Abstract
Recombinant avipox viruses are being widely evaluated as vaccines. To address how these viruses, which replicate poorly in mammalian cells, might be immunogenic, we studied how canarypox virus (ALVAC) interacts with primate antigen-presenting dendritic cells (DCs). When human and rhesus macaque monocyte-derived DCs were exposed to recombinant ALVAC, immature DCs were most susceptible to infection. However, many of the infected cells underwent apoptotic cell death, and dying infected cells were engulfed by uninfected DCs. Furthermore, a subset of DCs matured in the ALVAC-exposed DC cultures. DC maturation coincided with tumor necrosis factor alpha (TNF-α) secretion and was significantly blocked in the presence of anti-TNF-α antibodies. Interestingly, inhibition of apoptosis with a caspase 3 inhibitor also reduced some of the maturation induced by exposure to ALVAC. This indicates that both TNF-α and the presence of primarily apoptotic cells contributed to DC maturation. Therefore, infection of immature primate DCs with ALVAC results in apoptotic death of infected cells, which can be internalized by noninfected DCs driving DC maturation in the presence of the TNF-α secreted concomitantly by exposed cells. This suggests an important mechanism that may influence the immunogenicity of avipox virus vectors.
Recombinant avipox viruses, such as canarypox virus (ALVAC) or fowlpox virus, are of special interest in the field of vaccine development (reviewed in reference 38). These vectors are safe, since their replication is restricted to avian hosts. Even in immunocompromised mammalian hosts, systemic spread does not occur (57). In addition, they are large enough to be efficiently loaded with genes of different infectious or tumor agents. Finally, they can elicit immune responses in people previously exposed to vaccinia virus circumventing potential anti-vector immune responses in vaccinia virus-immune persons (10). Thus, avipox viruses provide a potentially useful vehicle to safely induce immune responses against a variety of antigens.
Since ALVAC has been shown to be more efficacious than a fowlpox virus-based vector, providing a comparable protective effect to that of a thymidine kinase-disrupted replication-competent vaccinia virus vector (58), many studies using different ALVAC constructs have been performed. ALVAC constructs encoding foreign proteins have been shown to induce humoral and cellular immune responses against a variety of infectious pathogens. These include rabies virus (19, 58), feline immunodeficiency virus (59), human immunodeficiency virus type (HIV-1) and HIV-2 (1, 4, 6, 15, 18, 35, 49), Japanese encephalitis virus (32, 43), rabbit hemorrhagic disease virus (17), and canine distemper virus (CDV) (39, 55), as well as tumor antigens (25, 34, 46). In fact, very recent studies revealed that mucosal application of ALVAC containing CDV genes induced high titers of neutralizing antibodies (Abs) and protection against a mucosal challenge with CDV (63). To potentially optimize the vaccine efficiency of ALVAC, we were particularly interested in how the virus imparts immunity in mammalian hosts without extensive replication, tissue damage, and subsequent spread of infection between cells.
Dendritic cells (DCs) are potent antigen-presenting cells central to immune activation and exist in an immature state at body surfaces and mucosal sites. Immature DCs can capture antigens such as extracellular and facultative intracellular bacteria, parasites, and dying cells (reviewed in references 5 and 54). This is followed by migration to the draining lymph nodes, where as mature DCs they present the antigen to lymphocytes. Furthermore, DCs express a variety of surface molecules necessary for the entry of viruses, such as CD4, chemokine receptors, for HIV and simian immunodeficiency virus (SIV), CD46 for measles virus (5), and DC-SIGN for the binding of HIV (22). Therefore, they can directly be infected by these and other viruses.
Since direct targeting of DCs with ALVAC might enhance immune responses against ALVAC-encoded antigens, we investigated the consequences of the interaction of ALVAC with DCs. While both immature and mature DCs were infected with ALVAC, immature DCs were more sensitive to infection. Many ALVAC-infected immature DCs rapidly underwent apoptotic cell death, and endocytosis of infected, dead or dying DCs by uninfected immature DCs was observed. Concurrently, a subpopulation of ALVAC-exposed DCs matured. Maturation was largely driven by the tumor necrosis factor alpha (TNF-α) secreted following exposure to ALVAC and partially by the ingestion of infected cell debris. These data suggest a mechanism for ALVAC-induced DC maturation, which involves apoptosis and TNF-α secretion, and this mechanism may augment the presentation of virus-encoded antigens and thereby have a significant impact on the efficiency of these viruses in vaccine development.
MATERIALS AND METHODS
Viral constructs.
All ALVAC constructs used in this study were engineered at Virogenetics, Troy, N.Y. Constructs include the strain vCP172, containing the sequence for the SIV gag (encoding for the SIV core protein p27) and pol (encoding for the viral reverse transcriptase and integrase), vCP180, which contains in addition to SIV gag-pol the gene for the viral envelope (env), vCP205 (HIV gag, protease, and env) and the parental strain (ALVAC). Heat-inactivated virus was obtained by incubating virus at 56°C for 30 min. Successful inactivation was confirmed by a lack of protein expression by DCs exposed to the heat-inactivated virus (data not shown). ALVAC was UV inactivated in 1 mM Tris in a 35-mm dish for 10 min with constant stirring. Inactivation was confirmed by performing a standard virus titration plaque assay.
Culture medium.
RPMI 1640 (Cellgro; Fisher Scientific, Springfield, N.J., or BioWhittaker, Walkersville, Md.), supplemented with 2 mM l-glutamine (GIBCO-BRL/Life Technologies [GIBCO-BRL], Grand Island, N.Y.), 50 μM 2-mercaptoethanol (Sigma Chemical Company, St. Louis, Mo.), 10 mM HEPES (GIBCO-BRL), penicillin (100 U/ml)-streptomycin (100 μg/ml) (GIBCO-BRL), and 1% human plasma (heparinized) or 10% heat-inactivated fetal calf serum (PAA Laboratories, Parker Ford, Pa.) screened negative for virus, mycoplasma, and below the detection limits for endotoxin.
Generation of human and macaque DCs.
Heparinized human blood was obtained from healthy donors or as leukopaks from the New York City Blood Bank or RH Labs, Baltimore, Md. Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation on Ficoll-Hypaque (Amersham Pharmacia Biotech AB, Uppsala, Sweden). T cells were removed by adhering 8 × 106 cells/well in a six-well tray (Falcon, Lincoln Park, N.Y.) for 1 to 2 h at 37°C. Nonadherent T cells were carefully washed away, and adherent monocytes were cultured for 6 to 7 days in 3 ml of medium containing 100 U of interleukin-4 (IL-4; R&D Systems, Minneapolis, Minn.) per ml and 1,000 U of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Immunex, Seattle, Wash.). In some experiments, immature human DCs were generated from a highly enriched population of CD14+ cells obtained by the magnetic bead selection of monocytes (Miltenyi Biotec, Auburn, Calif.). The cells were placed into tissue culture flasks or six-well plates at 106 cells/ml and cultured for 6 to 7 days in the presence of 100 to 1,000 U of IL-4 and 1,000 U of GM-CSF per ml.
Heparinized rhesus macaque (Macaca mulatta) blood was obtained from adult animals. All animals tested negative for Abs to SIV, type D retroviruses, and simian T-lymphotropic virus type 1, and were housed at the Tulane Regional Primate Research Center or the animal facilities at the Walter Reed Army Institute for Research. Animal care operations were in compliance with the regulations detailed under the animal welfare act, and in the Guide for the Care and Use of Laboratory Animals. PBMCs were separated by centrifugation on Ficoll-Hypaque. A total of 12 × 106 to 15 × 106 PBMCs were plated per well of a six-well tray and incubated for 1 to 2 h at 37°C. After careful removal of nonadherent cells (by washing with warm phosphate-buffered saline [PBS]), the adherent monocytes were cultured for 6 to 7 days in the presence of 100 U of IL-4 and 1,000 U of GM-CSF per ml. In some experiments, CD14+ monocytes were selected using the magnetic activated cell sorting (MACS) system (Miltenyi Biotec), plated at 106 cells/ml in six-well plates, and cultured in the presence of GM-CSF and IL-4 (see above).
For both human and monkey DCs, additional cytokines were added on days 2, 4, and 6. Immature DCs were obtained after the 6 to 7 days in culture with GM-CSF and IL-4. To generate mature DCs, 50% of the medium was substituted with monocyte-conditioned medium (MCM) on day 6 or 7, and the cells cultured for an additional 2 days. When immature and mature DCs were directly compared, immature DCs were cultured for the additional 2 days in the absence of MCM. MCM was generated using a slightly modified protocol from that previously described (45). Briefly, bacteriologic dishes (Falcon) were coated with 4 ml of a 100-μg/ml concentration of human gamma globulin (Bayer Corp., Elkhart, Ind.) in PBS and incubated for 10 min at room temperature before being washed four times with PBS. Then, 9 × 107 human PBMCs were plated in each dish and incubated for 1 h at 37°C. Nonadherent cells were removed, 8 ml of fresh medium was added, and the dishes were incubated for 24 h at 37°C. The 24-h cell-free supernatants were pooled, filtered, and frozen at −20°C before use.
The phenotype of the DCs was routinely monitored by two-color fluorescence-activated cell sorter (FACS) analysis on a FACScan flow cytometer (Becton Dickinson, Mountainview, Calif.). Fluorescein isothiocyanate (FITC)-conjugated monoclonal Abs (MAbs) against human major histocompatibility complex (MHC) class II (anti-HLA-DR-FITC) (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, Calif.) were used in combination with phycoerythrin (PE)-conjugated MAbs against human CD25 (BDIS), CD80 (BDIS), CD83 (Coulter Corp., Miami, Fla.), and CD86 (PharMingen, San Diego, Calif.). Immature DCs typically were HLA-DR++, CD86++, CD80+/low, CD83−/weak, and CD25−, while mature DCs expressed HLA-DR+++, CD86+++, CD25++, CD80++, and CD83++.
Infection of DCs with ALVAC.
DCs were counted and cultured at 105 large cells/well in 96-well round-bottom trays (Linbro). Either the original culture medium in which the DCs were generated (original medium) was reused without the addition of fresh cytokines or else fresh medium was prepared that contained 100 U of IL-4 and 1,000 U of GM-CSF per ml. DCs were infected with the indicated ALVAC construct at a multiplicity of infection (MOI) of 5 to 10. Where indicated, the cells were washed twice after 1 h at 37°C before being cultured for up to 4 days. When mature and immature DCs were compared, both DC preparations were resuspended in original medium. The DC phenotype was monitored by FACS analysis prior to and at various times after exposure to ALVAC. Uninfected control DCs were included and analyzed for comparison.
Alternatively, 2 × 106 to 5 × 106 immature human DCs were resuspended in 0.1 ml of culture medium in 15-ml conical tubes and incubated with vCP205 (MOI of 0.2 to 10) for 2 h at 37°C. Cells were washed and placed at 106 cells/ml in a 24-well plate (Costar; Corning, Inc., Corning, N.Y.). After 24 h, cell supernatants were collected and stored at −70°C for subsequent cytokine analysis. At the same time, DCs were harvested and counted and the viability was monitored by trypan blue staining.
In some experiments, ALVAC-infected or uninfected immature DCs (1 × 105 to 2 × 105) were placed into the wells of a 96-well culture tray (Millipore Multiscreen Filtration System, MAMCS9610). The upper filtration plate (Millipore MACMS4510) was positioned onto the culture tray, and uninfected DCs in cytokine-containing medium were then placed at various concentrations into the upper wells. The plates were cultured for up to 4 days at 37°C, and the DCs were collected from the upper (uninfected cells) and lower (infected versus uninfected cells) chambers at specific time points for FACS analysis.
Identification of ALVAC infection.
In order to identify recombinant ALVAC-infected cells, cells were stained intracellularly using a polyclonal rabbit anti-canarypox virus serum (Virogenetics) at a 1:10,000 dilution. Cells were washed twice and fixed in 4% paraformaldehyde-PBS (wt/vol) for 10 min at 4°C. After two washes, cells were incubated in 1% saponin (Sigma) for 30 min at 4°C, washed twice with PBS, and resuspended in 100 μl of 0.1% saponin containing the diluted rabbit serum. After 30 min of incubation at 4°C, the cells were washed twice with 0.1% saponin, resuspended in 100 μl of 0.1% saponin containing an FITC-conjugated donkey anti-rabbit immunoglobulin (Jackson ImmunoResearch Laboratories, West Grove, Pa.) at a 1:200 dilution, and incubated for 30 min at 4°C. Finally, cells were washed twice and monitored by FACS. Background staining was monitored on uninfected cells from the same donor and on cells incubated with normal rabbit immunoglobulin.
To detect expression of SIV proteins in infected cells, the viral constructs vCP172 (SIV gag and pol) or vCP180 (SIV gag, pol, and env) were used. Using the anti-SIV p27 MAb 55-2F12 (NIH AIDS Research and Reference Reagent Program), infection was monitored either by immunoperoxidase staining on cytospin preparations as described previously (26) or by intracellular FACS staining (see above). Here, biotinylated anti-SIV p27 MAb was used at a 1:400 dilution, and Fluorescein (DTAF)-conjugated streptavidin (Jackson ImmunoResearch) was used at 1:200.
To detect HIV p24, infected DCs were fixed and permeabilized using a 2% formaldehyde–0.1% saponin buffer. After a 20-min incubation, the cells were washed in ice-cold PBS–0.1% saponin. The cells were then incubated with a murine anti-p24 MAb (Dako, Carpinteria, Calif.) or the isotype control (Southern Biotechnology Associates, Birmingham, Ala.) at a 1:10 dilution followed by a PE-conjugated goat anti-mouse immunoglobulin (Southern Biotechnology Associates) at 1:200.
Apoptosis assays.
Apoptotic cells were identified using the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) technique, and assays were performed according to the manufacturer's guidelines (Boehringer Mannheim and Roche Molecular Biochemicals, Indianapolis, Ind.). In brief, cells were harvested at different time points after infection, washed twice with PBS in a 96-well V-bottomed tray (Linbro), and fixed in 4% paraformaldehyde–PBS (wt/vol) for 30 min at room temperature. After centrifugation (2,000 rpm for 2 to 3 min), cells were washed once with 200 μl of PBS, resuspended in 100 μl of permeabilization solution (0.1% Triton X-100 in 0.1 sodium citrate), and incubated for 2 min at 4°C. The cells were washed twice (2,000 rpm for 2 to 3 min) using cold PBS, resuspended in 50 μl of TUNEL reaction or control mixture, and incubated for 60 min at 37°C in 5% CO2. Cells were washed twice in PBS and analyzed by FACS.
Fluorescent labeling of DCs.
DCs were stained with the green fluorescent dye, 5-chloromethylfluorescein diacetate (CMFDA), or the red fluorescent dye, 5-(and-6)-{[(4-chloromethyl) benzoyl]amino}tetramethylrhodamine (CMTMR) (Molecular Probes, Eugene, Oreg.) according to the manufacturers' instructions. Cells were placed into a 1.5-ml Eppendorf tube, centrifuged, and resuspended in 100 μl of prewarmed (37°C) probe-containing (5 μM) RPMI. After 15 min at 37°C, the DCs were centrifuged, resuspended in prewarmed medium, and incubated for an additional 30 min at 37°C. The cells were centrifuged once more and then counted prior to use.
Confocal microscopy.
CMTMR (red)-stained infected DCs and CMFDA (green)-stained, uninfected DCs were cocultured at a ratio of 1:1 for 2 to 3 days. After culture, the cells were seeded in serum-free RPMI into alcian blue (Sigma) coated Lab Tek tissue culture chambers (Nunc, Naperville, Ill.) (27) at a minimum number of 104 cells/chamber. The chambers were incubated for 1 h at 37°C, the medium was aspirated, and the adhered cells were immediately fixed with 4% paraformaldehyde–PBS (wt/vol) for 20 min at room temperature. Specimens were examined by confocal laser scan microscopy (Zeiss) using adequate filter settings for fluorescein-rhodamine excitation and emission, respectively. Optical sections were approximately 0.75 μm thick, and images were overlaid using the microscope software provided by Zeiss.
Inhibition of apoptosis.
Apoptosis of infected cells was inhibited using the Caspase-3/CPP32 inhibitor Z-DEVD-FMK (Kamiya Biomedical Company, Seattle, Wash.) (28). Stock solutions (100 mM) of Z-DEVD-FMK in dimethyl sulfoxide (DMSO) were stored at 4°C. DCs were preincubated with Z-DEVD-FMK at a concentration of 250 or 500 μM for 30 min at 37°C in 5% CO2 before infection with recombinant ALVAC. To ensure that the drug did not interfere with DC maturation per se, a separate aliquot of DCs was pretreated with Z-DEVD-FMK prior to addition of MCM as the maturation stimulus.
TNF-α assays.
Cell supernatants were collected from ALVAC-exposed and unexposed DC cultures after 1 to 4 days of incubation and stored at −20°C. TNF-α concentrations were measured using commercially available ELISAs specific for human (R&D Systems) or monkey (U-Cytech, Utrecht, The Netherlands) TNF-α.
In some experiments, infected DCs were cultured in the presence of 20 μg of neutralizing anti-human TNF-α Abs (R&D Systems) or control immunoglobulin G1 (IgG1; PharMingen) per ml. Cell supernatants were collected 1 day after infection and analyzed for the presence of TNF-α. The DC phenotype was determined at days 1 and 3 by FACS analysis. Cells cultured for 3 to 4 days had additional Abs added at day 2 of cultures.
RESULTS
Infection of primate immature and mature DCs by recombinant ALVAC.
To determine whether DCs could be infected with ALVAC and to monitor the degree of infection, we chose the construct vCP172. This enabled us to monitor expression of both ALVAC and SIV antigens in the exposed DC populations. Mature and immature DCs were infected with vCP172 (MOI of 10). At multiple time points after infection, cells were stained intracellularly with either a polyclonal rabbit anti- ALVAC serum or the anti-SIVp27 MAb. Table 1 demonstrates the kinetics of infection obtained in a representative experiment using human DCs. Immature DCs exhibited a higher frequency of infection compared to mature DCs. A considerable percentage, but not all infected DCs (ALVAC positive), also expressed the protein encoded by the SIV gag gene in the viral construct. In subsequent experiments infections were mostly monitored at single time points. However, both human and rhesus macaque immature DCs always expressed ALVAC-specific proteins to a notably higher percentage (about two- to fourfold) than mature DCs, and numbers of ALVAC-positive cells were higher than those of p27-positive DCs (when infections using vCP172 or vCP180 were analyzed). This might simply reflect differences in the sensitivity of the Abs or that not all vCP172-infected, ALVAC-positive cells expressed SIV p27. It was unlikely that the ALVAC positivity reflected virus bound to the cell surface, since cells not permeabilized prior to staining were negative (data not shown).
TABLE 1.
Frequency of canarypox virus-infected immature and mature human DCs
| DC | Frequencya at (time postinfection [h]):
|
|||||
|---|---|---|---|---|---|---|
| 6 | 12 | 18 | 24 | 36 | 48 | |
| Mature | 8/0 | 19/3 | 33/4 | 15/3 | 14/4 | 11/2 |
| Immature | 28/14 | 42/34 | 64/41 | 43/30 | 48/12 | 48/8 |
Immature or mature DCs were infected with vCP172 (MOI of 10) and harvested at various time points after infection. Intracellular FACS staining using a polyclonal rabbit anti-canarypox virus serum and a MAb against SIV p27 was used to detect ALVAC-positive and p27-positive cells. The data are presented as the percent ALVAC-positive cells/percent p27-positive cells above background control staining.
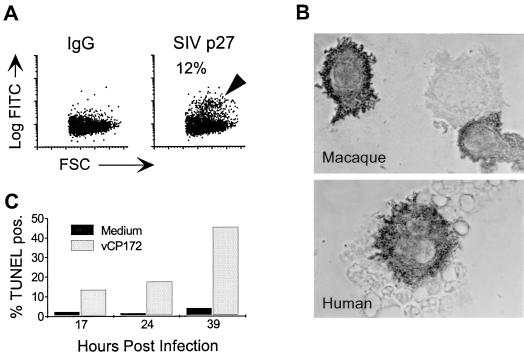
Immature and mature rhesus macaque DCs were also infected by recombinant ALVAC (Fig. 1). While immature DCs were more susceptible to infection, as seen with human DCs, the average numbers of infected macaque cells were as much as twofold lower than those detected in human DCs. Immunoperoxidase staining of cytospins for p27 expression identified SIV p27-positive DCs within infected rhesus macaque and human DCs.
FIG. 1.
ALVAC infection of immature DCs and subsequent apoptotic cell death. (A and B) Immature DCs were infected with vCP172 (MOI of 10). At 24 h after infection, p27 expression was monitored by either intracellular FACS staining (A, rhesus macaque) or by immunoperoxidase staining (dark reaction product) on cytospins (B; magnification, ×1,000). The log fluorescent intensity of FITC (Log FITC) is shown on the y axes, and the forward light scatter (FSC) is shown on the x axes. The percentages of SIV-p27-positive cells (arrowhead), above IgG control, are shown in panel A. These data are representative of more than five experiments. (C) vCP172-infected versus uninfected (Medium) immature human DCs were analyzed for the presence of apoptotic cells using a TUNEL FACS assay at the indicated times of culture. The percent TUNEL-positive cells at each time point of one representative experiment of three are shown.
When vCP172-infected immature DCs were cultured for 3 to 4 days, considerable cell death was observed. On average, uninfected cultures yielded approximately twice the numbers of viable cells on day 4 compared to infected cultures. This was further supported by the reduced forward scatter (size) and increased side scatter (granularity) of infected DCs that was evident upon FACS analysis. To investigate the mechanism of cell death, infected and uninfected DCs were harvested at multiple time points and FACS analysis was performed to detect apoptotic, TUNEL-positive cells. Figure 1C shows the kinetics of the appearance of TUNEL-positive cells after infection with ALVAC. These studies demonstrate a rapid increase in the numbers of apoptotic cells shortly after infection of immature DCs with recombinant ALVAC.
Infected DCs are phagocytosed by uninfected DCs.
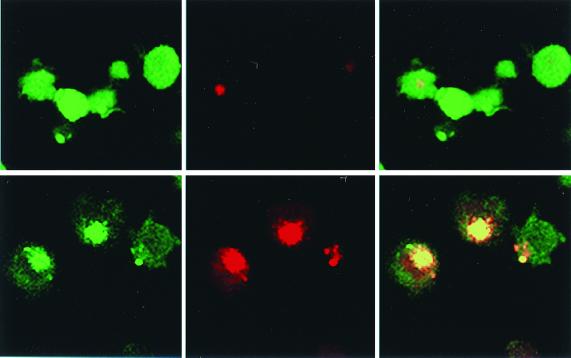
DCs have been shown to take up dead or dying cells (2, 3). Therefore, we examined whether the cell fragments from infected dying ALVAC-infected cells could be phagocytosed by uninfected DCs. CMFDA (green)-stained, uninfected DCs were cultured with CMTMR (red)-stained infected DCs for 3 days. FACS analysis of the cocultures revealed a significant proportion of green-red double-positive cells, as well as single green large cells in contrast to control cultures of uninfected red and uninfected green DC mixtures (data not shown). Confocal microscopic analysis of FACS-sorted double-positive or single-positive green fractions revealed that the double-positive fraction comprised mainly green (uninfected) cells with large red inclusions (Fig. 2, lower panels). While also many single-positive green cells contained red inclusions (Fig. 2, upper panels), they were considerably smaller than those detected in the double-positive fraction. Internalization of green, uninfected cells by red, infected DCs was not observed. Therefore, these results suggest that infected cells dying by apoptosis (Fig. 1C) were phagocytosed by uninfected DCs.
FIG. 2.
Phagocytosis of ALVAC-infected cells by immature, uninfected DCs. Green-stained, uninfected DCs were cocultured with red-stained, infected DCs. After 3 days, cells were sorted into single-positive green cells (top) or double-positive red-green cells (bottom). The cell populations were further analyzed using confocal laser scan microscopy. Demonstrated are images yielded with filter settings for fluorescein only (left), rhodamine only (middle), and with both images overlaid (right) (magnification, ×1,000).
DC maturation following ALVAC infection.
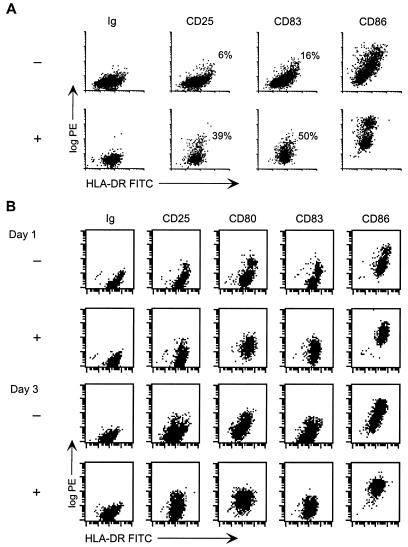
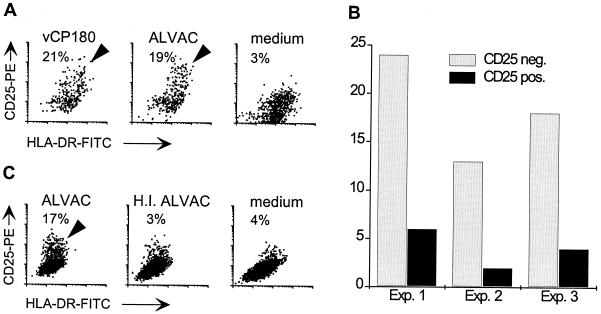
When cultures of ALVAC-infected immature DCs were observed more closely a second phenomenon was appreciated. Coincident with the cell death induced by exposure to ALVAC (Fig. 1C), there was a modulation of the DC phenotype (Fig. 3). Compared to the uninfected controls, increased expression of CD25, CD80, CD83, and CD86 was readily detected in at least a subset of cells after 3 to 4 days. In both human and monkey, the percentage of canarypox virus-exposed DCs expressing CD25 varied between donors and ranged between 5 and 35%. Uninfected control DCs sometimes contained a subset of spontaneously matured DCs, but this was always considerably smaller than that observed in ALVAC-infected cultures.
FIG. 3.
Maturation of DCs in vCP172-infected cultures. (A) Immature human DCs were infected with vCP172 (MOI of 10). Infected (+) and uninfected (−) DCs were cultured for 4 days. After culture, the cells were harvested and monitored for the expression of CD25-, CD83-, and CD86-PE (log PE y axes) versus HLA-DR–FITC (x axes). The percentage of large cells expressing CD25 and CD83 above the isotype control are indicated. (B) Immature rhesus macaque DCs were infected with vCP172 (+) (MOI of 10) or not (−) and cultured for 1 to 3 days before being harvested and analyzed. FACS analysis was performed on cells stained with FITC–anti-HLA-DR versus PE-immunoglobulin, -anti-CD25, -CD80, -CD83, or -CD86. Similar data were obtained from more than five experiments with human DCs and three different monkey donors.
More detailed examination of infected human and rhesus macaque immature DCs revealed that maturation could be detected within 1 day of exposure to the virus (Fig. 3B; see also Fig. 8B). Figure 3B illustrates the phenotypic changes detected in a rhesus macaque DC preparation pulsed with vCP172 1 to 3 days earlier. As early as 1 day postexposure, the levels of CD25, CD80, CD83, and CD86 had increased, and this was still evident at day 3. Although maturation began by day 1 after infection, the expression of maturation markers, particularly CD25, often increased over the next 2 to 3 days.
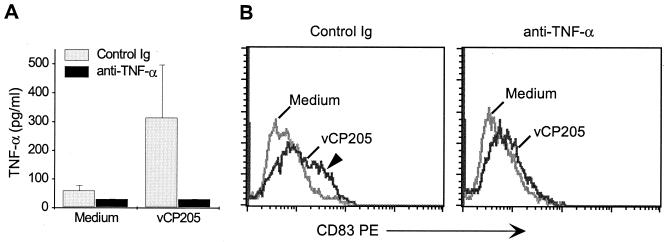
FIG. 8.
Canarypox virus-induced TNF-α production facilitates DC maturation. Immature human DCs were infected or not (Medium) with vCP205 (MOI of 5) and then incubated in the presence of a blocking anti-human TNF-α Ab or the control isotype immunoglobulin (20 μg/ml). (A) After 18 h the amount of TNF-α secreted into the supernatants was measured by enzyme-linked immunosorbent assay. (B) DCs were also collected at this time and stained for CD83 expression to monitor DC maturation. The level of CD83 expression (x axes) is shown for each of the populations. Medium-treated DCs are shown by a light gray line, and vCP205 is represented with a dark gray line. The CD83-positive subset seen in the immunoglobulin-treated, vCP205-pulsed DCs is indicated with an arrowhead. This subset is lost following anti-TNF-α treatment. The results represent one of three experiments.
To verify that it was the canarypox virus and not the foreign introduced genes driving maturation, immature DCs were infected with vCP180 (containing SIV gag, pol, and env) or with the parental strain not containing SIV-genes (ALVAC). Figure 4A shows that in both cultures a similar subset of DCs demonstrated increased CD25 expression (21 and 19%, respectively). In numerous experiments, different ALVAC constructs containing or lacking various viral genes were used, and maturation was observed with all constructs (data not shown). Hence, canarypox virus itself mediates induction of DC maturation and not by foreign genes (or the proteins encoded by those genes) that have been engineered into the vectors.
FIG. 4.
Induction of maturation is stimulated by viable canarypox virus. (A) Immature human DCs were infected with an MOI of 10 of either vCP180 or the parental strain (ALVAC) or left uninfected (medium). CD25 surface expression by large HLA-DR-positive cells was assessed 4 days after infection (CD25-PE, y axes; HLA-DR–FITC, x axes). The percentage of CD25-positive cells (above isotype control) are indicated in each panel (highlighted by arrowheads). (B) Immature human DCs that had been infected with vCP180 3 to 4 days earlier were sorted into CD25-negative (CD25 neg.) and CD25-positive (CD25 pos.) fractions. Each fraction was then immunostained for intracellular expression of p27 and analyzed by FACS. The percentage of SIV p27-positive cells, above the immunoglobulin control, is shown for each subset. (C) Immature DCs (human) were infected with live (ALVAC) or heat-inactivated (H.I.) ALVAC or left untreated (medium). After 4 days the DCs were examined for CD25 expression by FACS. The percentages of CD25-positive large cells (compared to the isotype control) are shown in each panel. The CD25-positive subset is highlighted by an arrowhead.
In an attempt to further characterize the maturing subset within ALVAC-exposed DC cultures, CD25-positive mature DCs were separated from CD25-negative immature DCs by cell sorting 3 to 4 days after infection with vCP180. Each subset was then analyzed for the expression of SIV p27 by intracellular FACS staining. Considerably fewer p27-positive cells were detected in the CD25-positive fraction compared to CD25-negative cells (Fig. 4B). Hence, viral infection or gene expression itself did not seem to be directly responsible for maturing the DCs. However, this phenomenon was dependent on viable virus. Heat inactivation of virus abrogated its ability to induce both cell death and maturation (Fig. 4C). To exclude the possibility that heat treatment inactivated a temperature-sensitive component of the virus stock that was inducing cell death and/or the maturation effect, UV-inactivated virus was also applied to DCs. As with heat-inactivated virus, UV-inactivated ALVAC did not induce DC maturation (data not shown).
Inhibition of apoptosis partially interferes with maturation.
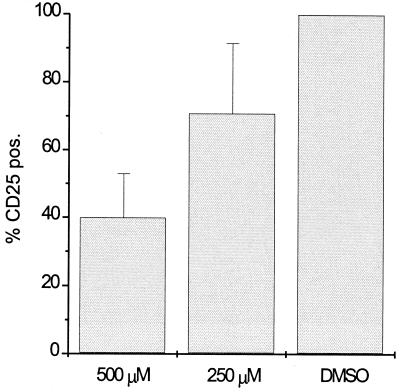
To investigate whether the induction of apoptotic cell death was a prerequisite for DC maturation, DCs were treated with the caspase-3 inhibitor Z-DEVD-FMK. Caspase-3 is believed to be involved in all pathways of apoptotic cell death (37). Immature DCs were pretreated with 250 or 500 μM Z-DEVD-FMK for 30 min. ALVAC was then added to the wells, and cultures were analyzed 4 days later for CD25 expression as a marker for mature DCs. Figure 5 shows that significantly less CD25 expression was detected in the presence of Z-DEVD-FMK (but not the diluent control). The addition of Z-DEVD-FMK to MCM-treated immature DCs did not interfere with MCM-induced maturation (data not shown). Z-DEVD-FMK did not modify the expression of ALVAC-specific proteins in exposed cultures as determined by intracellular FACS staining (data not shown). When apoptosis was monitored in ALVAC-exposed cultures in the presence of Z-DEVD-FMK, the percentage of TUNEL-positive cells was reduced by approximately 70% (data not shown), while higher drug concentrations repeatedly lead to drug-induced cell death. Thus, blocking apoptotic cell death in the ALVAC-infected cultures reduced the amount of DC maturation, indicating that the presence of primarily apoptotic cells rather than a minor population of primarily necrotic DCs drove DC maturation, at least partially.
FIG. 5.
Inhibition of maturation of ALVAC-infected DCs by the addition of a caspase 3 inhibitor. A total of 250 or 500 μM Z-DEVD-FMK, or the equivalent dilution of DMSO diluent for the high dose (DMSO), were added to immature DCs 30 min before infection with ALVAC. Inhibition of maturation was assessed by the lack of CD25 expression 4 days after infection using the DMSO-treated cells as the 100% matured population. The data represent the mean percentages of CD25-expression (% CD25 pos.) of three experiments.
TNF-α secreted by ALVAC-exposed DCs facilitates DC maturation.
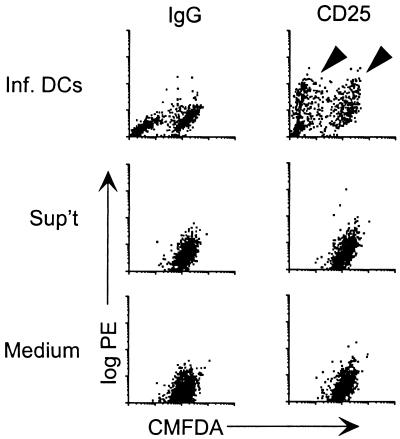
The previous findings suggested that apoptosis contributed to maturation. However, even high doses of a caspase-3 inhibitor did not completely reverse the maturation effect of ALVAC infection (Fig. 5). The evidence that a soluble factor released by ALVAC-pulsed DCs might contribute to the maturation of healthy DCs was initially obtained using two different strategies. First, immature DCs were infected with ALVAC, free virus was washed off, and the infected cells were added to uninfected, immature DCs, which had been previously stained green using CMFDA. After 4 days, DC cultures were monitored for maturation (CD25-PE staining) in the infected (CMFDA-negative) and uninfected (CMFDA-positive) populations. Figure 6, upper right, shows that similar maturation (CD25 expression) was detected in both infected (CMFDA-negative) and uninfected (CMFDA-positive) DCs. The addition of supernatant, middle panel, from infected cells harvested directly after washing out the virus did not change the phenotype of uninfected cells. Thus, the maturation effect was dependent on the presence of infected cells, and residual cell-free virus could be excluded as a mediator.
FIG. 6.
Maturation of uninfected DCs by ALVAC-infected immature DCs. Immature DCs were infected with ALVAC, and free virus was washed out. Uninfected immature DCs that had been stained with the green fluorescent dye CMFDA were added to the infected (unstained) DCs at a ratio of 1:1 (Inf. DCs). As controls, the supernatant from infected cells (collected directly after washing off the virus) was added to CMFDA-stained cells (Sup't) or the green-uninfected cells were kept in medium (Medium). Maturation (highlighted by arrowheads) was assessed by CD25 expression 4 days later. The log PE is expressed on the y axes (CD25 versus the IgG control), and the CMFDA fluorescence intensity is expressed on the x axes. The results from one of two similar experiments are provided.
A second approach also suggested that soluble products produced by ALVAC-exposed DCs were able to promote DC maturation. Immature DCs cultured on the other side of a porous membrane were induced to mature only when ALVAC-infected DCs were present in the opposing chamber (data not shown). Hence, the infected population released a factor(s) that was able to induce maturation of the uninfected DCs.
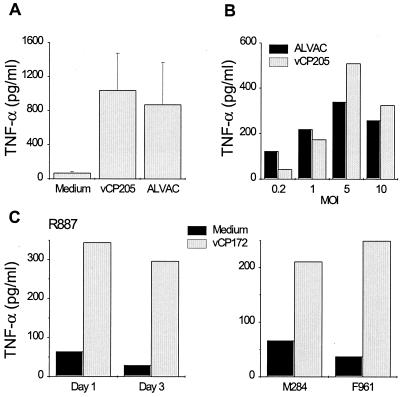
TNF-α is secreted in response to viral infections (44), as well as being able to induce DC maturation in vitro (5). Consequently, the presence of TNF-α in the ALVAC-infected DC cultures was investigated. Cell supernatants were collected from infected and uninfected DC cultures 1 to 3 days following exposure to ALVAC. Significant TNF-α production occurred within 1 day of exposure of immature human DCs to canarypox virus and was dependent on the infectious dose used to infect the DCs but was not influenced by the presence (or absence) of the foreign genes in the construct (Fig. 7A and B). Similar TNF-α secretion was detected in vCP172-infected rhesus macaque DCs (Fig. 7C). Interestingly, inclusion of neutralizing anti-human TNF-α Abs in the infected human DC cultures resulted in diminished TNF-α detection and prevented much of the DC maturation measured by CD83 expression within a day of infection (Fig. 8). Partial block of DC maturation was also observed at day 3 (data not shown). Therefore, TNF-α secreted by immature DCs in response to infection with ALVAC contributed significantly to maturation of uninfected DCs.
FIG. 7.
TNF-α secretion by canarypox virus-infected primate DCs. (A) Immature human DCs were infected with either ALVAC or vCP205 (MOI of 5) or left uninfected (Medium). Cell supernatants were collected after 24 h and assessed for the presence of TNF-α. The mean TNF-α production from three separate experiments using different donors is shown. (B) Immature human DCs were infected with ALVAC or vCP205 at the indicated MOIs, and the TNF-α production was measured 24 h later. (C) Immature rhesus macaque DCs were pulsed with vCP172 (MOI of 10) or not (Medium) and cultured for 1 to 3 days. The TNF-α produced after 1 and 3 days is shown for animal R887 and after 1 day for animals M284 and F961.
DISCUSSION
In recent years there has been an immense increase in the number of publications investigating the interaction of DCs with various viruses. These encompass measles virus (20, 29, 30, 51, 52, 60), vaccinia virus (14, 16), herpes simplex virus (47), polyomavirus (13), and most extensively the immunodeficiency viruses HIV and SIV (7, 9, 23, 26, 31, 41, 42, 62, 65). While DCs may play a role in the pathogenesis of these infections, they are also likely to be critical in activating virus-specific immune responses. Recombinant avipox virus constructs have proven to be both immunogenic and safe in humans (6, 15, 19, 32, 34, 49) and are, therefore, of great interest in vaccine research. One study was published on the use of recombinant fowlpox virus for the transfer of genes to DCs (8). However, little detail has been placed on understanding the interactions between DCs and potential viral vectors, nor on what influence such infections could impart on normal DC function. In the present study, we investigated the impact of direct in vitro infection of ex vivo generated DCs with ALVAC in order to determine how to possibly enhance their immunogenicity for applications in future vaccine development.
Our studies confirmed that significant numbers of immature human and rhesus macaque DCs could be infected with ALVAC (Table 1 and Fig. 1A and B). This was comparable to the number of immature DCs that can be infected by fowlpox virus constructs (8). However, monitoring the infected DCs over the next few days revealed that considerable cell death was detected in cultures of ALVAC-infected immature human and macaque DCs. The major mechanism of death in ALVAC-infected cultures was primarily via apoptosis (Fig. 1C); however, trypan blue staining also showed that many cells underwent further secondary necrosis (data not shown). On the other hand, mature DCs seemed to be more resistant to both viral infection and ALVAC-induced cell death. This finding was intriguing, especially since the virus was interacting with a nonpermissive host (mammalian) cells. Furthermore, similar cytopathic effects were not reported for fowlpox virus-infected DCs (8). Interestingly, it has recently become clear that various DNA and RNA viruses can inhibit apoptosis of infected cells. Such antiapoptotic genes have been reported for various poxviruses such as vaccinia virus, myxoma virus, and tanapoxvirus (12, 24), but not for ALVAC. Our results suggest that the cytopathic effects of ALVAC did not require the completion of the full virus life cycle, since an abortive infection, as seen in mammalian cells, was effective in damaging infected immature primate DCs. In contrast, Taylor et al. have reported (57) no serious side effects after ALVAC infection of various mammalian cell lines in vitro or following infection of laboratory animals. Notably, when rabbits or squirrel monkeys were injected with higher doses of ALVAC via the intradermal (i.d.) route, temporary poxvirus-like skin lesions developed. Furthermore, virus was recovered from the injection site for up to 4 days postinjection. This could reflect a similar phenomenon as we observed in our primary DC system. Considering the high density of immature DCs in the dermis, DCs may have been infected with the i.d. injected ALVAC, resulting in DC death thereby contributing to lesion formation.
When infected “red” DCs were cocultured with uninfected “green” DCs, large red inclusions were detected in the sorted red-green double-positive fraction (Fig. 2). Single green cells contained (at most) low numbers of much smaller red inclusions. This suggested that healthy, uninfected DCs had phagocytosed infected, dying DCs. It is important to note that the double-positive cells showed an intracellular colocalization of green and red fluorescence, while the rest of the cell surface seemed rather weakly stained with the green dye. This most likely indicates that a significant proportion of green-stained cell membrane of the uninfected DCs was internalized during the process of phagocytosis. The internalization of the engulfing cells' membrane with the captured red-labeled infected DC debris would result in their colocalization within the cell. Because extensive areas of the residual membrane of the uninfected cells would have to be renewed, the green staining of the rest of the cell appears fainter as a result. The mechanism via which the infected DC debris was internalized is not known. However, one receptor, the αvβ5 integrin is primarily expressed by immature DCs and has been shown to be involved in the uptake of apoptotic cells by immature DCs (2).
Further analysis revealed that ALVAC infection of primate DCs lead to the expression of a more mature DC phenotype, i.e., increased levels of CD25, CD80, CD83, and CD86 (Fig. 3), as well as another DC maturation marker, DC-LAMP (11) (data not shown). While CD80, CD83, and CD86 often appeared to be upregulated on the entire DC population, CD25 expression was restricted to a subset of DCs. Recent studies indicated that the expression of CD25 correlated with a fully differentiated mature DC phenotype, as monitored by the stabilization of MHC-peptide complexes on the DC surface (E. Kampgen, 6th International DC Meeting, May 2000). Therefore, this suggests that at least a subset of DCs was induced to completely mature following infection with ALVAC, while the bulk populations appeared to be somewhat activated. Fewer infected cells were actually detected in the mature CD25-positive fraction compared to the CD25-negative and CD25-weak subset (Fig. 4B). This suggests that infection per se was not directly causing DC maturation in the cultures. The maturation effects were solely mediated by the virus and not influenced by the presence or absence of foreign genes (Fig. 4A). Potential contaminants within the virus stocks that could induce maturation, such as lipopolysaccharide (5) or mycoplasma (48) were ruled out using the limulus (BioWhittaker, Walkersville, Md.) and mycoplasma detection (Gen-Probe Rapid Detection System; Fisher Scientific, Pittsburgh, Pa.) assays (data not shown).
To clarify whether the presence of apoptotic cells contributed to DC maturation, we treated the infected cultures with an inhibitor of apoptosis. Caspases have been found to be the major molecules whose activation leads to apoptotic cell death (37). Two main pathways have been described. One involves caspase-8, which is mainly activated after signaling through receptors belonging to the Fas/TNF-receptor family. In contrast, the cytochrome c/Apaf-1 pathway requires caspase-9. Both pathways ultimately activate caspase-3 as the “effector caspase.” Therefore, we targeted caspase-3 with the inhibitor Z-DEVD-FMK, which has been shown to block apoptosis in vitro (28). In the presence of the caspase 3 inhibitor, there was a significantly lower number of DCs induced to express CD25 following ALVAC exposure (Fig. 5). Hence, induction of apoptosis as a result of ALVAC infection contributed significantly to maturation of DCs within these cultures. To date, only the uptake of necrotic but not primarily apoptotic cells has been shown to induce DC maturation (21, 50). The contradictions may reflect differences in the way that the cells were induced to die and, more importantly, that virus-induced cell death might be very different to freeze-thaw-induced death. Of note, influenza virus-infected apoptotic monocytes did also fail to induce DC maturation in those experiments (50). This could be due to the use of different virus strains in that study as opposed to our study. Furthermore, as mentioned above, apoptotic ALVAC-infected DCs subsequently underwent secondary necrosis, and this also could influence the outcome.
Not all the maturation effect of ALVAC infection was blocked in the above assays, suggesting either that the inhibition of apoptosis was incomplete, or that there were additional apoptosis-independent mechanisms inducing DC maturation. Subsequent examination revealed that ALVAC-mediated TNF-α secretion by immature DCs was a significant player in DC maturation, especially during the early stages (Fig. 7 and 8). Signaling through the TNF receptor family represents one of the more potent stimuli for DC maturation in vitro and in vivo resulting in NF-κB activation (5). Interestingly, supernatants from necrotic cell lines have recently been reported to also induce DC maturation; however, these supernatants did not contain TNF-α (50). Again, this implies that there might be critical differences in the manner of DC maturation induced by artificially “killed” cells and virus-mediated events. While the addition of anti-TNF Abs, which neutralize both secreted and membrane-bound TNF-α to the ALVAC-infected DCs limited DC maturation, the effect was not 100% (Fig. 8). Therefore, our data suggest that both TNF-α secretion and apoptotic cell death induced by ALVAC infection contribute significantly to the observed DC maturation. It is possible that other candidate cytokines such as alpha interferon (IFN-α) may be involved. IFN-α has been shown to act synergistically with TNF-α on DC maturation (33, 45). In addition, virus-infected cells, including immature DCs (40, 56), secrete IFN-α. Therefore, we cannot exclude that trace amounts of this and other factors may also be at work here.
How do our findings relate to how ALVAC behaves in vivo? ALVAC-infected DCs may act directly and present antigens in vivo. However, it has recently been shown that if a virus does not target the professional antigen-presenting cells, the activation of antiviral cytotoxic T cells requires presentation of exogenous antigen on MHC class I molecules via the alternative pathway by bone-marrow-derived cells (53). Additional studies demonstrated that bystander DCs are able to present bacterial antigens derived from apoptotic Salmonella-infected macrophages in the context of both MHC class I and class II molecules (64). Therefore, cross-presentation of viral antigens by DCs which have matured after engulfing dying infected cells could contribute to the immunogenicity of ALVAC in mammals. The concomitantly secreted TNF-α could also induce migration of ALVAC-loaded DCs to the draining lymph nodes (61) and thereby facilitate immune stimulation. This could be particularly important when administering ALVAC via the i.d. route. The skin comprises numerous immature DCs in the epidermis (Langerhans cells) and dermis (dermal DCs), which would provide suitable targets for injected ALVAC. Of note, the i.d. route is the same route used to eradicate smallpox using a very similar virus and possibly via similar mechanisms, since vaccinia virus could be detected in Langerhans cells at the sites of inoculation (36). In vivo presentation of ALVAC-borne antigens by DCs is currently under investigation.
ACKNOWLEDGMENTS
We thank Agegnehu Gettie (Aaron Diamond AIDS Research Center and Tulane Regional Primate Research Center) and Mark Lewis (Southern Research Institute) for providing rhesus macaque blood samples; Judy Adams for help with graphics; and Heidi Cleven, Mark Louder, John Mealy, and David Schaer for expert technical assistance.
This work was supported by the Dorothy Schiff Foundation, the Irma T. Hirschl Trust, and NIH grants AI 40877 and AI 44335 (to M.P.).
REFERENCES
- 1.Abimiku A G, Franchini G, Tartaglia J, Aldrich K, Myagkikh M, Markham P D, Chong P, Klein M, Kieny M P, Paoletti E, et al. HIV-1 recombinant poxvirus vaccine induces cross-protection against HIV-2 challenge in rhesus macaques. Nat Med. 1995;1:321–329. doi: 10.1038/nm0495-321. [DOI] [PubMed] [Google Scholar]
- 2.Albert M L, Pearce S F A, Francisco L M, Sauter B, Roy P, Silverstein R L, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 4.Andersson S, Makitalo B, Thorstensson R, Franchini G, Tartaglia J, Limbach K, Paoletti E, Putkonen P, Biberfeld G. Immunogenicity and protective efficacy of a human immunodeficiency virus type 2 recombinant canarypox (ALVAC) vaccine candidate in cynomolgus monkeys. J Infect Dis. 1996;174:977–985. doi: 10.1093/infdis/174.5.977. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Belshe R B, Gorse G J, Mulligan M J, Evans T G, Keefer M C, Excler J L, Duliege A M, Tartaglia J, Cox W I, McNamara J, Hwang K L, Bradney A, Montefiori D, Weinhold K J. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. NIAID AIDS Vaccine Evaluation Group. AIDS. 1998;12:2407–2415. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Blauvelt A, Asada H, Saville M W, Klaus-Kovtun V, Altman D J, Yarchoan R, Katz S I. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J Clin Investig. 1997;100:2043–2053. doi: 10.1172/JCI119737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown D, Davies D, Skinner M, Bowen G, Hollingsworth S, Mufti G, Arrand J, Stacey S. Antigen transfer to cultured human dendritic cells using recombinant avipoxvirus vectors. Cancer Gene Ther. 1999;6:238–245. doi: 10.1038/sj.cgt.7700014. [DOI] [PubMed] [Google Scholar]
- 9.Cameron P U, Freudenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 10.Clements-Mann M L, Weinhold K, Matthews T J, Graham B S, Gorse G J, Keefer M C, McElrath M J, Hsieh R H, Mestecky J, Zolla-Pazner S, Mascola J, Schwartz D, Siliciano R, Corey L, Wright P F, Belshe R, Dolin R, Jackson S, Xu S, Fast P, Walker M C, Stablein D, Excler J L, Tartaglia J, Paoletti E, et al. Immune responses to human immunodeficiency virus (HIV) type 1 induced by canarypox expressing HIV-1MN gp120, HIV-1SF2 recombinant gp120, or both vaccines in seronegative adults. NIAID AIDS Vaccine Evaluation Group. J Infect Dis. 1998;177:1230–1246. doi: 10.1086/515288. [DOI] [PubMed] [Google Scholar]
- 11.de Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin J-J, Ait-Yahia S, Patel S, Mattei M-G, Banchereau J, Zurawski S, Davoust J, Caux C, Lebecque S. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 12.Dobbelstein M, Shenk T. Protection against apoptosis by the vaccinia virus SPI-2 [B13R] gene product. J Virol. 1996;70:6479–6485. doi: 10.1128/jvi.70.9.6479-6485.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drake D R, III, Moser J M, Hadley A, Altman J D, Maliszewski C, Butz E, Lukacher A E. Polyomavirus-infected dendritic cells induce antiviral CD8+ T lymphocytes. J Virol. 2000;74:4093–4101. doi: 10.1128/jvi.74.9.4093-4101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drillien R, Spehner D, Bohbot A, Hanau D. Vaccinia virus-related events and phenotypic changes after infection of dendritic cells derived from human monocytes. Virology. 2000;268:471–481. doi: 10.1006/viro.2000.0203. [DOI] [PubMed] [Google Scholar]
- 15.Egan M A, Pavlat W A, Tartaglia J, Paoletti E, Weinhold K J, Clements M L, Siliciano R F. Induction of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T lymphocyte responses in seronegative adults by a nonreplicating, host-range-restricted canarypox vector (ALVAC) carrying the HIV-1MN env gene. J Infect Dis. 1995;171:1623–1627. doi: 10.1093/infdis/171.6.1623. [DOI] [PubMed] [Google Scholar]
- 16.Engelmayer J, Larsson M, Subklewe M, Chahroudi A, Schmaljohn A, William C, Steinman R M, Bhardwaj N. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol. 1999;163:6762–6768. [PubMed] [Google Scholar]
- 17.Fischer L, Le Gros F X, Mason P W, Paoletti E. A recombinant canarypox virus protects rabbits against a lethal rabbit hemorrhagic disease virus (RHDV) challenge. Vaccine. 1997;15:90–96. doi: 10.1016/s0264-410x(96)00102-8. [DOI] [PubMed] [Google Scholar]
- 18.Franchini G, Robert-Guroff M, Tartaglia J, Aggarwal A, Abimiku A, Benson J, Markham P, Limbach K, Hurteau G, Fullen J, Wills M, Arp J, Dekaban G, Paoletti E, Gallo R C. Highly attenuated HIV type 2 recombinant poxviruses, but not HIV-2 recombinant Salmonella vaccines, induce long-lasting protection in rhesus macaques. AIDS Res Hum Retrovir. 1995;11:909–920. doi: 10.1089/aid.1995.11.909. [DOI] [PubMed] [Google Scholar]
- 19.Fries L F, Tartaglia J, Taylor J, Kauffman E K, Meignier B, Paoletti E, Plotkin S. Human safety and immunogenicity of a canarypox-rabies glycoprotein recombinant vaccine: an alternative poxvirus vector system. Vaccine. 1996;14:428–434. doi: 10.1016/0264-410x(95)00171-v. [DOI] [PubMed] [Google Scholar]
- 20.Fugier-Vivier I, Rivailler P, Rissoan M-C, Liu Y-J, Rabourdin-Combe C, Warnarr S O, van de Velde C J H, Melief C J M. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 22.Geijtenbeek T B H, Kwon D S, Torensma R, van Vliet S J, van Duijnhoven G C F, Middel J, Cornelissen I L M H A, Nottet H S L M, KewalRamani V N, Littman D R, Figdor C G, van Kooyk Y. DC-SIGN, a dendritic cell specific HIV-1 binding protein that enhances TRANS-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 23.Granelli-Piperno A, Pope M, Inaba K, Steinman R M. Coexpression of NFKB/ReL and Sp1 transcription factors in HIV-1 induced, dendritic cell-T cell syncytia. Proc Natl Acad Sci USA. 1995;92:10944–10948. doi: 10.1073/pnas.92.24.10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbein G, O'Brien W A. Tumor necrosis factor (TNF)-alpha and TNF receptors in viral pathogenesis. Proc Soc Exp Biol Med. 2000;223:241–257. doi: 10.1177/153537020022300305. [DOI] [PubMed] [Google Scholar]
- 25.Hodge J W, McLaughlin J P, Kantor J A, Schlom J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15:759–768. doi: 10.1016/s0264-410x(96)00238-1. [DOI] [PubMed] [Google Scholar]
- 26.Ignatius R, Isdell F, O'Doherty U, Pope M. Dendritic cells from skin and blood of macaques both promote SIV replication with T cells from different anatomical sites. J Med Primatol. 1998;27:121–128. doi: 10.1111/j.1600-0684.1998.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 27.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, Albert M, Bhardwaj N, Mellman I, Steinman R M. Efficient presentation of phagocytosed cellular fragments on the MHC class II products of dendritic cells. J Exp Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasai T, Ohguchi K, Nakashima S, Ito Y, Naganawa T, Kondo N, Nozawa Y. Increased activity of oleate-dependent type phospholipase D during actinomycin D-induced apoptosis in Jurkat T cells. J Immunol. 1998;161:6469–6474. [PubMed] [Google Scholar]
- 29.Kitov P I, Sadowska J M, Mulvey G, Armstrong G D, Ling H, Pannu N S, Read R J, Bundle D R. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature. 2000;403:669–672. doi: 10.1038/35001095. [DOI] [PubMed] [Google Scholar]
- 30.Klagge I M, Schneider-Schaulies S. Virus interactions with dendritic cells. J Gen Virol. 1999;80:823–833. doi: 10.1099/0022-1317-80-4-823. [DOI] [PubMed] [Google Scholar]
- 31.Knight S C, Macatonia S E, Patterson S. HIV-1 infection of dendritic cells. Int Rev Immunol. 1990;6:163–175. doi: 10.3109/08830189009056627. [DOI] [PubMed] [Google Scholar]
- 32.Konishi E, Kurane I, Mason P W, Shope R E, Kanesa-Thasan N, Smucny J J, Hoke C H, Jr, Ennis F A. Induction of Japanese encephalitis virus-specific cytotoxic T lymphocytes in humans by poxvirus-based JE vaccine candidates. Vaccine. 1998;16:842–849. doi: 10.1016/s0264-410x(97)00265-x. . (Erratum, 17:I, 1999.) [DOI] [PubMed] [Google Scholar]
- 33.Luft T, Pang K C, Thomas E, Hertzog P, Hart D N J, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- 34.Marshall J L, Hawkins M J, Tsang K Y, Richmond E, Pedicano J E, Zhu M Z, Schlom J. Phase I study in cancer patients of a replication-defective avipox recombinant vaccine that expresses human carcinoembryonic antigen. J Clin Oncol. 1999;17:332–337. doi: 10.1200/JCO.1999.17.1.332. [DOI] [PubMed] [Google Scholar]
- 35.Myagkikh M, Alipanah S, Markham P D, Tartaglia J, Paoletti E, Gallo R C, Franchini G, Robert-Guroff M. Multiple immunizations with attenuated poxvirus HIV type 2 recombinants and subunit boosts required for protection of rhesus macaques. AIDS Res Hum Retrovir. 1996;12:985–992. doi: 10.1089/aid.1996.12.985. [DOI] [PubMed] [Google Scholar]
- 36.Nagao S, Inaba S, Iijima S. Langerhans cells at the sites of vaccinia virus inoculation. Arch Dermatol Res. 1976;256:23–31. doi: 10.1007/BF00561177. [DOI] [PubMed] [Google Scholar]
- 37.Nunez G, Benedict M A, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 38.Paoletti E. Applications of pox virus vectors to vaccination: an update. Proc Natl Acad Sci USA. 1996;93:11354–11358. doi: 10.1073/pnas.93.21.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pardo M C, Bauman J E, Mackowiak M. Protection of dogs against canine distemper by vaccination with a canarypox virus recombinant expressing canine distemper virus fusion and hemagglutinin glycoproteins. Am J Vet Res. 1997;58:833–836. [PubMed] [Google Scholar]
- 40.Pfeffer L M, Dinarello C A, Herberman R B, Williams B R, Borden E C, Bordens R, Walter M R, Nagabhushan T L, Trotta P P, Pestka S. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 1998;58:2489–2499. [PubMed] [Google Scholar]
- 41.Pope M, Betjes M G H, Romani N, Hirmand H, Cameron P U, Hoffman L, Gezelter S, Schuler G, Steinman R M. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 42.Pope M, Elmore D, Ho D, Marx P. Dendritic cell—T cell mixtures, isolated from the skin and mucosae of macaques, support the replication of SIV. AIDS Res Hum Retrovir. 1997;13:819–827. doi: 10.1089/aid.1997.13.819. [DOI] [PubMed] [Google Scholar]
- 43.Raengsakulrach B, Nisalak A, Gettayacamin M, Thirawuth V, Young G D, Myint K S A, Ferguson L M, Hoke C H, Innis B L, Vaughn D W. An intranasal challenge model for testing Japanese encephalitis vaccines in rhesus monkeys. Am J Trop Med Hyg. 1999;60:329–337. doi: 10.4269/ajtmh.1999.60.329. [DOI] [PubMed] [Google Scholar]
- 44.Ramshaw I A, Ramsay A J, Karupiah G, Rolph M S, Mahalingam S, Ruby J C. Cytokines and immunity to viral infections. Immunol Rev. 1997;159:119–135. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 45.Reddy A, Sapp M, Feldman M, Subklewe M, Bhardwaj N. A monocyte conditioned medium is more effective than defined cytokines in mediating the terminal maturation of human dendritic cells. Blood. 1997;90:3640–3646. [PubMed] [Google Scholar]
- 46.Roth J, Dittmer D, Rea D, Tartaglia J, Paoletti E, Levine A J. p53 as a target for cancer vaccines: recombinant canarypox virus vectors expressing p53 protect mice against lethal tumor cell challenge. Proc Natl Acad Sci USA. 1996;93:4781–4786. doi: 10.1073/pnas.93.10.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salio M, Cella M, Suter M, Lanzavecchia A. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol. 1999;29:3245–3253. doi: 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 48.Salio M, Cerundolo V, Lanzavecchia A. Dendritic cell maturation is induced by mycoplasma infection but not by necrotic cells. Eur J Immunol. 2000;30:705–708. doi: 10.1002/1521-4141(200002)30:2<705::AID-IMMU705>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 49.Salmon-Ceron D, Excler J L, Finkielsztejn L, Autran B, Gluckman J C, Sicard D, Matthews T J, Meignier B, Valentin C, El Habib R, Blondeau C, Raux M, Moog C, Tartaglia J, Chong P, Klein M, Milcamps B, Heshmati F, Plotkin S. Safety and immunogenicity of a live recombinant canarypox virus expressing HIV type 1 gp120 MN MN tm/gag/protease LAI (ALVAC-HIV, vCP205) followed by a p24E-V3 MN synthetic peptide (CLTB-36) administered in healthy volunteers at low risk for HIV infection. AGIS Group and L'Agence Nationale de Recherches sur Le Sida. AIDS Res Hum Retrovir. 1999;15:633–645. doi: 10.1089/088922299310935. [DOI] [PubMed] [Google Scholar]
- 50.Sauter B, Albert M L, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death. Exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnorr J-J, Xanthakos S, Keikavoussi P, Kämpgen E, Meulen V T, Schneider-Schaulies S. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc Natl Acad Sci USA. 1997;94:5326–5331. doi: 10.1073/pnas.94.10.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Servet-Delprat C, Vidalain P O, Azocar O, Le Deist F, Fischer A, Rabourdin-Combe C. Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J Virol. 2000;74:4387–4393. doi: 10.1128/jvi.74.9.4387-4393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sigal L J, Crotty S, Andino R, Rock K L. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 54.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 55.Stephensen C B, Welter J, Thaker S R, Taylor J, Tartaglia J, Paoletti E. Canine distemper virus (CDV) infection of ferrets as a model for testing morbillivirus vaccine strategies: NYVAC- and ALVAC-based CDV recombinants protect against symptomatic infection. J Virol. 1997;71:1506–1513. doi: 10.1128/jvi.71.2.1506-1513.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Svensson H, Johannisson A, Nikkila T, Alm G V, Cederblad B. The cell surface phenotype of human natural interferon-alpha producing cells as determined by flow cytometry. Scand J Immunol. 1996;44:164–172. doi: 10.1046/j.1365-3083.1996.d01-289.x. [DOI] [PubMed] [Google Scholar]
- 57.Taylor J, Meignier B, Tartaglia J, Languet B, VanderHoeven J, Franchini G, Trimarchi C, Paoletti E. Biological and immunogenic properties of a canarypox-rabies recombinant, ALVAC-RG (vCP65) in non-avian species. Vaccine. 1995;13:539–549. doi: 10.1016/0264-410x(94)00028-l. [DOI] [PubMed] [Google Scholar]
- 58.Taylor J, Trimarchi C, Weinberg R, Languet B, Guillemin F, Desmettre P, Paoletti E. Efficacy studies on a canarypox-rabies recombinant virus. Vaccine. 1991;9:190–193. doi: 10.1016/0264-410x(91)90152-v. [DOI] [PubMed] [Google Scholar]
- 59.Tellier M C, Pu R, Pollock D, Vitsky A, Tartaglia J, Paoletti E, Yamamoto J K. Efficacy evaluation of prime-boost protocol: canarypoxvirus-based feline immunodeficiency virus (FIV) vaccine and inactivated FIV-infected cell vaccine against heterologous FIV challenge in cats. AIDS. 1998;12:11–18. doi: 10.1097/00002030-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Vidalain P O, Azocar O, Lamouille B, Astier A, Rabourdin-Combe C, Servet-Delprat C. Measles virus induces functional TRAIL production by human dendritic cells. J Virol. 2000;74:556–559. doi: 10.1128/jvi.74.1.556-559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang B, Fujisawa H, Zhuang L, Kondo S, Shivji G M, Kim C S, Mak T W, Sauder D N. Depressed Langerhans cell migration and reduced contact hypersensitivity response in mice lacking TNF receptor p75. J Immunol. 1997;159:6148–6155. [PubMed] [Google Scholar]
- 62.Weissman D, Li Y, Ananworanich J, Zhou L-J, Adelsberger J, Tedder T F, Baseler M, Fauci A S. Three populations of cells with dendritic morphology exist in peripheral blood, only one of which is infectable with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1995;92:826–830. doi: 10.1073/pnas.92.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Welter J, Taylor J, Tartaglia J, Paoletti E, Stephensen C B. Mucosal vaccination with recombinant poxvirus vaccines protects ferrets against symptomatic CDV infection. Vaccine. 1999;17:308–318. doi: 10.1016/s0264-410x(98)00211-4. [DOI] [PubMed] [Google Scholar]
- 64.Yrlid U, Wick M J. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J Exp Med. 2000;191:613–623. doi: 10.1084/jem.191.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zoeteweij J P, Blauvelt A. HIV-Dendritic cell interactions promote efficient viral infection of T cells. J Biomed Sci. 1998;5:253–259. doi: 10.1007/BF02255856. [DOI] [PubMed] [Google Scholar]