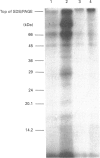
Abstract
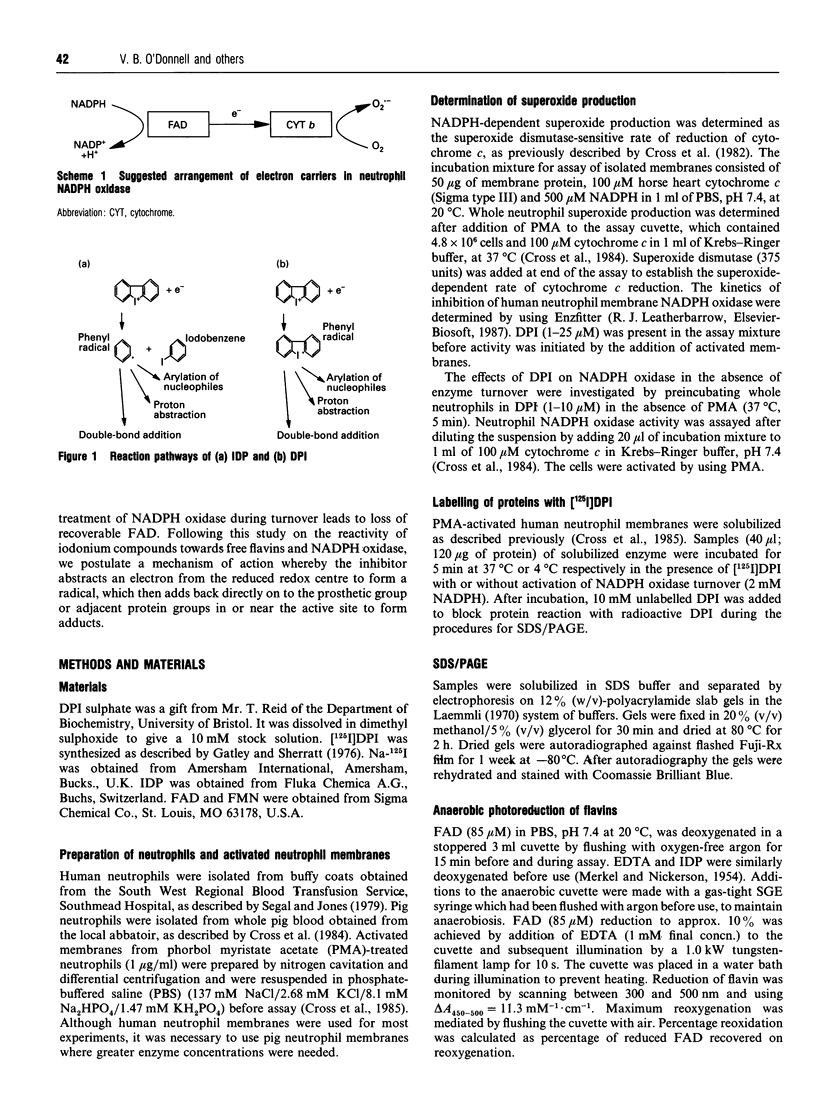
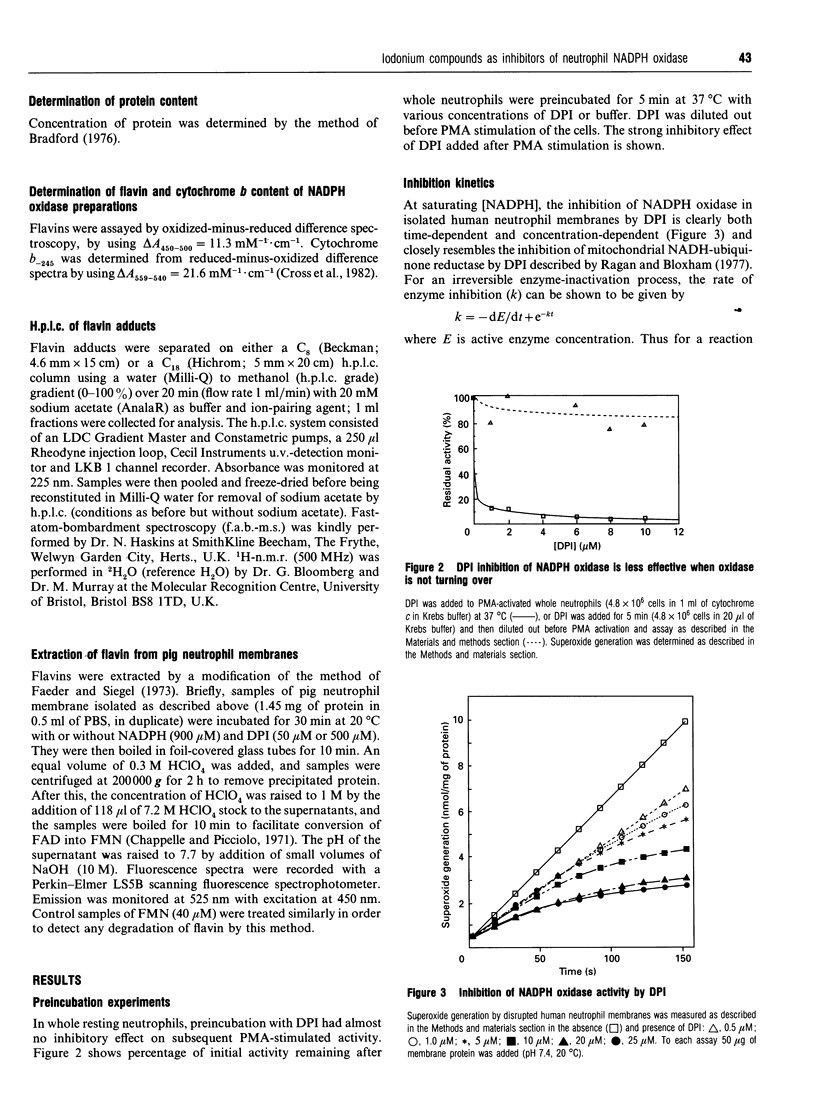
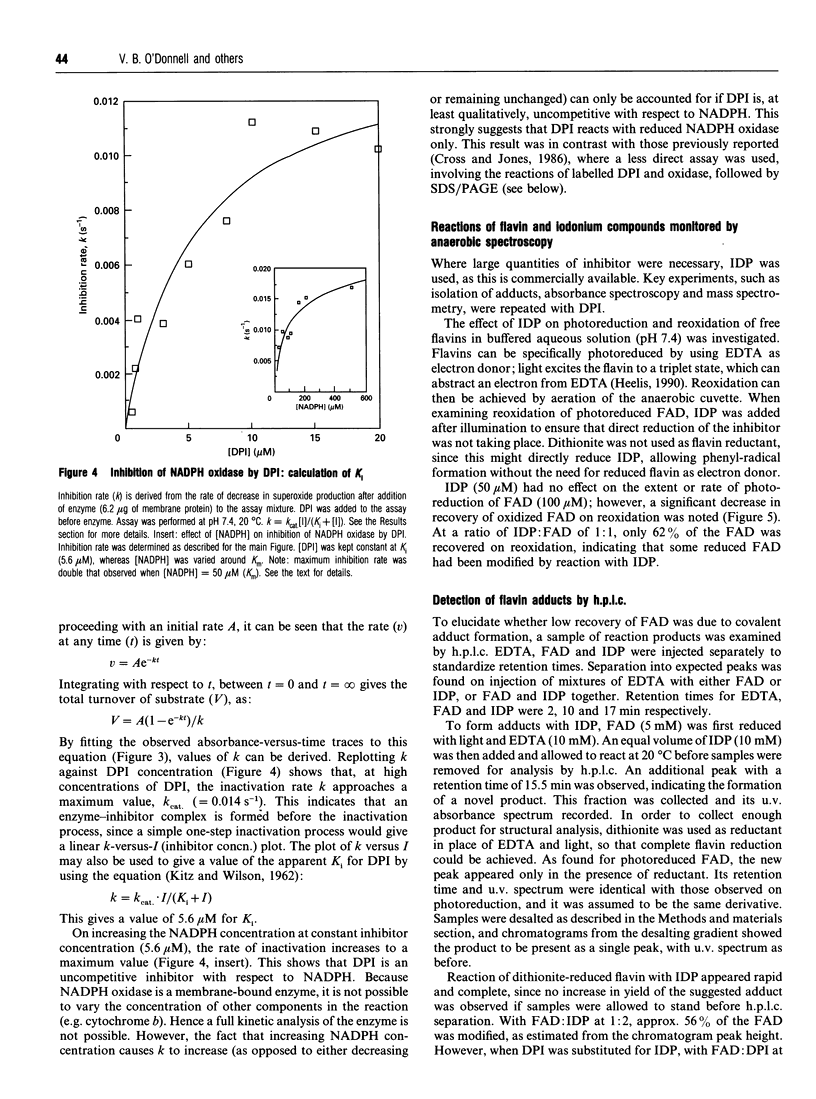
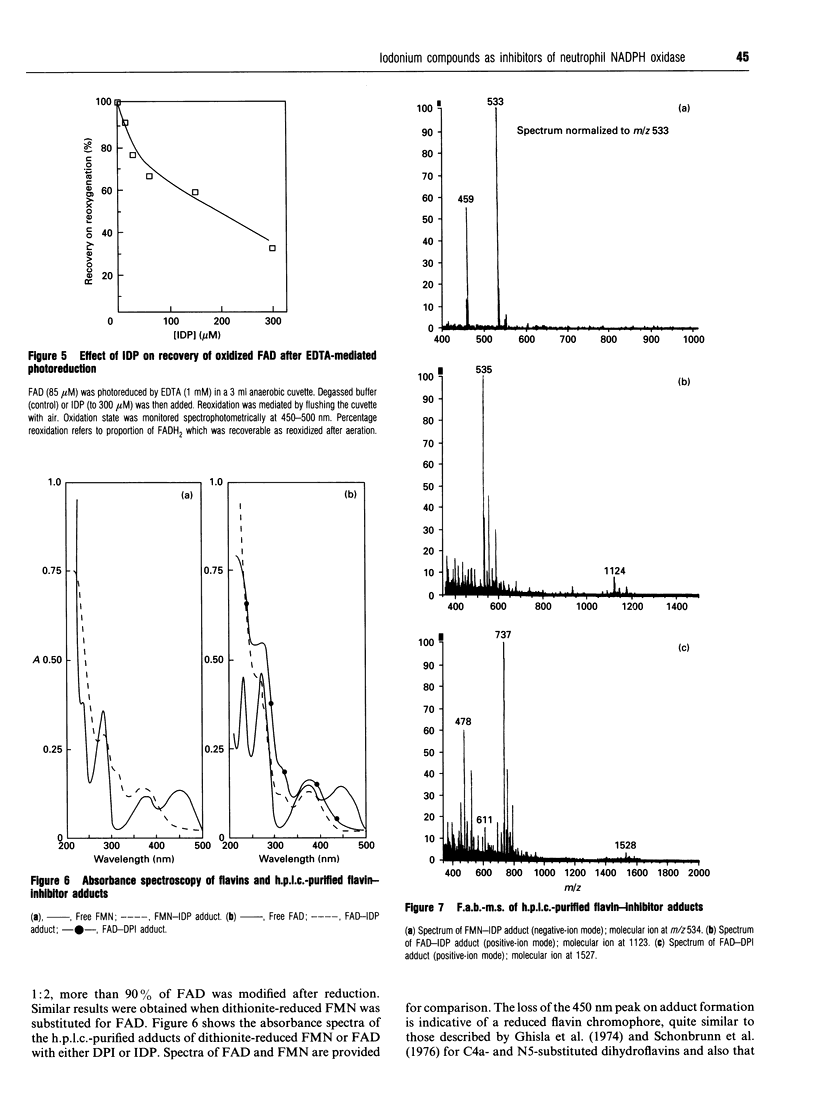
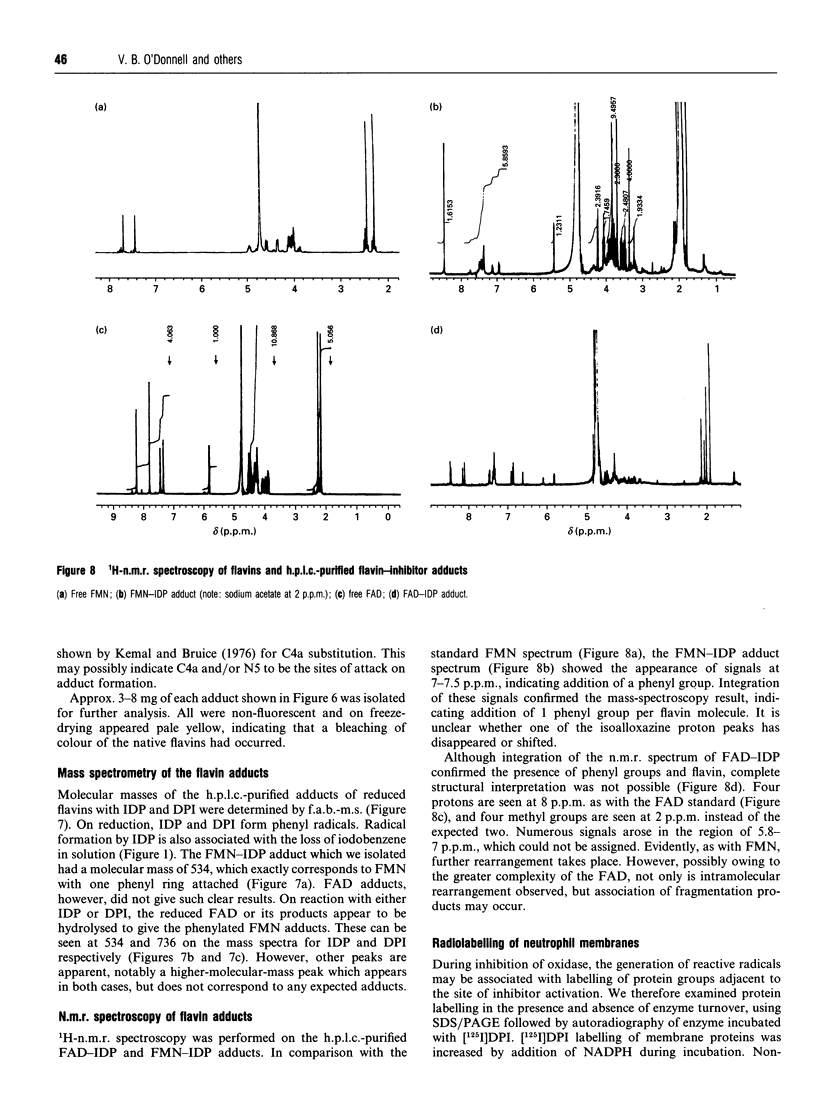
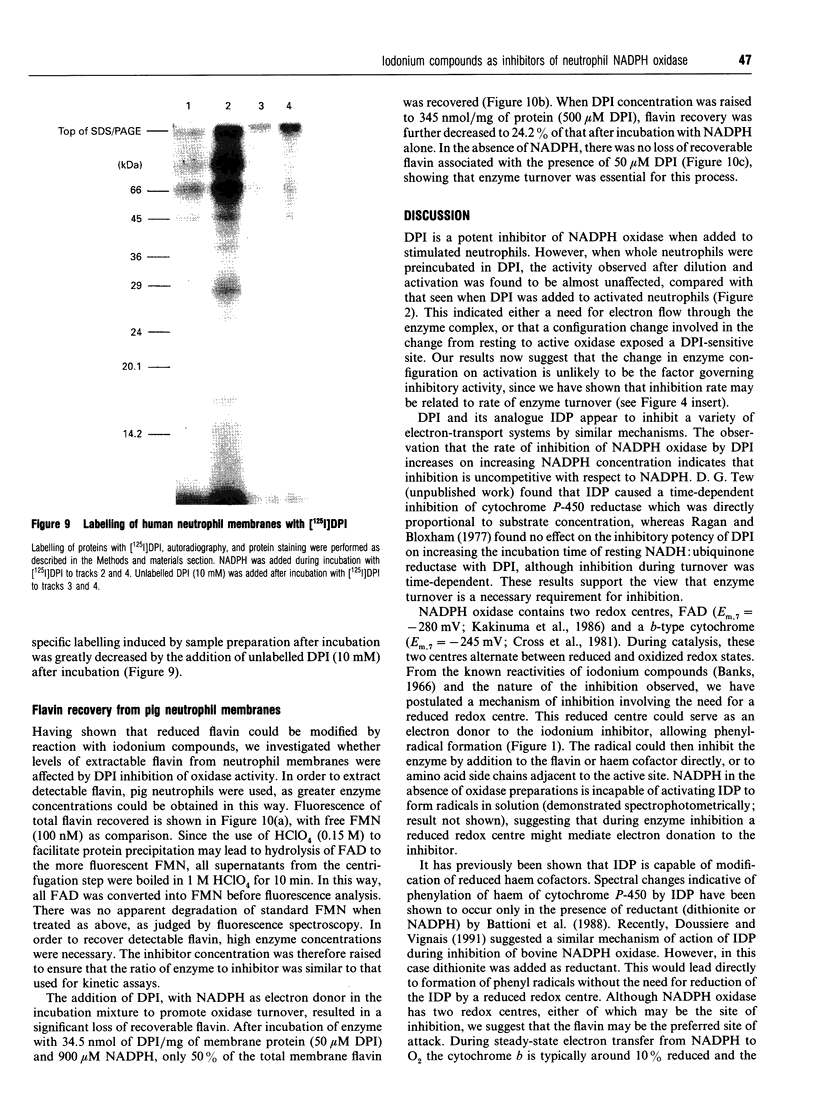
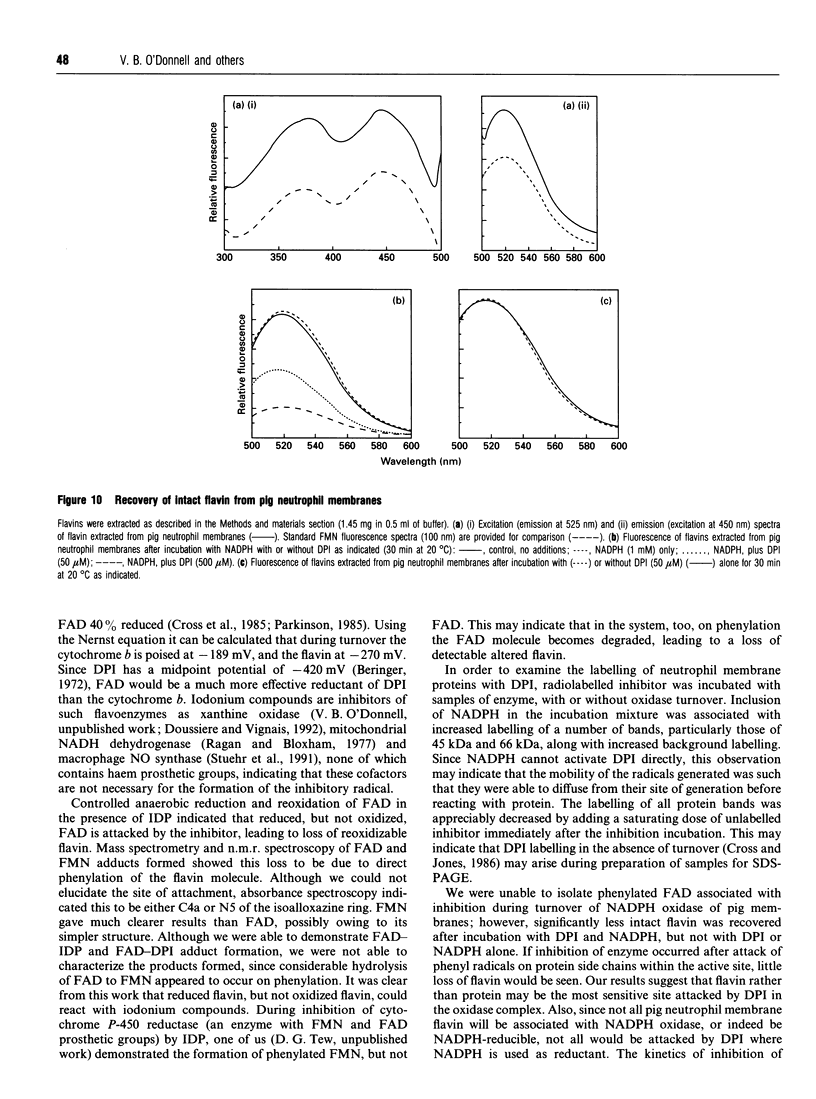
Diphenyleneiodonium (DPI) and its analogues have been previously shown to react via a radical mechanism whereby an electron is abstracted from a nucleophile to form a radical, which then adds back to the nucleophile to form covalent adducts [Banks (1966) Chem. Rev. 66, 243-266]. We propose that the inhibition of neutrophil NADPH oxidase by DPI occurs via a similar mechanism. A reduced redox centre in the oxidase could serve as electron donor to DPI, and inhibition would occur after direct phenylation of the redox cofactor, or of adjacent amino acid groups by the DPI radical. In the absence of an activatory stimulus, human neutrophil NADPH-oxidase was not inhibited by DPI. The Ki for time-dependent inhibition by DPI of human neutrophil membrane NADPH oxidase was found to be 5.6 microM. Inhibitory potency of DPI was shown to be directly related to rate of enzyme turnover, indicating the need for a reduced redox centre. Adducts were formed between photoreduced flavin (FAD or FMN) and inhibitor (DPI or diphenyliodonium). These were separated by h.p.l.c. and characterized by absorbance spectroscopy, 1H-n.m.r. and fast-atom-bombardment m.s. and found to have properties consistent with substituted 4a,5-dihydroflavins. After incubation of pig neutrophil membranes with DPI, the quantity of recoverable intact flavin was greatly diminished when NADPH was present to initiate oxidase turnover, indicating that the flavin may be the site of DPI activation. These results may provide a common mechanism of action for iodonium compounds as inhibitors of other flavoenzymes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandsch R., Bichler V. Covalent flavinylation of 6-hydroxy-D-nicotine oxidase involves an energy-requiring process. FEBS Lett. 1987 Nov 16;224(1):121–124. doi: 10.1016/0014-5793(87)80433-7. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Higson F. K., Jones O. T., Harper A. M., Segal A. W. The enzymic reduction and kinetics of oxidation of cytochrome b-245 of neutrophils. Biochem J. 1982 May 15;204(2):479–485. doi: 10.1042/bj2040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. Enzymic mechanisms of superoxide production. Biochim Biophys Acta. 1991 May 6;1057(3):281–298. doi: 10.1016/s0005-2728(05)80140-9. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Harper A. M., Segal A. W. Oxidation-reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem J. 1981 Feb 15;194(2):599–606. doi: 10.1042/bj1940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986 Jul 1;237(1):111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Parkinson J. F., Jones O. T. Mechanism of the superoxide-producing oxidase of neutrophils. O2 is necessary for the fast reduction of cytochrome b-245 by NADPH. Biochem J. 1985 Mar 15;226(3):881–884. doi: 10.1042/bj2260881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Parkinson J. F., Jones O. T. The superoxide-generating oxidase of leucocytes. NADPH-dependent reduction of flavin and cytochrome b in solubilized preparations. Biochem J. 1984 Oct 15;223(2):337–344. doi: 10.1042/bj2230337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R. The inhibitory effects of some iodonium compounds on the superoxide generating system of neutrophils and their failure to inhibit diaphorase activity. Biochem Pharmacol. 1987 Feb 15;36(4):489–493. doi: 10.1016/0006-2952(87)90356-x. [DOI] [PubMed] [Google Scholar]
- Doussière J., Vignais P. V. Diphenylene iodonium as an inhibitor of the NADPH oxidase complex of bovine neutrophils. Factors controlling the inhibitory potency of diphenylene iodonium in a cell-free system of oxidase activation. Eur J Biochem. 1992 Aug 15;208(1):61–71. doi: 10.1111/j.1432-1033.1992.tb17159.x. [DOI] [PubMed] [Google Scholar]
- Ellis J. A., Mayer S. J., Jones O. T. The effect of the NADPH oxidase inhibitor diphenyleneiodonium on aerobic and anaerobic microbicidal activities of human neutrophils. Biochem J. 1988 May 1;251(3):887–891. doi: 10.1042/bj2510887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faeder E. J., Siegel L. M. A rapid micromethod for determination of FMN and FAD in mixtures. Anal Biochem. 1973 May;53(1):332–336. doi: 10.1016/0003-2697(73)90442-9. [DOI] [PubMed] [Google Scholar]
- Gatley S. J., Sherratt S. A. The effects of diphenyleneiodonium on mitochondrial reactions. Relation of binding of diphenylene[125I]iodonium to mitochondria to the extent of inhibition of oxygen uptake. Biochem J. 1976 Aug 15;158(2):307–315. doi: 10.1042/bj1580307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisla S., Massey V., Lhoste J. M., Mayhew S. G. Fluorescence and optical characteristics of reduced flavines and flavoproteins. Biochemistry. 1974 Jan 29;13(3):589–597. doi: 10.1021/bi00700a029. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Hancock J. T., Jones O. T. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem J. 1987 Feb 15;242(1):103–107. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Kakinuma K., Kaneda M., Chiba T., Ohnishi T. Electron spin resonance studies on a flavoprotein in neutrophil plasma membranes. Redox potentials of the flavin and its participation in NADPH oxidase. J Biol Chem. 1986 Jul 15;261(20):9426–9432. [PubMed] [Google Scholar]
- Kemal C., Bruice T. C. Simple synthesis of a 4a-hydroperoxy adduct of a 1,5-dihydroflavine: preliminary studies of a model for bacterial luciferase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):995–999. doi: 10.1073/pnas.73.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MERKEL J. R., NICKERSON W. J. Riboflavin as a photocatalyst and hydrogen carrier in photochemical reduction. Biochim Biophys Acta. 1954 Jul;14(3):303–311. doi: 10.1016/0006-3002(54)90188-2. [DOI] [PubMed] [Google Scholar]
- Ragan C. I., Bloxham D. P. Specific labelling of a constituent polypeptide of bovine heart mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone reductase by the inhibitor diphenyleneiodonium. Biochem J. 1977 Jun 1;163(3):605–615. doi: 10.1042/bj1630605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbrunn A., Abeles R. H., Walsh C. T., Ghisla S., Ogata H., Massey V. The structure of the covalent flavin adduct formed between lactate oxidase and the suicide substrate 2-hydroxy-3-butynoate. Biochemistry. 1976 May 4;15(9):1798–1807. doi: 10.1021/bi00654a003. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. The subcellular distribution and some properties of the cytochrome b component of the microbicidal oxidase system of human neutrophils. Biochem J. 1979 Jul 15;182(1):181–188. doi: 10.1042/bj1820181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., West I., Wientjes F., Nugent J. H., Chavan A. J., Haley B., Garcia R. C., Rosen H., Scrace G. Cytochrome b-245 is a flavocytochrome containing FAD and the NADPH-binding site of the microbicidal oxidase of phagocytes. Biochem J. 1992 Jun 15;284(Pt 3):781–788. doi: 10.1042/bj2840781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. M., Curnutte J. T. Molecular basis of chronic granulomatous disease. Blood. 1991 Feb 15;77(4):673–686. [PubMed] [Google Scholar]
- Stuehr D. J., Fasehun O. A., Kwon N. S., Gross S. S., Gonzalez J. A., Levi R., Nathan C. F. Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J. 1991 Jan;5(1):98–103. doi: 10.1096/fasebj.5.1.1703974. [DOI] [PubMed] [Google Scholar]