 0.001; risk ratio, 95% CI: 0.14, 0.04 to 0.59), and 3 of 39 (7.7%) patients in group RB (vs. group C, P = 0.003; risk ratio, 95% CI: 0.22, 0.07 to 0.71). Ten of 40 patients in group C, 2 of 40 patients in group RC (vs. group C, P = 0.012; risk ratio, 95% CI: 0.20, 0.05 to 0.86), and 2 of 39 patients in group RB (vs. group C, P = 0.014; risk ratio, 95% CI: 0.21, 0.05 to 0.88) needed rescue propofol. No differences in the recovery time and adverse effects were detected.
Conclusions:
Both continuous infusion and single bolus administration of remimazolam can effectively reduce the occurrence of emergence delirium in children.
In a three-arm randomized controlled trial comparing incidence of emergence delirium in children aged 1 to 6 yr undergoing laparoscopic hernia repair, those randomized to continuous intraoperative infusion of remimazolam and those randomized to a single bolus of remimazolam at the end of surgery both had a lower incidence of emergence delirium compared to placebo controls.
Editor’s Perspective.
What We Already Know about This Topic
In children, emergence delirium may occur after sevoflurane anesthesia. A range of agents has been found to reduce the risk of delirium including midazolam. Remimazolam is a novel short-acting benzodiazepine.
What This Article Tells Us That Is New
In a three-arm randomized controlled trial comparing incidence of emergence delirium in children aged 1 to 6 yr undergoing laparoscopic hernia repair, those randomized to continuous intraoperative infusion of remimazolam and those randomized to a single bolus of remimazolam at the end of surgery both had a lower incidence of emergence delirium compared to placebo controls.
Sevoflurane is a commonly employed inhalational anesthetic in pediatric surgery, with properties of respiratory tolerance, rapid onset, rapid offset, and hemodynamic stability. However, emergence delirium may occur frequently in children after sevoflurane anesthesia, with most frequently reported rates ranging between 10 and 20%.1–4 The presence of emergence delirium can not only delay recover and increase healthcare costs but may also decrease parent satisfaction scores with anesthesia.5–7
Various pharmacologic interventions have been explored to prevent or treat emergence delirium, including α2-adrenergic receptor agonists, opioids, and sedative agents.8–10 Previously, midazolam has been reported to reduce the incidence of emergence delirium in children.11,12 Compared to intranasal or oral routes, IV administration of midazolam is the most commonly utilized method to prevent emergence delirium.11 A single bolus of midazolam at 0.5 mg · kg−1 at the beginning or end of surgery has been reported to reduce emergence delirium.12 However, the primary concern with using midazolam to prevent emergence delirium in children is the potential for delaying anesthesia recovery.13 This is attributed to midazolam having a relatively long half-life (ranging from 0.79 to 2.83 h), and its main metabolite, 1-hydroxymidazolam, has been reported to be pharmacologically active.14
Remimazolam, a novel, ultrashort-acting benzodiazepine, has been successfully used for the induction and maintenance of procedural sedation and general anesthesia. Similar to remifentanil, remimazolam is rapidly hydrolyzed to a pharmacologically inactive metabolite (CNS 7054) via nonspecific tissue esterase activity. This process induces a unique and favorable pharmacologic profile, including rapid onset and offset of sedation and a predictable duration of action. Additionally, it has the advantages of less respiratory and circulatory depression than opioids and propofol.15 Compared to midazolam, intravenous infusion remimazolam showed good controllability with a high median (interquartile range) clearance of 15.9 (12.9, 18.2) ml · kg−1 · min−1 and a short terminal half-life of 67 (49, 85) min.16
Despite its potential and advantages, the role of remimazolam in preventing emergence delirium in children has not been thoroughly studied. Yang et al. reported that remimazolam at 0.2 mg · kg−1 at the end of adenotonsillectomy under sevoflurane anesthesia reduced the incidence of emergence delirium in children.17 However, tonsillectomy procedures are often accompanied by severe postoperative pain,18 which could potentially affect the assessment of emergence delirium. Additionally, the study did not observe the impact of continuous infusion of remimazolam on emergence delirium. Therefore, this study aimed to investigate the hypothesis that a continuous infusion or a single bolus of remimazolam can reduce the incidence of emergence delirium in children after laparoscopic surgery.
Materials and Methods
Study Design
The prospective, randomized, double-blind study was performed after getting approval from the Institutional Review Board of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (reference No. LCKY 2020-267; November 9, 2020) and registering at https://clinicaltrials.gov (NCT04621305; principal investigator, Hua-Cheng Liu; registered on November 16, 2020). Written informed consent was obtained from the parents or legal guardians of children enrolled in the trial.
Study Population
Inclusion Criteria
A total of 120 children, aged 1 to 6 yr, American Society of Anesthesiologists (Schaumburg, Illinois) Physical Status I or II, with a body mass index for age between the 25th and 85th percentiles,19 who were scheduled for laparoscopic inguinal hernia repair under general anesthesia with inhaled sevoflurane, were enrolled in the study.
Exclusion Criteria
We excluded children with American Society of Anesthesiologists Physical Status III or IV, those with abnormal liver or kidney function, cardiovascular or endocrine dysfunction, known allergy or hypersensitivity to remimazolam, recent respiratory infection, developmental delay, or autism. Additionally, children were excluded if they were under specialized care or lived in social welfare institutions, or there were any other factors that could affect their ability to participate in the study.
Data Collection
A paper case report form was designed for registration of clinical data and study results. Data were stored in a password-protected computer to maintain patients’ confidentiality. The guidelines of Good Clinical Practice were closely followed during the study. One investigator was assigned to follow the patients throughout the entire study, from the preoperative setting until recovery room discharge, specifically to collect, store, and transfer data. A second investigator was responsible for verifying the accuracy and safety of the stored data.
Randomization and Blinding
Randomization was performed in a 1:1:1 ratio according to a computer-generated randomization table (SPSS Inc., USA) generated before commencing the study.
Blinding and Drug Preparation
Group assignments were concealed using sequentially numbered, opaque, sealed envelopes, which were maintained by an independent investigator. All the study drugs (remimazolam) or the saline were prepared by an independent pharmacist. The powder of remimazolam besylate (25 mg, Yichang Humanwell Pharmaceutical Co., Ltd., China) was dissolved in saline to achieve a concentration of 1 mg · ml−1. The study drug and saline were indistinguishable in color and syringe size and dispensed in identical containers to ensure blinding. During the maintenance period, each group of children received either the study drug or normal saline at a rate of 1 ml · kg−1 · h−1. Additionally, a single bolus of the study drug (dose, 0.2 ml · kg−1) or placebo was administered to each group 5 min before the end of the surgery. Study participants, observers, and attending anesthesiologists were all blinded to the group allocation and study drug administered, adhering to the principles of blinding.
Standardized Procedure
Induction and Maintenance of Anesthesia
Participant characteristics and modified Yale Preoperative Anxiety Scale were recorded in the preoperative waiting area. No premedication was administered.
Standard anesthesia monitoring including continuous electrocardiography, pulse oximetry, Bispectral Index (BIS), and noninvasive blood pressure was begun before induction of anesthesia. The values were recorded every 5 min. Anesthesia was induced with fentanyl (2 to 2.5 μg · kg−1), propofol (2 to 2.5 mg · kg−1), and cisatracurium (0.1 mg · kg−1) with 100% oxygen at a flow rate of 6 l · min–1. After successful anesthesia induction, a laryngeal mask airway (Ambu AuraOnce, China) was placed after the children’s muscles were relaxed and they were unconscious.
The randomization assignment for the child was opened after induction of anesthesia as follows:
Control group (group C): Continuous infusion of normal saline at a rate of 1 ml · kg−1 · h−1 was begun and stopped at the time of beginning wound closure (about 5 min before the end of the operation). After the continuous infusion was stopped, a single IV bolus of 0.2 ml · kg−1 normal saline (time, 30 s or less) was administered.
Remimazolam continuous infusion group (group RC): Continuous infusion of remimazolam at a rate of 1 mg · kg−1 · h−1 (1 ml · kg−1 · h−1) was begun and stopped at the time of beginning wound closure (about 5 min before the end of the operation). After the continuous infusion was stopped, a single IV bolus of 0.2 ml · kg−1 normal saline (time, 30 s or less) was administered.
Remimazolam bolus group (group RB): Continuous infusion of normal saline at a rate of 1 ml · kg−1 · h−1 was begun and stopped at the time of beginning wound closure (about 5 min before the end of the operation). After the continuous infusion was stopped, a single IV bolus of 0.2 mg · kg−1 remimazolam (time, 30 s or less) was administered.
During maintenance of anesthesia, sevoflurane was dynamically adjusted to maintain the BIS near 50 (range, 40 to 60) and the vital signs within 20% of their baseline values. An air/oxygen (50%) mixture was delivered at a flow rate of 2 l · min−1. The end-tidal sevoflurane concentrations and vital signs were recorded every 5 min after the remimazolam or normal saline infusion was initiated. The end-tidal pressure of carbon dioxide was maintained between 35 and 45 mmHg by adjusting the respiratory rate and tidal volume. Supplemental doses of 0.5 to 1 μg · kg−1 of fentanyl were used based on the anesthesiologist’s judgment, particularly if the blood pressure or heart rate (HR) exceeded 20% of baseline values, yet insufficient sedation was ruled out as a cause.
Analgesia was supplemented by a 0.5 mg · kg−1 single IV bolus of ketorolac tromethamine and 0.2 ml · kg−1 0.75% ropivacaine local infiltration at the wound site.20 All patients received an intravenous dose of 0.15 mg · kg−1 dexamethasone after the induction of anesthesia to prevent postoperative nausea and vomiting.21 At the end of the surgery, sevoflurane was discontinued, and the oxygen flow was increased to 5 l · min−1. Muscle blockade was antagonized with neostigmine 0.02 mg · kg−1 and atropine 0.01 mg · kg−1. The laryngeal mask airway was removed when patients resumed spontaneous breathing with adequate tidal volume (6 ml · kg−1 or greater) and a respiratory frequency of 15 breaths · min–1 or more.
Recovery from Anesthesia
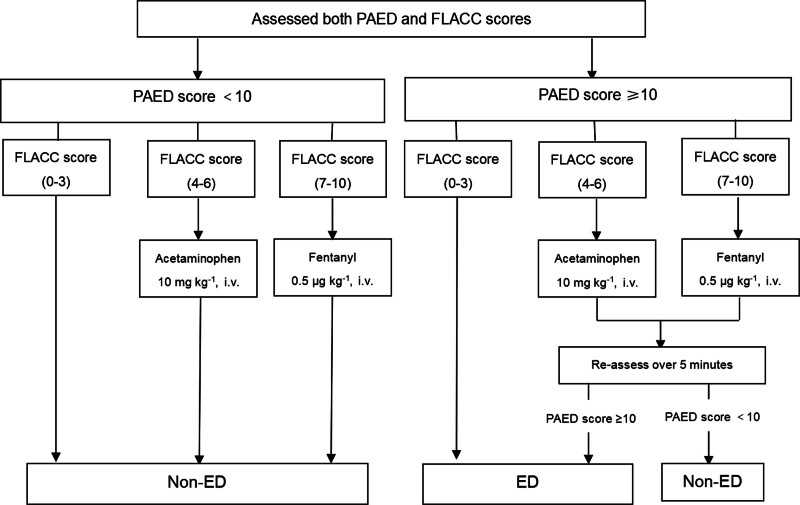
In the PACU, a single trained study member, blinded to the group allocations, was responsible for documenting the Pediatric Anesthesia Emergence Delirium scale (PAED) and Face, Legs, Activity, Cry, Consolability (FLACC) scores at 0, 5, 10, 20, and 30 min after emergence from anesthesia. Additionally, if any signs suggestive of emergence delirium appeared during these intervals, immediate assessments using the PAED and FLACC scales were conducted. The diagnostic process of emergence delirium and the treatment procedure is schematically presented in figure 1.
Fig. 1.
The diagnostic process of emergence delirium. ED, emergence delirium; FLACC, Face, Legs, Activity, Cry, Consolability; PAED, Pediatric Anesthesia Emergence Delirium scale.
PAED less than 10, FLACC 1 to 3: Patients who were not experiencing emergence delirium or pain. No special intervention required.
PAED less than 10, FLACC 4 to 6: Patients with moderate pain but no emergence delirium. Managed with intravenous acetaminophen at a dosage of 10 mg · kg−1.
PAED less than 10, FLACC 7 to 9: Patients with severe pain but no emergence delirium. Treated with intravenous fentanyl at a dosage of 0.5 μg · kg−1.
PAED 10 or greater, FLACC 1 to 3: Patients with emergence delirium but no pain.
PAED 10 or greater, FLACC 4 to 6: Patients with moderate pain. Pain was treated by intravenous administration of 10 mg · kg–1 acetaminophen. After 5 min, the PAED score was reassessed. The child was diagnosed with emergence delirium if the reassessed PAED score remained 10 or higher,22,23 regardless of the FLACC score.
PAED 10 or greater, FLACC 7 to 9: Patients with severe pain. Pain was treated by intravenous administration of 0.5 μg · kg–1 fentanyl. After 5 min, the PAED score was reassessed. The child was diagnosed with emergence delirium if the reassessed PAED score remained 10 or higher, regardless of the FLACC score.
Children diagnosed with emergence delirium received initial comfort measures. If these were ineffective, 1 mg · kg−1 propofol was administered.
The following adverse events were recorded. Hypotension was defined as a systolic arterial pressure less than (70 mmHg + 2 times age in years),24 bradycardia as a HR less than 60 beats · min−1. All episodes of vomiting, respiratory depression (respiratory rate fewer than 10 breaths · min−1 or oxygen saturation less than 90% for more than 1 min),25 and laryngospasm were recorded. Children were observed in the recovery room for at least 30 min after emergence from anesthesia. When a modified Aldrete score of 9 was reached, the child was discharged from the PACU.
Study Outcomes
The primary outcome was the incidence of emergence delirium. Figure 1 shows the diagnostic process of emergence delirium. If the PAED score was 10 or higher and the FLACC score was less than 4, the child was diagnosed with emergence delirium. If the PAED score was 10 or higher and the FLACC score was 4 or greater, the child first received analgesic treatment—intravenous administration of 10 mg · kg−1 acetaminophen for FLACC scores between 4 and 6, and intravenous administration of 0.5 μg · kg−1 fentanyl for FLACC scores of 7 or higher. The child was reassessed 5 min after analgesic treatment. The diagnosis of emergence delirium was confirmed if the reassessed PAED score remained 10 or higher,22,26 regardless of the FLACC score.
The secondary outcomes were as follows:
The number of rescue doses of propofol administered in the PACU.
The end-tidal concentration of sevoflurane required to maintain a BIS within 40 to 60.
Blood pressure and HR at 5 min, 10 min, 15 min, and 20 min after the drug infusion started.
Blood pressure and HR immediately before (0 min) and at 1 min, 3 min, and 5 min after a single bolus of remimazolam or saline.
At the occurrence of postoperative pain, as defined by FLACC scores 4 or greater; the FLACC scores were evaluated at 0, 5, 10, 20, and 30 min after emergence from anesthesia.
Recovery time was the time from the cessation of sevoflurane to the point when the child was awakened and responded readily to their name spoken in a normal tone of voice. This includes instances in which the child’s response may have been delayed due to emergence delirium.
The incidence of adverse events.
Outcome Measures
The diagnostic process of emergence delirium is presented in figure 1, assessed by the PAED scale (Supplemental Table S1, https://links.lww.com/ALN/D572).22,26 Postoperative pain was measured using the FLACC pain score (Supplemental Table S2, https://links.lww.com/ALN/D572).27 The modified Yale Preoperative Anxiety Scale (Supplemental Table S3, https://links.lww.com/ALN/D572) is a score designed to assess anxiety in children before surgery.28
Anesthesia time was defined as the time between initial administration of anesthesia and the cessation of sevoflurane. Surgery time was the time from the first incision to completing the final sutures.
Sample Size
In our pilot study involving 40 participants, we observed a 55% incidence of emergence delirium in the control group (11 of 20) and 20% in each treatment group (2 of 10). Therefore, we predicted emergence delirium rates of 55% for the control group and 20% for the treatment groups. Assuming that the α error is 0.025 (0.05/2) and the power is 0.80, the required number of patients is 96. Estimating a 20% dropout rate, 120 children were needed in this study (PASS 15.0).
Statistical Methods
The data were analyzed by SPSS version 24.0 for Windows (SPSS Inc.). The normality of distribution of continuous variables was tested by Shapiro–Wilk test. Continuous variables with normal distribution are presented as mean ± SD, whereas nonnormal continuous variables are presented as median (interquartile range). Continuous variables include FLACC score, postoperative fentanyl consumption, recovery time, end-tidal concentration of sevoflurane, blood pressure, and HR. Continuous variables with a normal distribution were compared among the three groups using one-way ANOVA. Only if the ANOVA test was significant, the P value for pairwise comparisons was calculated using Student’s t test with Bonferroni correction. Kruskal–Wallis nonparametric tests, with Mann–Whitney tests for pairwise comparisons, were used for variables not conforming to normal distribution. Median differences (and 95% CI) were calculated with Hodges–Lehmann estimators. Categorical variables include the incidence of emergence delirium, incidence of rescue propofol for emergence delirium, incidence of postoperative pain, incidence of rescue fentanyl, and incidence of rescue acetaminophen. Categorical variables are presented as number and percentage, and their intergroup comparison were performed by chi-square test or Fisher exact test. Repeated-measures data, including blood pressure and HR, were analyzed using mixed-effects model analysis. All statistical tests were two-sided, and a P value <0.05 was considered significant. In multiple comparisons, the pairwise comparison test level was adjusted to 0.05/number of comparisons.
Results
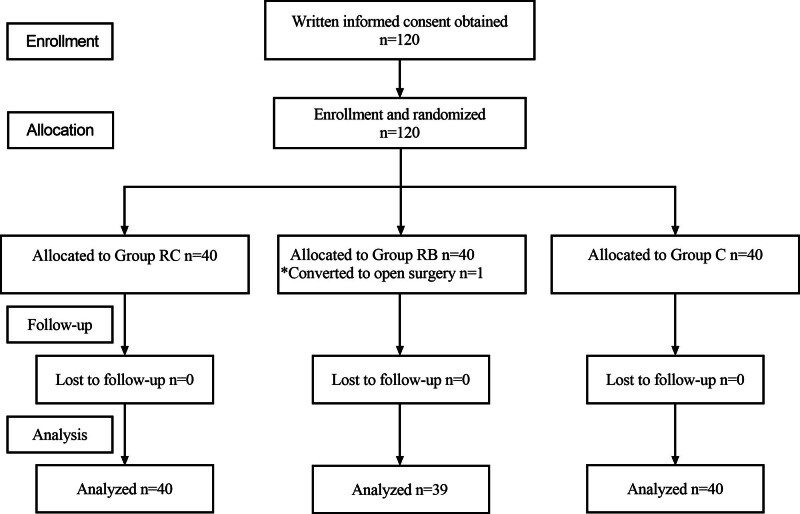
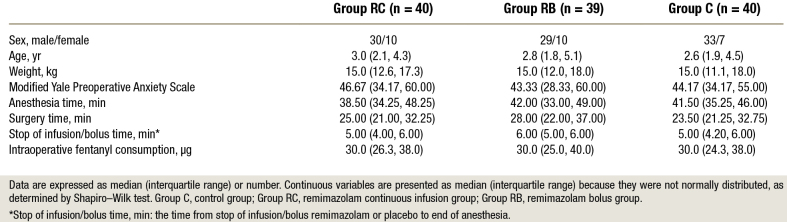
A total of 120 children were randomly allocated in this clinical trial. One child in group RB was excluded due to the need for conversion from laparoscopic to open surgery. A total of 119 children were included in the final analysis (fig. 2). The baseline characteristics, anesthesia and surgical times, intraoperative fentanyl consumption, and stop of the infusion/bolus times of the three groups were similar (table 1).
Fig. 2.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram. Group C, control group; Group RC, remimazolam continuous infusion group; Group RB, remimazolam bolus group.
Table 1.
Subject Characteristics and Clinical Data
Incidence of Emergence Delirium, FLACC, and Recovery Time
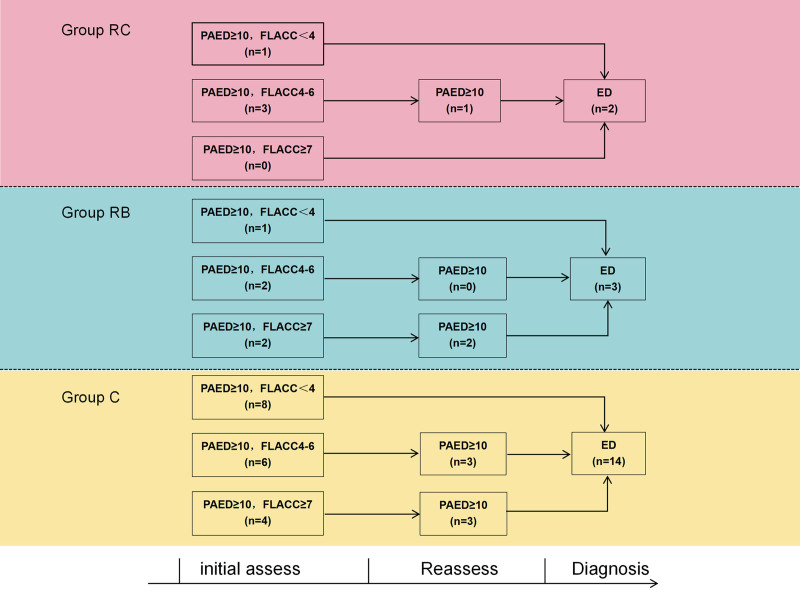
In the group RC, there were three children initially having PAED 10 or greater with FLACC in the range of 4 to 6. After acetaminophen treatment, two of these children no longer met the criteria for delirium due to the reassessed PAED scores decreasing to less than 10. In the group RB, there were two children initially having PAED 10 or greater with FLACC in the range of 4 to 6, and two children having PAED 10 or greater with FLACC 7 or greater. After analgesia treatment, the two children treated with acetaminophen no longer met the criteria for delirium, while the two children who received fentanyl continued to exhibit PAED scores 10 or greater, leading to a diagnosis of emergence delirium. In group C, there were six children initially having PAED 10 or greater with FLACC in the range of 4 to 6, and four children having PAED 10 or greater with FLACC 7 or greater. After analgesia treatment, four of these children (three treated with acetaminophen and one with fentanyl) no longer met the criteria for delirium. For details on the diagnostic process and results, see figure 3.
Fig. 3.
The detailed results of the diagnostic process of emergence delirium. Group C, control group; Group RC, remimazolam continuous infusion group; Group RB, remimazolam bolus group. ED, emergence delirium; FLACC, Face, Legs, Activity, Cry, Consolability; PAED, Pediatric Anesthesia Emergence Delirium scale.
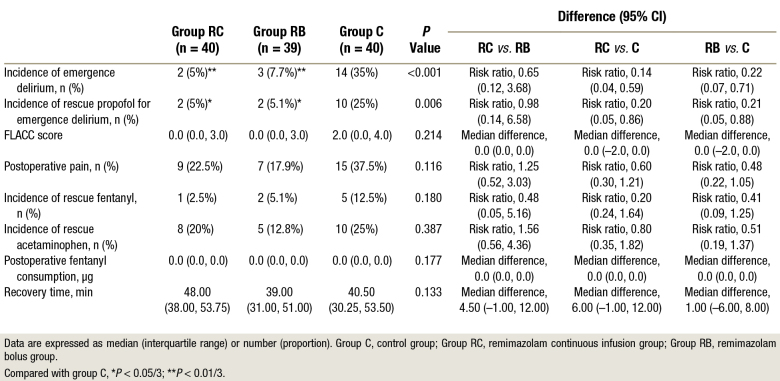
Emergence delirium was observed in 14 of 40 (35%) patients in group C, 2 of 40 (5%) patients in group RC (vs. group C, P = 0.001; risk ratio, 95% CI: 0.14, 0.04 to 0.59), and 3 of 39 (7.7%) patients in group RB (vs. group C, P = 0.003; risk ratio, 95% CI: 0.22, 0.07 to 0.71). Ten of 40 (25%) patients in group C, 2 of 40 (5%) patients in group RC (vs. group C, P = 0.012; risk ratio, 95% CI: 0.20, 0.05 to 0.86), and 2 of 39 (5.1%) patients in group RB (vs. group C, P = 0.014; risk ratio, 95% CI: 0.21, 0.05 to 0.88) needed rescue propofol. There was no significant difference among the three groups in recovery time and FLACC scores in the PACU (table 2).
Table 2.
Postoperative Assessments
Remimazolam and Sevoflurane
The area under the curve for the end-tidal concentration of sevoflurane from 0 to the end of surgery in group RC was significantly reduced by approximately 20% compared to groups RB and C (group RC vs. group RB, median difference, –11.13; 95% CI, –20.75 to –0.25, P = 0.013; group RC vs. group C, median difference, –9.00; 95% CI, –16.75 to –1.50; P = 0.003).
Hemodynamics
After continuous infusion of drug was started, there was no significant main effect of the group or interaction effect of time with group in systolic blood pressure, diastolic blood pressure, mean arterial pressure, and HR (Supplemental Table S4, https://links.lww.com/ALN/D572). After administering a single IV bolus of drug, there was no significant main effect of the group or interaction effect of time with group in systolic blood pressure, diastolic blood pressure, or mean arterial pressure; there was a significant main effect of the group (P < 0.001), but the interaction between the group and time was not significant (P = 0.174) in HR. Compared with group C, children in group RB had significantly higher mean HR values at 3 and 5 min after administering a single bolus of remimazolam (P = 0.004; P < 0.001; Supplemental Table S5, https://links.lww.com/ALN/D572). Children in group RC had significantly higher mean HR values at 0, 1, 3, and 5 min after stopping infusion of remimazolam than in group C (P = 0.007, P = 0.009, P = 0.001, P < 0.001, respectively; Supplemental Table S5, https://links.lww.com/ALN/D572).
Safety
One child experienced vomiting in group RC. None of the children experienced bradycardia, hypotension, hypoxemia, or laryngospasm. No other severe adverse events were noted.
Discussion
This prospective randomized double-blind study aimed to evaluate whether continuous infusion or a single bolus of remimazolam can reduce the incidence of emergence delirium in children undergoing laparoscopic surgery with sevoflurane anesthesia. We found that both a continuous infusion and a single bolus of remimazolam effectively reduces the incidence of emergence delirium with stable hemodynamics and few adverse reactions.
In this study, we found that both continuous infusion and a single bolus of remimazolam exhibited similar efficacy in preventing emergence delirium in children, reducing the incidence from 35% to 5% and 7.5%, respectively. These results align with a previous study, which demonstrated that a single bolus of remimazolam reduced the incidence of emergence delirium from 44% to 12% in children undergoing tonsillectomy and adenoidectomy with sevoflurane anesthesia.17 Notably, our study suggested a reduced incidence of emergence delirium, possibly attributed to the use of minimally invasive laparoscopic surgery, which is a less painful surgical procedure than tonsillectomy. In this study, the preventive effect of remimazolam on emergence delirium appears to be similar to the effects observed with commonly used drugs in other studies. A previous study reported that single dose of dexmedetomidine and propofol reduced the incidence of emergence delirium from 40.6% to 9.4% and 13.9%, respectively.29 Future research could explore the relative merits and drawbacks of remimazolam in preventing emergence delirium, aiming to offer a more comprehensive evaluation of diverse preventive strategies. In adults, remimazolam-based total intravenous anesthesia provided a similar quality of recovery to propofol undergoing thyroid surgery.30 Further research is needed to investigate whether remimazolam as a primary agent for anesthesia would yield favorable postoperative recovery outcomes in children.
In terms of postoperative recovery, the recovery time in the group RC was 9 min longer than that observed in the other two groups. This finding contradicts what one would expect from the rapid metabolism pharmacokinetic profile of remimazolam and is inconsistent with previous studies involving the administration of remimazolam as a single agent.16 Since flumazenil was not employed in our study,31 we cannot definitively attribute the delayed awakening solely to remimazolam accumulation. Further research is warranted to confirm whether children undergoing continuous remimazolam infusion experience longer postoperative recovery times compared to anesthesia with sevoflurane or propofol.
Postoperative pain is a major confounding factor for emergence delirium.6,22 To minimize the impact of pain on the diagnosis of emergence delirium, several measures were taken. First, we selected laparoscopic surgery, which is associated with minimal pain stimuli, and provided the same analgesic regimen to all patient groups. The results showed no statistical difference in pain levels among the three groups. Second, we employed both FLACC and PAED scoring to distinguish between postoperative pain and delirium in the postoperative phase. However, the “Restless” and “Inconsolable” aspects of the PAED scale overlap with the symptoms of pain.22,32 Therefore, we did not immediately diagnose children with an initial PAED score 10 or greater and FLACC score 4 or greater as having postoperative delirium; instead, we reassessed the PAED score 5 min after analgesic administration to diagnose whether emergence delirium had occurred, aiming to mitigate the potential influence of pain on postoperative emergence delirium assessment.22 Nevertheless, we observed a higher incidence of postoperative pain in the control group, which may be related to the higher incidence of emergence delirium in this group, because some cases of emergence delirium may be misdiagnosed as postoperative pain, just as some cases of postoperative pain may be misdiagnosed as emergence delirium.22
To ensure consistent anesthesia depth among the three groups, BIS was used to monitor the depth of anesthesia. However, controversy exists regarding the correlation between remimazolam and BIS. Hence, when the BIS was maintained at 40 to 60, some children in the RC group may have achieved undesired deeper sedation. Furthermore, continuous infusion of remimazolam significantly reduced the consumption of sevoflurane with BIS-guided anesthesia. The results of a recent cohort study demonstrated that sevoflurane dose was not associated with delirium severity or incidence.33
Two studies reported that both continuous infusion and a single bolus of remimazolam could increase HR in healthy volunteers.34,35 Similar results were found in groups RC and RB, which may be attributed to the pharmacologic properties of remimazolam. However, the increase in HR observed in the group RC after discontinuation of the drug may be attributed to a rapid decline in anesthesia depth. Overall, the observed changes in HR are minor and acceptable.
Both continuous infusion and a single bolus of remimazolam can effectively reduce the incidence of emergence delirium in children. Compared with continuous infusion, a single bolus of remimazolam is more convenient and does not delay postoperative recovery. However, continuous infusion of remimazolam can reduce intraoperative sevoflurane consumption by about 20%. Therefore, compared with a single bolus of remimazolam, continuous infusion may reduce patient costs and the environmental impact of sevoflurane.
Limitations
Although we attempted to ensure a comparable depth of anesthesia with the BIS monitor, the relationship between sevoflurane with and without remimazolam and the BIS are poorly understood in children. Second, short-term or long-term postoperative effects on the patient’s behavior and cognition were not evaluated in this study. Third, pharmacokinetic data and information on clinical application of remimazolam in children are limited; the dosage in this study was based on adult data and our pilot study data. Fourth, we chose intravenous fentanyl for patients in severe pain, and fentanyl itself has sedative effects, which might skew our study result. Despite the fact that we specifically selected surgical procedures with minimal pain stimuli and implemented measures to prevent postoperative pain, it is important to emphasize that measuring emergence delirium in surgeries where pain is not a prominent feature is preferred.36 Last, this study is a single-center study, and the conclusion should be validated by multicenter studies.
Conclusions
Both continuous infusion and a single bolus of remimazolam can effectively reduce emergence delirium in children by sevoflurane anesthesia with stable hemodynamics and few adverse reactions.
Acknowledgments
The authors thank all participants in this study and extend their grateful acknowledgment to anonymous reviewers for their indispensable assistance.
Research Support
This study was supported by the Major Science Technology Projects of Wenzhou (Wenzhou, China; ZY2023030), the Social Development Science and Technology Project of Taizhou City of China (Taizhou, China; 20ywa59), and the Wenzhou Science and Technology Bureau (Wenzhou, China; Y20220948).
Competing Interests
The authors declare no competing interests.
Reproducible Science
Full protocol available at: huachengliu@163.com. Raw data available at: huachengliu@163.com.
Supplemental Digital Content
Supplemental tables, https://links.lww.com/ALN/D572
Supplementary Material
Footnotes
Published online first on May 16, 2024.
This article has been selected for the Anesthesiology CME Program (www.asahq.org/JCME2024SEP). Learning objectives and disclosure and ordering information can be found in the CME section at the front of this issue.
This article is featured in “This Month in Anesthesiology,” page A1.
This article is accompanied by an editorial on p. 434.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are available in both the HTML and PDF versions of this article. Links to the digital files are provided in the HTML text of this article on the Journal’s Web site (www.anesthesiology.org).
Y.-H.C. and J.W.Z. contributed equally to this article.
The article processing charge was funded by the Major Science Technology Projects of Wenzhou, the Social Development Science and Technology Project of Taizhou City of China, and the Wenzhou Science and Technology Bureau.
Contributor Information
John Wei Zhong, Email: John.zhong@utsouthwestern.edu.
Hong-Yu Ma, Email: mhy15612484069@163.com.
Peter Szmuk, Email: Peter.Szmuk@UTSouthwestern.edu.
Cheng-Yu Wang, Email: wangzhen@hmc.edu.cn.
Zhen Wang, Email: wangzhen@hmc.edu.cn.
Xu-Lin Zhang, Email: 867237394@qq.com.
Le-Qi Dong, Email: 1040596063@qq.com.
Hua-Cheng Liu, Email: huachengliu@163.com.
References
- 1.Banchs RJ, Lerman J: Preoperative anxiety management, emergence delirium, and postoperative behavior. Anesthesiol Clin 2014; 32:1–23 [DOI] [PubMed] [Google Scholar]
- 2.Bortone L, Bertolizio G, Engelhardt T, Frawley G, Somaini M, Ingelmo PM: The effect of fentanyl and clonidine on early postoperative negative behavior in children: A double-blind placebo controlled trial. Paediatr Anaesth 2014; 24:614–9 [DOI] [PubMed] [Google Scholar]
- 3.Doerrfuss JI, Kramer S, Tafelski S, Spies CD, Wernecke KD, Nachtigall I: Frequency, predictive factors and therapy of emergence delirium: Data from a large observational clinical trial in a broad spectrum of postoperative pediatric patients. Minerva Anestesiol 2019; 85:617–24 [DOI] [PubMed] [Google Scholar]
- 4.Malarbi S, Stargatt R, Howard K, Davidson A: Characterizing the behavior of children emerging with delirium from general anesthesia. Paediatr Anaesth 2011; 21:942–50 [DOI] [PubMed] [Google Scholar]
- 5.Costi D, Cyna AM, Ahmed S, et al. : Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst Rev 2014; 2014:CD007084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mason KP: Paediatric emergence delirium: A comprehensive review and interpretation of the literature. Br J Anaesth 2017; 118:335–43 [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Fan Q, Zhang J, et al. : Effect of ultrasound-guided lumbar plexus block on emergence agitation in children undergoing hip surgery: Study protocol for a randomized controlled trial. Trials 2019; 20:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MS, Moon BE, Kim H, Lee JR: Comparison of propofol and fentanyl administered at the end of anaesthesia for prevention of emergence agitation after sevoflurane anaesthesia in children. Br J Anaesth 2013; 110:274–80 [DOI] [PubMed] [Google Scholar]
- 9.Lin Y, Chen Y, Huang J, et al. : Efficacy of premedication with intranasal dexmedetomidine on inhalational induction and postoperative emergence agitation in pediatric undergoing cataract surgery with sevoflurane. J Clin Anesth 2016; 33:289–95 [DOI] [PubMed] [Google Scholar]
- 10.Hauber JA, Davis PJ, Bendel LP, et al. : Dexmedetomidine as a rapid bolus for treatment and prophylactic prevention of emergence agitation in anesthetized children. Anesth Analg 2015; 121:1308–15 [DOI] [PubMed] [Google Scholar]
- 11.Kawai M, Kurata S, Sanuki T, et al. : The effect of midazolam administration for the prevention of emergence agitation in pediatric patients with extreme fear and non-cooperation undergoing dental treatment under sevoflurane anesthesia, a double-blind, randomized study. Drug Design Dev Ther 2019; 13:1729–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho EJ, Yoon SZ, Cho JE, Lee HW: Comparison of the effects of 0.03 and 0.05 mg/kg midazolam with placebo on prevention of emergence agitation in children having strabismus surgery. Anesthesiology 2014; 120:1354–61 [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Yoon SZ, Lim HJ, Yoon SM: Prophylactic use of midazolam or propofol at the end of surgery may reduce the incidence of emergence agitation after sevoflurane anaesthesia. Anaesth Intensive Care 2011; 39:904–8 [DOI] [PubMed] [Google Scholar]
- 14.Salonen M, Kanto J, Iisalo E, Himberg JJ: Midazolam as an induction agent in children: A pharmacokinetic and clinical study. Anesth Analg 1987; 66:625–8 [PubMed] [Google Scholar]
- 15.Kleiman RB, Darpo B, Thorn M, Stoehr T, Schippers F: Potential strategy for assessing QT/QTc interval for drugs that produce rapid changes in heart rate: Electrocardiographic assessment of the effects of intravenous remimazolam on cardiac repolarization. Br J Clin Pharmacol 2020; 86:1600–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao YQ, Ihmsen H, Hu ZY, et al. : Pharmacokinetics of remimazolam after intravenous infusion in anaesthetised children. Br J Anaesth 2023; 131:914–20 [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Lin C, Chen S, Huang Y, Cheng Q, Yao Y: Remimazolam for the prevention of emergence delirium in children following tonsillectomy and adenoidectomy under sevoflurane anesthesia: A randomized controlled study. Drug Design Dev Ther 2022; 16:3413–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldamluji N, Burgess A, Pogatzki-Zahn E, Raeder J, Beloeil H; PROSPECT Working Group Collaborators: PROSPECT guideline for tonsillectomy: Systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia 2021; 76:947–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Guo SS, et al. : 2000 CDC growth charts for the United States: Methods and development. Vital Health Stat 2002; 11:1–190 [PubMed] [Google Scholar]
- 20.Tamura T, Kaneko K, Yokota S, et al. : Comparison between rectus sheath block with 0.25% ropivacaine and local anesthetic infiltration with 0.5% ropivacaine for laparoscopic inguinal hernia repair in children. Nagoya J Med Sci 2019; 81:341–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czarnetzki C, Elia N, Lysakowski C, et al. : Dexamethasone and risk of nausea and vomiting and postoperative bleeding after tonsillectomy in children: A randomized trial. JAMA 2008; 300:2621–30 [DOI] [PubMed] [Google Scholar]
- 22.Somaini M, Engelhardt T, Fumagalli R, Ingelmo PM: Emergence delirium or pain after anaesthesia–How to distinguish between the two in young children: A retrospective analysis of observational studies. Br J Anaesth 2016; 116:377–83 [DOI] [PubMed] [Google Scholar]
- 23.Somaini M, Sahillioğlu E, Marzorati C, Lovisari F, Engelhardt T, Ingelmo PM: Emergence delirium, pain or both? A challenge for clinicians. Paediatr Anaesth 2015; 25:524–9 [DOI] [PubMed] [Google Scholar]
- 24.Yanagida N, Sato S, Asaumi T, Ogura K, Ebisawa M: Risk factors for severe reactions during double-blind placebo-controlled food challenges. Int Arch Allergy Immunol 2017; 172:173–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh SK, Lee IO, Lim BG, et al. : Comparison of the analgesic effect of sufentanil versus fentanyl in intravenous patient-controlled analgesia after total laparoscopic hysterectomy: A randomized, double-blind, prospective study. Int J Med Sci 2019; 16:1439–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sikich N, Lerman J: Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology 2004; 100:1138–45 [DOI] [PubMed] [Google Scholar]
- 27.Crellin DJ, Harrison D, Santamaria N, Babl FE: Systematic review of the Face, Legs, Activity, Cry and Consolability scale for assessing pain in infants and children: Is it reliable, valid, and feasible for use? Pain 2015; 156:2132–51 [DOI] [PubMed] [Google Scholar]
- 28.Kain ZN, Mayes LC, Cicchetti DV, Bagnall AL, Finley JD, Hofstadter MB: The Yale Preoperative Anxiety Scale: How does it compare with a “gold standard”? Anesth Analg 1997; 85:783–8 [DOI] [PubMed] [Google Scholar]
- 29.Makkar JK, Bhatia N, Bala I, Dwivedi D, Singh PM: A comparison of single dose dexmedetomidine with propofol for the prevention of emergence delirium after desflurane anaesthesia in children. Anaesthesia 2016; 71:50–7 [DOI] [PubMed] [Google Scholar]
- 30.Choi JY, Lee HS, Kim JY, et al. : Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: A randomized non-inferiority trial. J Clin Anesth 2022; 82:110955. [DOI] [PubMed] [Google Scholar]
- 31.Wesolowski AM, Zaccagnino MP, Malapero RJ, Kaye AD, Urman RD: Remimazolam: Pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy 2016; 36:1021–7 [DOI] [PubMed] [Google Scholar]
- 32.Büttner W, Finke W: Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: A comprehensive report on seven consecutive studies. Paediatr Anaesth 2000; 10:303–18 [DOI] [PubMed] [Google Scholar]
- 33.Taylor J, Payne T, Casey C, et al. : Sevoflurane dose and postoperative delirium: A prospective cohort analysis. Br J Anaesth 2023; 130:e289–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H: Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology 2020; 132:636–51 [DOI] [PubMed] [Google Scholar]
- 35.Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM: A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg 2012; 115:274–83 [DOI] [PubMed] [Google Scholar]
- 36.Lerman J, Ingelmo P: Emergence delirium in children: Do the studies reflect reality? Paediatr Anaesth 2024; 34:493–4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.