Abstract
Nanoparticles, mRNA, and ultraviolet light combine to reprogram specific immune cells directly in the body.
The COVID-19 pandemic has seen the first mRNA vaccines put into global use with administration to billions of people. mRNA vaccines, such as the Pfizer-BioNTech and Moderna COVID-19 vaccines, work by mimicking infection-induced immunity (1). In infection-induced immunity, immune cells—including dendritic cells and T cells—work together to help the body fight infection and disease. The process starts with dendritic cells (DCs), which are scavenger cells that forage for pathogens in the body. When a DC finds a pathogen, it takes up the pathogen, degrades the pathogen, and then displays pieces of the degraded pathogen (called antigens) on its surface via molecules called major histocompatibility complexes (MHCs). The antigen-loaded MHCs act like beacons for T cells, white blood cells that are targeted for one specific antigen. Though each T cell wards off only one type of antigen, the body has billions of T cells, allowing for these immune cells to eliminate almost any antigen. However, the type of MHC on which the antigen is presented determines which subset of T cells are called to action. MHCIs interact with cytotoxic T cells, whereas MHCIIs interact with helper T cells. After the MHC–T cell interaction, cytotoxic T cells activate to kill cells infected with the pathogens, and helper T cells support the production of pathogen-neutralizing antibodies. In mRNA vaccines, the mRNA is taken up by DCs and translated into antigenic peptide, which is then presented on the DC surface to stimulate T cells as in infection-induced immunity (1). The vaccines encapsulate the mRNA in minuscule fat droplets, called lipid nanoparticles (LNPs), to provide stability in the body and facilitate delivery into DCs after intramuscular injection (1).
In this issue of Science Advances, Su et al. (2) explore another promising application of LNP-mRNA therapeutics: direct delivery of mRNA to T cells in the body. Precise targeting of T cells could lead to breakthroughs in the treatment of cancer, infection, and immune disease, but this challenging task is fraught with numerous biological barriers. Using innovative optical techniques, Su et al. (2) generated LNPs with the ability to overcome these barriers and deliver mRNA cargo to specific subsets of T cells.
T cell delivery challenges
Delivering therapeutic mRNA to T cells is much more challenging than delivering it to DCs. Many DCs reside in the skin near the site of an intramuscular injection, and—because their job as antigen-presenting cells requires that they forage for pathogens to take up—they readily engulf the injected LNPs. In contrast, most T cells reside in lymphoid tissue, mucosal sites, and human peripheral blood. This means that intravenous administration of mRNA, rather than an intramuscular injection, is required for sufficient T cell delivery. Intravenous administration, unfortunately, presents numerous challenges. One is that intravenously administered LNPs will accumulate in the liver, preventing them from reaching T cells. Even if an LNP does reach a T cell, the T cell will not take up the LNP as readily as a DC would because foraging is not part of a T cell’s job.
To overcome these issues, researchers are creating nanoparticles with surface modifications, such as antibody fragments, that allow specific binding to T cells. By attaching T cell–specific antibody fragments to the nanoparticle surface, researchers have engineered targeted nanoparticles for T cell delivery (3, 4). When tested, these targeted nanoparticles can efficiently deliver mRNA cargo to T cells (4). Particles such as these—that broadly target all T cells regardless of antigen specificity—would be useful to mediate a strong, total T cell response for applications in cancer therapy.
When treating certain viral infections or virus-mediated cancers, however, it is often desirable to target only T cells that are specific to the disease instead of all T cells. Enhancing only disease-specific T cells would help the immune system to maintain self-tolerance and avoid inflammation resulting from overactivity (2). Toward this goal, researchers have worked to develop nanoparticles that have antigenic peptide-MHCI (pMHCI) complexes conjugated to their surfaces (5–7). Mimicking DC–T cell interactions, particles engineered in this manner can only interact with the cytotoxic T cells that are specific to the chosen antigenic peptides. The research published to date has focused mainly on activating disease-specific T cells.
Using light to create targeted LNP technology
Su et al. (2) attached pMHCI molecules to LNPs for the delivery of mRNA to disease-specific T cells—with the goal of reprogramming the cells instead of only activating them. This is valuable because T cells can be reprogrammed with mRNA to make new proteins, such as therapeutic receptors or stimulatory proteins.
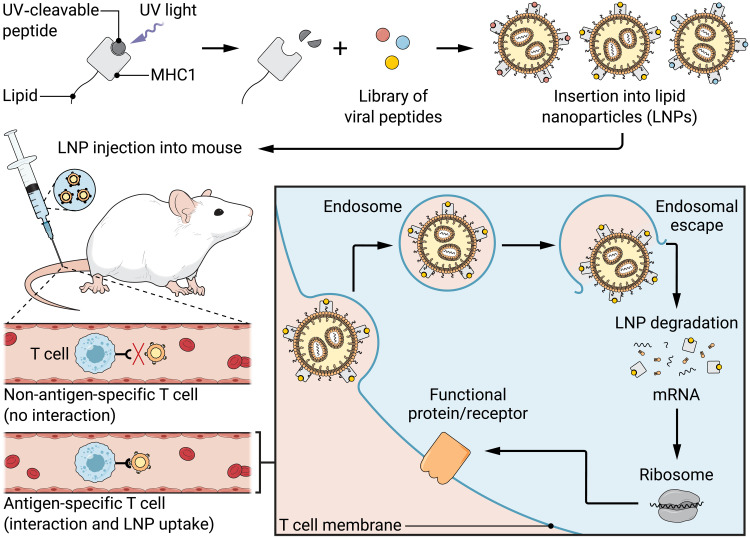
Su et al. (2) first created the LNPs—the minuscule fat droplets—by combining a fat mixture with mRNA in a microfluidic mixing channel. Then, they worked to synthesize and incorporate the disease-specific pMHCIs. Historically, researchers synthesized pMHCI molecules in a complicated, stepwise process, which rendered generating large libraries of pMHCIs prohibitively time-consuming (8). Fortunately, strategies to efficiently swap different peptides into pMHCIs have recently been developed, such as ultraviolet (UV) light–mediated peptide exchange (8). Using this strategy, Su et al. (2) synthesized the MHCIs with a placeholder peptide. The placeholder peptide degrades when exposed to UV light, exposing the MHCI presentation groove and allowing a new antigenic peptide to bind (8). This means that a single batch of placeholder-MHCI can be synthesized and exposed to UV light, and then multiple antigenic peptides can be added at once to generate a library of pMHCI molecules in one step. Su et al. (2) conjugated their UV-exchanged pMHCI molecules to fat molecules, which acted like tethers to secure the pMHCIs to the surface of their mRNA containing LNPs to create antigen-presenting nanoparticles (APNs) (Fig. 1).
Fig. 1. Ultraviolet light generates a library of peptide-MHCI molecules, which are then inserted into lipid nanoparticles to create antigen-presenting nanoparticles.
These nanoparticles deliver mRNA to specific groups of T cells (2). This technology could help make new treatments for certain cancers and viruses a reality. Credit: Ashley Mastin/Science Advances.
Targeted LNP validation in mice
Su et al. (2) first compared their UV-exchanged pMHCI APNs with conventionally refolded (the long, stepwise process) pMHCI APNs in three mouse models and found that both APNs successfully targeted and delivered their cargo mRNA to antigen-specific cytotoxic T cells. This shows that their new, easier to produce, UV-exchanged pMHCIs work just as well as conventionally refolded pMHCIs to target specific groups of cytotoxic T cells. Second, Su et al. (2) gave mice an intravenous injection of a mixture of three types of UV-exchanged APNs, each carrying a different influenza A antigenic peptide. They observed mRNA delivery to the three antigen-specific cytotoxic T cell populations at significantly higher rates compared to other cell populations, demonstrating that these APNs can target multiple cytotoxic T cell populations simultaneously.
Impact and future directions
This approach improves the precision with which mRNA containing LNPs can target and deliver their cargo to specific cytotoxic T cell populations and can be efficiently scaled to generate large libraries of APNs. In their study, Su et al. (2) lay the groundwork for applications in influenza, but their APNs could also be adapted for cancer immunotherapy by using pMHCIs with tumor antigens to target cancer-specific cytotoxic T cells. However, this study only considers the functionalization of LNPs with pMHCIs for engineering cytotoxic T cells. Perhaps LNPs could similarly be functionalized with pMHCIIs for engineering helper T cells, which might have important therapeutic applications for the treatment of autoimmune diseases or the malignant tumor microenvironment. Furthermore, some immunotherapies are most effective when both cytotoxic and helper T cells participate (9), so the ability to target both cell types via a mixture of pMHCI and pMHCII APNs could prove beneficial. However, Su et al. (2) hypothesize that the generation of pMHCII APNs may be more difficult than the production of pMHCI APNs since MHCIIs interact more weakly with both peptides and T cells than do MHCIs. Nevertheless, pMHCI APN technology (2) is an exciting step toward illuminating the goal of creating personalized immune cell therapies and vaccines.
REFERENCES
- 1.Chaudhary N., Weissman D., Whitehead K. A., mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20, 817–838 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su F.-Y., Zhao Q., Dahotre S. N., Gamboa L., Bawage S. S., Silva Trenkle A. D., Zamat A., Phuengkham H., Ahmed R., Santangelo P. J., Kwong G. A., In vivo mRNA delivery to virus-specific T cells by light-induced ligand exchange of MHC class I antigen-presenting nanoparticles. Sci. Adv. 8, eabm7950 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith T. T., Stephan S. B., Moffett H. F., McKnight L. E., Ji W., Reiman D., Bonagofski E., Wohlfahrt M. E., Pillai S. P. S., Stephan M. T., In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotech. 12, 813–820 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parayath N. N., Stephan S. B., Koehne A. L., Nelson P. S., Stephan M. T., In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 11, 6080 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L., Song S., Jin X., Wan X., Shahzad K. A., Pei W., Zhao C., Shen C., An artificial antigen-presenting cell delivering 11 immune molecules expands tumor antigen-specific CTLs in ex vivo and in vivo murine melanoma models. Cancer Immunol. Res. 7, 1188–1201 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Zhang X., Luo M., Dastagir S. R., Nixon M., Khamhoung A., Schmidt A., Lee A., Subbiah N., McLaughlin D. C., Moore C. L., Gribble M., Bayhi N.,Amin V., Pepi R., Pawar S., Lyford T. J., Soman V., Mellen J., Carpenter C. L., Turka L. A., Wickham T. J., Chen T. F., Engineered red blood cells as an off-the-shelf allogeneic anti-tumor therapeutic. Nat. Commun. 12, 2637 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ugel S., Zoso A., de Santo C., Li Y., Marigo I., Zanovello P., Scarselli E., Cipriani B., Oelke M., Schneck J. P., Bronte V., In vivo administration of artificial antigen-presenting cells activates low-avidity T cells for treatment of cancer. Cancer Res. 69, 9376–9384 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodenko B., Toebes M., Hadrup S. R., van Esch W. J. E., Molenaar A. M., Schumacher T. N. M., Ovaa H., Generation of peptide-MHC class I complexes through UV-mediated ligand exchange. Nat. Protoc. 1, 1120–1132 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Sommermeyer D., Hudecek M., Kosasih P. L., Gogishvili T., Maloney D. G., Turtle C. J., Riddell S. R., Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 30, 492–500 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]