Abstract
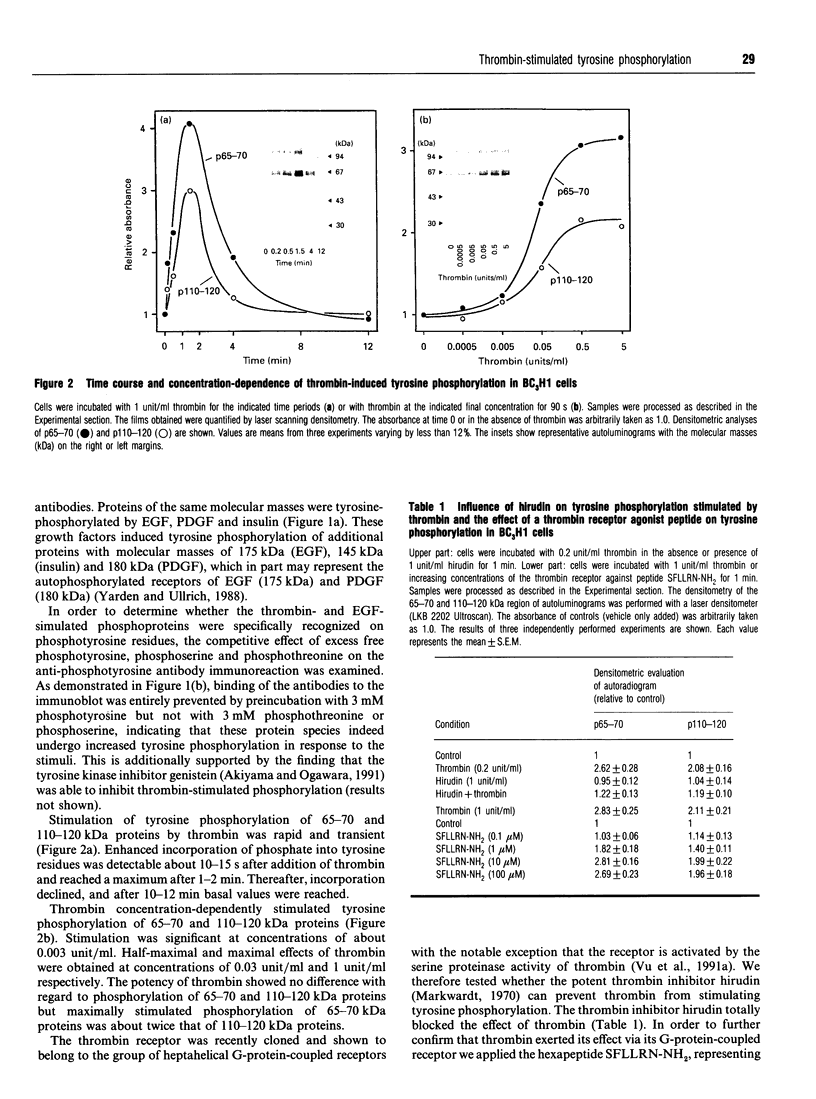
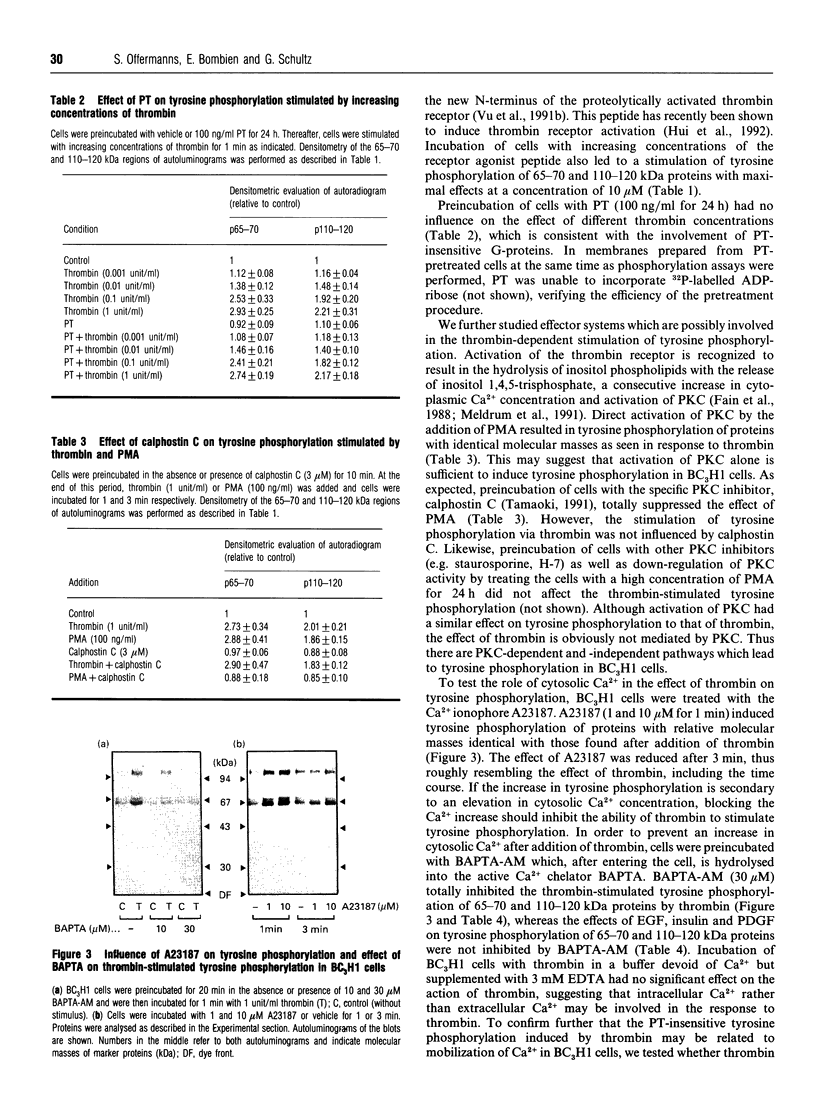
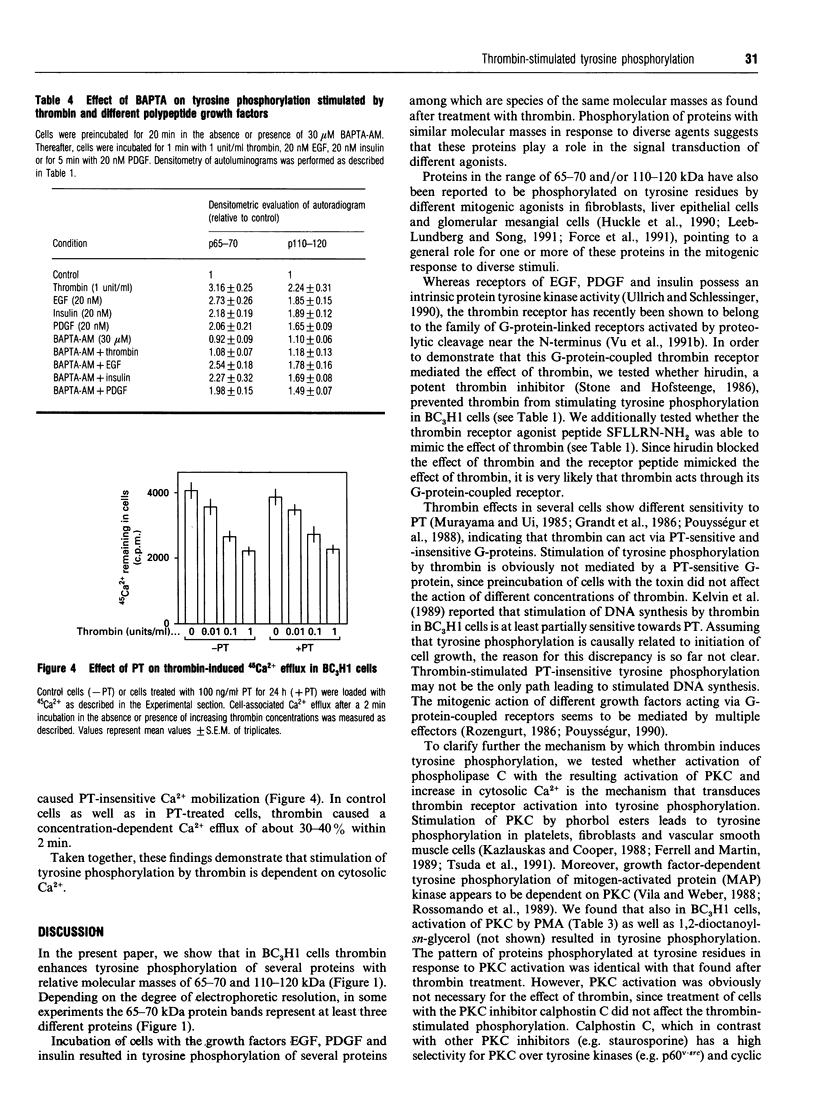
The proteinase thrombin, known to act via heptahelical G-protein-coupled receptors, is a mitogenic agent for different cell types, including the mouse muscle cell line BC3H1. In this study, the effect of thrombin on tyrosine phosphorylation was examined using anti-phosphotyrosine antibodies. Thrombin was found to induce phosphorylation of 65-70 and 110-120 kDa proteins in BC3H1 cells. The effect of thrombin was concentration-dependent, being half-maximal and maximal at concentrations of 0.03 and 1 unit/ml respectively. The thrombin-induced increase in phosphorylation was rapid (< or = 10 s) and transient, with a peak response after about 1-2 min. The effect of thrombin could be mimicked by the thrombin receptor agonist peptide SFLLRN-NH2. Preincubation of cells with pertussis toxin (PT) had no effect on thrombin-induced tyrosine phosphorylation. Epidermal growth factor, platelet-derived growth factor and insulin stimulated tyrosine phosphorylation of different proteins, among which were 65-70 and 110-120 kDa proteins. The phorbol ester 12-myristate 13-acetate (PMA) as well as the Ca2+ ionophore A23187 both stimulated tyrosine phosphorylation of proteins identical to those phosphorylated by thrombin, suggesting that activation of protein kinase C (PKC) and elevation of the cytosolic Ca2+ concentration alone are sufficient to induce tyrosine phosphorylation. However, calphostin C and other PKC inhibitors, which completely inhibited tyrosine phosphorylation induced by PMA, had no influence on the effect of thrombin, whereas loading of cells with the intracellular Ca2+ chelator bis-(O-aminophenoxy)ethane-NNN'N'-tetra-acetic acid totally blocked thrombin-stimulated tyrosine phosphorylation. Thus tyrosine phosphorylation stimulated by thrombin is an early PT-insensitive cellular response which is either directly mediated by elevation of cytosolic Ca2+ concentration or by a presently unknown mechanism that requires an elevated cytosolic Ca2+ concentration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ogawara H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- Berkow R. L., Dodson R. W. Alterations in tyrosine protein kinase activities upon activation of human neutrophils. J Leukoc Biol. 1991 Jun;49(6):599–604. doi: 10.1002/jlb.49.6.599. [DOI] [PubMed] [Google Scholar]
- Brown R. D., Berger K. D., Taylor P. Alpha 1-adrenergic receptor activation mobilizes cellular Ca2+ in a muscle cell line. J Biol Chem. 1984 Jun 25;259(12):7554–7562. [PubMed] [Google Scholar]
- Chambard J. C., Paris S., L'Allemain G., Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987 Apr 23;326(6115):800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar A., Paul A. K., Shukla S. D. Platelet-activating factor stimulation of tyrosine kinase and its relationship to phospholipase C in rabbit platelets: studies with genistein and monoclonal antibody to phosphotyrosine. Mol Pharmacol. 1990 Apr;37(4):519–525. [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Wallace M. A., Wojcikiewicz R. J. Evidence for involvement of guanine nucleotide-binding regulatory proteins in the activation of phospholipases by hormones. FASEB J. 1988 Jul;2(10):2569–2574. doi: 10.1096/fasebj.2.10.2838362. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Thrombin stimulates the activities of multiple previously unidentified protein kinases in platelets. J Biol Chem. 1989 Dec 5;264(34):20723–20729. [PubMed] [Google Scholar]
- Force T., Kyriakis J. M., Avruch J., Bonventre J. V. Endothelin, vasopressin, and angiotensin II enhance tyrosine phosphorylation by protein kinase C-dependent and -independent pathways in glomerular mesangial cells. J Biol Chem. 1991 Apr 5;266(10):6650–6656. [PubMed] [Google Scholar]
- Gilmore T., Martin G. S. Phorbol ester and diacylglycerol induce protein phosphorylation at tyrosine. Nature. 1983 Dec 1;306(5942):487–490. doi: 10.1038/306487a0. [DOI] [PubMed] [Google Scholar]
- Golden A., Brugge J. S. Thrombin treatment induces rapid changes in tyrosine phosphorylation in platelets. Proc Natl Acad Sci U S A. 1989 Feb;86(3):901–905. doi: 10.1073/pnas.86.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Wang E., Johnson G., Huang C. K., Sha'afi R. I. Platelet-activating factor induces tyrosine phosphorylation in human neutrophils. J Biol Chem. 1991 Apr 5;266(10):6240–6245. [PubMed] [Google Scholar]
- Grandt R., Aktories K., Jakobs K. H. Evidence for two GTPases activated by thrombin in membranes of human platelets. Biochem J. 1986 Aug 1;237(3):669–674. doi: 10.1042/bj2370669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. K., Bonak V., Laramee G. R., Casnellie J. E. Protein tyrosine phosphorylation in rabbit peritoneal neutrophils. Biochem J. 1990 Jul 15;269(2):431–436. doi: 10.1042/bj2690431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckle W. R., Prokop C. A., Dy R. C., Herman B., Earp S. Angiotensin II stimulates protein-tyrosine phosphorylation in a calcium-dependent manner. Mol Cell Biol. 1990 Dec;10(12):6290–6298. doi: 10.1128/mcb.10.12.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K. Y., Jakubowski J. A., Wyss V. L., Angleton E. L. Minimal sequence requirement of thrombin receptor agonist peptide. Biochem Biophys Res Commun. 1992 Apr 30;184(2):790–796. doi: 10.1016/0006-291x(92)90659-9. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Protein kinase C mediates platelet-derived growth factor-induced tyrosine phosphorylation of p42. J Cell Biol. 1988 Apr;106(4):1395–1402. doi: 10.1083/jcb.106.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelvin D. J., Simard G., Sue-A-Quan A., Connolly J. A. Growth factors, signaling pathways, and the regulation of proliferation and differentiation in BC3H1 muscle cells. II. Two signaling pathways distinguished by pertussis toxin and a potential role for the ras oncogene. J Cell Biol. 1989 Jan;108(1):169–176. doi: 10.1083/jcb.108.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Song X. H. Bradykinin and bombesin rapidly stimulate tyrosine phosphorylation of a 120-kDa group of proteins in Swiss 3T3 cells. J Biol Chem. 1991 Apr 25;266(12):7746–7749. [PubMed] [Google Scholar]
- Meldrum E., Parker P. J., Carozzi A. The PtdIns-PLC superfamily and signal transduction. Biochim Biophys Acta. 1991 Mar 19;1092(1):49–71. doi: 10.1016/0167-4889(91)90177-y. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Receptor-mediated inhibition of adenylate cyclase and stimulation of arachidonic acid release in 3T3 fibroblasts. Selective susceptibility to islet-activating protein, pertussis toxin. J Biol Chem. 1985 Jun 25;260(12):7226–7233. [PubMed] [Google Scholar]
- Offermanns S., Seifert R., Metzger J. W., Jung G., Lieberknecht A., Schmidt U., Schultz G. Lipopeptides are effective stimulators of tyrosine phosphorylation in human myeloid cells. Biochem J. 1992 Mar 1;282(Pt 2):551–557. doi: 10.1042/bj2820551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Chambard J. C., L'Allemain G., Magnaldo I., Seuwen K. Transmembrane signalling pathways initiating cell growth in fibroblasts. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):427–436. doi: 10.1098/rstb.1988.0086. [DOI] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Sharp J. D., White D. L., Chiou X. G., Goodson T., Gamboa G. C., McClure D., Burgett S., Hoskins J., Skatrud P. L., Sportsman J. R. Molecular cloning and expression of human Ca(2+)-sensitive cytosolic phospholipase A2. J Biol Chem. 1991 Aug 15;266(23):14850–14853. [PubMed] [Google Scholar]
- Stone S. R., Hofsteenge J. Kinetics of the inhibition of thrombin by hirudin. Biochemistry. 1986 Aug 12;25(16):4622–4628. doi: 10.1021/bi00364a025. [DOI] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Yanagisawa M., Yamashita K., Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989 May 15;264(14):7856–7861. [PubMed] [Google Scholar]
- Tamaoki T. Use and specificity of staurosporine, UCN-01, and calphostin C as protein kinase inhibitors. Methods Enzymol. 1991;201:340–347. doi: 10.1016/0076-6879(91)01030-6. [DOI] [PubMed] [Google Scholar]
- Thompson N. T., Bonser R. W., Garland L. G. Receptor-coupled phospholipase D and its inhibition. Trends Pharmacol Sci. 1991 Nov;12(11):404–408. doi: 10.1016/0165-6147(91)90617-2. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Kawahara Y., Shii K., Koide M., Ishida Y., Yokoyama M. Vasoconstrictor-induced protein-tyrosine phosphorylation in cultured vascular smooth muscle cells. FEBS Lett. 1991 Jul 8;285(1):44–48. doi: 10.1016/0014-5793(91)80721-e. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Vila J., Weber M. J. Mitogen-stimulated tyrosine phosphorylation of a 42-kD cellular protein: evidence for a protein kinase-C requirement. J Cell Physiol. 1988 May;135(2):285–292. doi: 10.1002/jcp.1041350216. [DOI] [PubMed] [Google Scholar]
- Vostal J. G., Jackson W. L., Shulman N. R. Cytosolic and stored calcium antagonistically control tyrosine phosphorylation of specific platelet proteins. J Biol Chem. 1991 Sep 5;266(25):16911–16916. [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Wheaton V. I., Hung D. T., Charo I., Coughlin S. R. Domains specifying thrombin-receptor interaction. Nature. 1991 Oct 17;353(6345):674–677. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- Zachary I., Gil J., Lehmann W., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation in intact Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4577–4581. doi: 10.1073/pnas.88.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Woll P. J., Rozengurt E. A role for neuropeptides in the control of cell proliferation. Dev Biol. 1987 Dec;124(2):295–308. doi: 10.1016/0012-1606(87)90483-0. [DOI] [PubMed] [Google Scholar]





