Abstract
Introduction:
Spine fixation surgery for traumatic vertebral fractures is associated with severe pain and is often difficult to control. Traditionally systemic opioids have been the mainstay of analgesia for these procedures, which can lead to hyperalgesia, nausea, ileus, sedation, cognitive impairment, dependence, etc., limiting usage of opioids. The Erector spinae plane block (ESPB) is a novel ultrasound-guided procedure with easily identifiable sonoanatomy. We hypothesized that a multimodal approach involving ESPB to a conventional analgesic regimen with local infiltration for patients undergoing major traumatic spine surgeries might provide better perioperative analgesia and reduce the need for postoperative opioid requirements.
Material and Methods:
A randomized control prospective trial was conducted on 34 ASA grade I –II patients aged 18 to 65 years who were scheduled to undergo elective posterior spine fixation surgery with ASIA B to E after traumatic spine fracture under general anesthesia. Patients were randomized to Group A which included patients who received general anesthesia with ESPB, and Group B, or the control group, included patients who received general anesthesia with systemic analgesics and postoperative local infiltration without ESPB. Intraoperative total fentanyl consumption, VAS score at 0, 3, 6, 12, 18, and 24 hours, time to activate patient-controlled analgesia (PCA) pump, total morphine consumption, and opioid-related side effects were monitored and compared in both groups.
Results:
Postoperative PCA morphine consumption was significantly lower in group A patients who received ESPB than those in the control group (17.06 ± 9.59 vs 37.82 ± 9.88 P value = <0.0001). VAS scores at rest and movement at 0, 3, 6, 9, 12, 18, and 24 hours were significantly lower (P value = 0.05) in the ESPB group compared with the control group at all time points.
Conclusion:
Bilateral ultrasound-guided Erector spinae plane block, when administered in traumatic spine patients undergoing spine fixation surgery, provides better analgesia with statistically decreased VAS scores and less postoperative opioid requirement.
Keywords: Analgesia, erector spinae plane block, traumatic spine fracture
Introduction
According to WHO, every year, around the world, between 250,000 and 800,000 people suffer from traumatic spinal injuries, which constitutes 5%–25% of all trauma patients.[1] Recent advances in surgical techniques have led to a paradigm shift towards more surgery and the early discharge of these patients. However, pain associated with these surgeries is a deterrent factor for early ambulation and discharge from the hospital.[2] Spine surgery is associated with severe postoperative pain and was ranked the second (spinal fixation one to two segments) and third (dorsal spinal fusion, three or more segments) most painful surgical act among 179 other procedures.[3]
Conventionally, analgesia in these surgeries is usually provided by local infiltration at the end of surgery and systemic opioids at our institute. These surgeries require opioids and are associated with numerous adverse effects of opioids.[4] Other techniques, like neuraxial, have been tried to enhance the multimodal approach and decrease the use of opioids. Although neuraxial blockade reduces opioid use, these blocks cause hemodynamic instability and interfere with the necessary neurological evaluation. Moreover, the neuraxial technique involves a midline plane at the surgical site and is not always preferred by many surgeons.[5]
The erector spinae plane (ESP) block is a novel ultrasound (US) guided procedure where the local anesthetic is injected into the musculofascial plane between the erector spinae muscle (ESM) and the tip of the transverse process.[6] The injectate spreads along the fibers of the erector spinae muscle, which extends across the entire length of the thoracolumbar spine, thus permitting extensive craniocaudal spread covering multiple dermatomes.[7] This capacity to cover multiple dermatomes with a single injection at a vertebral level transverse process gives it a unique advantage for spine fixation at multiple levels without interfering with midline surgical incision.[4] We here in our study compared the effect of ESPB as an opioid-sparing adjunct and part of multimodal perioperative analgesia to the conventional approach of local infiltration with systemic opioids in traumatic spine surgery.
Material and Methods
A prospective, randomized controlled trial was conducted in the Jai Prakash Narayan Apex Trauma Centre, All India Institute of Medical Sciences, New Delhi, after obtaining Institutional Ethics Committees approval (REF/2018/12/022605) and registration with CTRI (CTRI registration no. CTRI/2019/03/018206). All the ASA grade I – II patients between 18–65 years of age with spine injuries ASIA grade B to E, planned for posterior spine fixation surgery, were enrolled in the study. Patients who refused to participate or give consent, had abnormal spine anatomy, infection at the site of injection, pregnancy, incision involving more than six intervertebral spaces, coagulopathy (prothrombin time INR >1.5, platelet <100,000/mm), or had an allergy to local anesthetic drugs were excluded from the study.
A day before the surgery, written informed consent was obtained from all the patients after explaining the possible risks and benefits of the intervention. They were explained the Visual Analogue Scale (VAS) of 0 to 10 for pain assessment, where 0 stands for least and 10 for most severe pain. Patients were also explained and trained to use a patient-controlled analgesia (PCA) pump of morphine in the postoperative period. A preoperative neurological assessment was also conducted and confirmed with the surgeons for ASIA grading.
Allocation and blinding
All the participants were randomly allocated to either of the two groups after computer-based randomization- that is, General anesthesia with ESPB (Group A) or General anesthesia with local infiltration (Group B). A triple-blind study was formulated where the anesthetist in the operation theatre assessing the post-operative outcomes differed from one who performed the block and was blinded to the intervention. Similarly, the statistician who analyzed the data and the patient was also blinded.
Anesthesia technique
After arrival in the operation theatre, a wide-bore IV cannula was inserted, and standard ASA monitors (NIBP, SaO2, ECG) +/– IBP) were attached. All patients were pre-oxygenated with 100% oxygen for 3 minutes, followed by standard intravenous induction (Fentanyl 2 mcg/kg, Propofol 2 mg/kg, and Atracurium 0.5 mg/kg). The airway was secured by an appropriately sized cuffed endotracheal tube. All patients were positioned prone for surgery, taking care of the endotracheal tube, pressure points, eye, spine, and possible nerve injuries. A mixture of O2 + N2O and Sevoflurane or Isoflurane was used for intraoperative maintenance of anesthesia. Vital parameters such as the patient’s heart rate (HR), non-invasive blood pressure (NIBP), invasive blood pressure (IBP), and oxygen saturation were recorded by the principal investigator. After positioning, Group A patients received ESP blocks before incision with systemic opioids. In contrast, opioids with local infiltration at the end of surgery were used in Group B for postoperative analgesia.
Intervention
Group A- ESP Block- High frequency linear or curved array probe (Sonosite Turbo M) was selected depending on the patient’s body habitus and the depth of the transverse process. The level at the anticipated midpoint of the surgical incision or above or below was chosen per the convenience of Sono anatomy for the block. The transducer was placed in a para-sagittal plane 2–3 cm lateral to the midline to identify the tip of the transverse process and ESM. A 5 or 8-cm echogenic blunt tip needle was inserted in real time and in-plane to reach the tip of the transverse process below the ESM. After slight needle retraction, 20 ml of 0.25% Bupivacaine with 1 mcg/kg Clonidine was injected into the interspace. The location of the needle was further confirmed by visible fluid spread and lifting of the erector spinae muscle (ESM) of the hyperechoic bony shadow of the transverse process. The same procedure was repeated on the contralateral side.
Group B- Local Infiltration- At the end of the surgery, patients recruited to Group B received 15–20 mL 0.25% Bupivacaine infiltration along the surgical incision by the surgeon.
Analgesic regimen
Fentanyl 1 mcg/kg was supplemented if blood pressure (BP) or heart rate (HR) was >20% of the baseline during surgery. One gram of intravenous paracetamol was also given to the patients forty-five minutes before the end of surgery. All the patients were extubated after complete neuromuscular blockade reversal. In the postoperative period, all the patients received intravenous paracetamol 1 gm every eighth hour. Patient-controlled analgesia (PCA) morphine pump was initiated in both groups with the following settings: Concentration-1mg/ml, Patient bolus-1mg, Lockout-10 minutes, and dose limit-12mg/4 hrs.
Postoperative parameters
The primary objective of our study was to assess and compare Visual Analogue score (VAS) for pain assessment at rest and on movement recorded at 0, 3, 6, 12, 18, and 24 hours postoperatively. Time to first patient bolus was noted, and the number of morphine demands and total consumption in milligrams in 24 hours were recorded. Patients were also assessed for any possible adverse effects of opioids or regional anesthesia.
Sample size calculation
Based on previous similar studies, the average consumption of morphine was approximately 31.6+/– 12.5 mg for 24 hours.[8] Anticipating approximately 30% less average consumption in our intervention group, for a 5% level of significance with 80% power and a two-sided test, the total sample size required for the study was N = 34. These patients were randomly allocated in equal numbers, 17 cases in Group A and 17 cases in Group B.
Statistical analysis
Statistical analysis was done using Statistical Package for Social Science (SPSS) version 21.0.
Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± SD. The Kolmogorov-Smirnov test tested the normality of data. If the normality was rejected, then non-parametric tests were used. Quantitative variables were compared using the t-test or Mann- Whitney Test. Qualitative variables were compared using the Chi-Square test or Fischer’s Exact test.
Results
In this prospective randomized study, thirty-four patients were eligible and enrolled to participate. Seventeen patients were allocated each to Group A and to Group B [Figure 1. CONSORT diagram].
Figure 1.
CONSORT Diagram
We compared demographic profiles, ASA status, ASIA grading, and duration of anesthesia and surgery in both groups. All the baseline characteristics were comparable in both groups. (Table 1. Comparison of Demography and other baseline characteristics). It was also seen that most patients undergoing surgery had fractures at multiple levels (N = 17), out of which the most common site was T12-L1 burst # (N = 9). Single-point burst fractures were mostly at the level of the lumbar region (N = 9). Also, the distribution of fracture levels was similar in both groups [Table 2]. The total duration of anesthesia was comparable between the two groups (Group A-225.88 ± 67.29 mins vs. Group B- 257.06 ± 67.76 mins, P = 0.18). Group A had 2, 4, and 11 patients belonging to ASIA grades B, D, and E, and group B had 6, 3, and 8 patients belonging to ASIA grades B, D, and E. ASIA grading between the two groups were comparable. There was also no significant difference in the ASA status of patients belonging to both groups (Group A- ASA I: 12 patients i.e., 70.59%, ASA II: 5 patients i.e., 20.41%, Group B- ASA I: 14 patients i.e., 82.35%, ASA II: 3 patients i.e., 17.65%, P = 0.336).
Table 1.
Comparison of Demography and other baseline characteristics
| Parameters | Group A | Group B | P | |||
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age (Years) (Mean±SD) | 33.65±10.99 | 30.53±10.01 | 0.393 | |||
| Gender (%) | ||||||
| Male | n=12 (7.59%) | n=11 (64.71%) | 0.714 | |||
| Female | n=5 (29.41%) | n=6 (35.29%) | ||||
| Body mass index (kg/m2) (Mean±SD) | 26.2±2.77 | 26.94±2.54 | 0.422 | |||
| ASA (%) | ||||||
| I | 12 (70.59%) | 14 (82.35%) | 0.688 | |||
| II | 5 (29.41%) | 3 (17.65%) | ||||
| ASIA (%) | ||||||
| B | 2 (11.76%) | 6 (35.29%) | 0.336 | |||
| D | 4 (23.53%) | 3 (17.65) | ||||
| E | 11 (64.71%) | 8 (47.06%) | ||||
| Duration of surgery (mins) (Mean±SD) | 144.12±63.82 | 177.35±72.29 | 0.165 | |||
| Duration of anesthesia (mins) (Mean±SD) | 225.88±65.05 | 257.06±67.76 | 0.18 |
Table 2.
Comparison of distribution of level of fracture between Group A and Group B
| #Level | Group A | Group B | ||
|---|---|---|---|---|
| Lumbar # | 4 | 5 | ||
| Thoracic # | 4 | 4 | ||
| Multilevel # | 9 | 8 |
As the patient was shifted to the postoperative care unit, the time to the first activation of the PCA pump was significantly earlier (P = 0.0003) in Group B (9.41 ± 11.43 minutes) as compared to Group A (53.82 ± 46.25 minutes). Patients allocated to Group B also needed significantly (P < 0.0001) more morphine requests/demands (151 ± 68 times) than patients of Group A (54 ± 45) [Figure 2]. Since the number of requests was higher in Group B compared to Group A, postoperative morphine consumption was also significantly higher in Group B (37.82 ± 9.88 mg) as compared with Group A (17.06 ± 9.59 mg) with a p-valve = <0.0001 [Figure 3].
Figure 2.
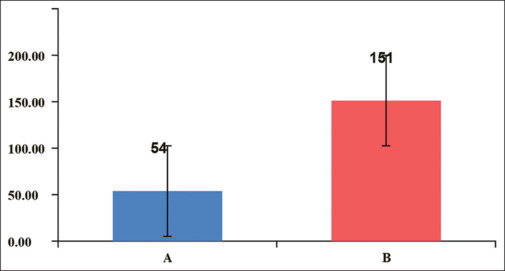
Comparison of total morphine demand (mg) between Group A and B
Figure 3.
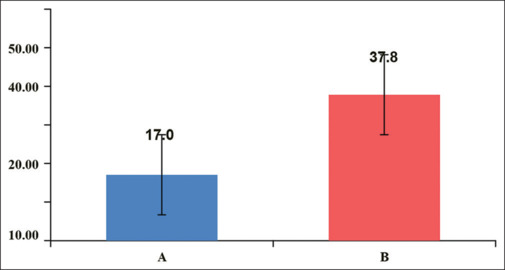
Comparison of total morphine consumption (mg) between group A and B
We also assessed and compared postoperative pain between the groups at rest and with movement using the VAS score at the following time points: 0, 3, 6, 12, and 24 hours after surgery. The VAS scores at rest and movement were significantly lower in Group A compared to Group B at all-time points that is, 0, 3, 6, 12, and 24 hours [Figure 4] with a p-valve = <0.05 at all-time points.
Figure 4.
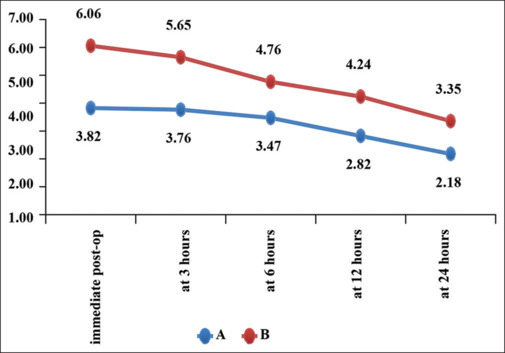
Comparison of VAS at rest between group A and B
Intraoperative fentanyl consumption was also compared between the two groups. The total intraoperative fentanyl requirement was significantly higher for Group B (342.35 ± 152.22 mcg) than for Group A (211.76 ± 59.37 mcg). The difference was statistically significant (P < 0.0003).
Discussion
Traumatic spinal injuries were responsible for the loss of an estimated 9.5 million disability-adjusted life-years globally between 1990 and 2016.[9,10] With a better understanding of the pathophysiology of spinal cord injury, anesthetic and surgical techniques have evolved and become much safer. The focus has, therefore, increasingly been shifted to surgical intervention and improving the outcome.[11,12]
Postoperative pain in patients undergoing posterior traumatic spine surgery ranks amongst the most painful surgical procedures and is a serious deterrent to enhanced recovery after surgery. It is also challenging to get good analgesic control with systemic analgesics without considering their adverse effects for an anesthetist. Recently, with the increasing use of ultrasound, there has been a renewed focus on adding regional to multimodal analgesic regimens and decreasing the use of opioids. Regional techniques, however, are lacking for traumatic spine surgeries.
The paraspinal muscles and posterior processes, arches, and ligaments are the sources of pain in posterior spine surgery. These parts belong to the dorsal compartment of the vertebral column and are innervated by the dorsal rami of the spinal nerves. Interfacial plane blocks, like ESP, have emerged as a possible and promising regional technique for posterior spine surgery.[13]
The ESP plane block directly targets dorsal rami, thus providing analgesia for cutaneous and musculoskeletal structures of the back during spine surgery. A local anesthetic may also seep through perforating channels and act indirectly on the spinal nerve roots, ventral rami, or epidural space. This lack of direct injection at target neural structures leads to relatively low concentration at nerves resulting in selective nociception without motor and other side effects.[13] Further adding to the safety of ESP block is its relative ease of locating, and performing, and relative lack of any neurovascular structures. Real-time in-plane sonographic visualization of complete needle trajectory further improves safety.
Since the first description of ESP Block by Forero et al.,[7] several studies have shown it provides good postoperative analgesia after breast, abdominal, bariatric, and thoracic surgery. However, limited quality literature is available for spine surgeries, and complete lack of studies for traumatic spine fixation. We, therefore conducted a prospective randomized trial comparing the efficacy of bilateral erector spinae plane block to conventional postoperative surgical site infiltration with local anesthetics on postoperative pain relief in traumatic spine surgery.
The frequency and severity of pain following any surgery are directly influenced by the type and duration of surgery, which may affect the requirement for analgesia and lead to confounding. The average duration of surgery (P = 0.165) and ASIA grading of injuries and other confounding demographic variables were comparable in both groups (Group A, Group B) of our study. Also, in our study, it was seen that the majority of patients had fractures in more than one level (N = 16) of which the most common site was T12-L1.
We administered fentanyl at induction and as a rescue analgesic during surgery. Total intraoperative fentanyl doses were calculated and compared between the two groups. Intraoperative fentanyl requirement was significantly higher for the control group (342.35 ± 152.22) as compared to patients with a block (211.76 ± 59.3, P < 0.0003). We noted that fentanyl was initially needed in the ESP block group during the surgery as the effect of plane blocks starts after half an hour to 45 minutes.[14] Later, the analgesic effect of ESP block sets in, and the fentanyl requirement significantly decreases in patients with ESP block.
We trained the patients to use a PCA pump with systemic morphine and recorded the time for its activation. PCA was activated soon after the surgery, as early as 9–10 mins in postoperative patients with local infiltration. In comparison, it took approximately 1 hour (53.82 ± 46.25, P = 0.0003) with ESP block. We then followed and checked the number of times the patient demanded morphine. Patients not given block demanded 151 times in 24 hours, more than the pump could deliver, while those with ESP block demanded a significantly lesser number of times (N = 54). This resulted in significantly higher morphine consumption in patients without block (A = 17.06 ± 9.59, B = 37.82 ± 9.88, P = <0.0001). We compared pain scores between the groups at rest and with movement at 0, 3,6,12, and 24 hours to assess and compare the multimodal analgesic efficacy of PCA morphine with infiltration or block. Analgesic scores at rest and movement, even with PCA morphine, were significantly lower in the group with ESP Block than in group B with infiltration at all time intervals in 24 hours. We thus infer that ESP block provides superior analgesic control than local surgical site infiltration with local anesthetics in patients undergoing traumatic spine surgery.
We searched the literature for ESP block’s efficacy for other surgeries. In a systemic review and meta-analysis comparing ESP block for breast surgeries, the block showed promising results. Patients with ESP block required minimal morphine as low as 0.12 mg to 5 mg within post-operative 24 hours. It also showed minimal demands (24 events in 125 patients) of morphine in patients with block.[15]
We found three studies by Tulgar et al., Ozdemir et al., and Altıparmak et al. for patients undergoing Laparoscopic Cholecystectomy under General anesthesia and ESP block at lower thoracic levels.[16,17,18] Tulgar and colleagues[16] observed that the analgesic score was better in patients with ESP block compared to the control group only for the initial 3 hours and lesser tramadol consumption in the first 12 hours. Halime et al.[17] also found that although ESP blocks with tramadol PCA provided better analgesia than subcostal transverses abdominis plane block, patients asked for the first dose of tramadol within the second postoperative hour. Atiparma k et al.[18] also found that patients with ESP block for postoperative analgesia needed 4 mg of morphine within the first 4 hours and 24 mg of Morphine within 24 hours, even with a background PCA of tramadol.
We also searched for literature on ESP block for surgeries further at lower lumbar levels, In a study by Tulgar et al. ESP block was performed at a fourth lumbar vertebral level for patients undergoing Hip surgery.[19] They concluded that the ESP block provided better analgesia than the control for the first six hours, and postoperative tramadol PCA consumption was also significantly less for First 12 hours.
We noticed that for surgeries like the breast, where ESP block was performed at the thoracic level, it provided a pain-free period with minimal supplemental analgesia for 24 to 48 hours. When the ESP block was performed at lower thoracic levels for laparoscopic cholecystectomy, it provided better analgesia than the control. However, its effect was less remarkable than breast surgeries, and patients asked for analgesic supplements within 2 to 4 hours, when ESP block was performed at even lower lumbar levels for hip surgery, ESP block only provided significant benefit for the initial 6 to 12 hours compared to the control. We also found similar results. Patients with ESP block had significantly better pain control at rest and movement for 24 hours. They also demanded significantly less morphine, and therefore consumption was significantly less. However, our patients with ESP block asked for Morphine within the first hour. Morphine consumption and demands were also more frequent than previously described surgeries at other levels.
These differences can be attributed to the following reasons. There are anatomical differences as we go down from upper thoracic to lower thoracic and lumbar levels. Erector Spinae muscles become bulkier thus, sonographic anatomy becomes more difficult to visualize and locate than upper thoracic levels. More tendinous attachments at lower levels also make it difficult to hydro dissect at this plane and decrease the extent of spread at lower levels. Most patients in our study had fractures at more than one level (N = 17) most common being at the T12-L1 vertebra (N = 9). Also, single-level fractures were more in number in the lumbar region (N = 10) as compared to thoracic (N = 6). Surgeries for spine fractures at more than one level are associated with severe postoperative pain compared to other surgeries like breast, laparoscopic cholecystectomy, or hip surgeries. Moreover, we used a PCA pump for morphine, a subjective patient-based approach, rather than other studies where rescue analgesia was only administered when pain scores were considered unacceptable by the treating physician or nurse.
We found a systemic review and meta-analysis for ESP block performed at lumbar levels for spine surgery.[20] They included six studies, and similar to our findings, they revealed that ESP block provided superior analgesia to control or sham block for the first 24 hours and decreased the need for opioids. Unlike opioid requirements and consumption in our study, it was lesser in their studies. This may be because they mostly included lesser painful decompression or discectomy surgery as compared to ours where surgeries were performed for spine fractures at more than one level. Their analysis was limited by the no. of studies and the sample size in each study. Their studies lacked the primary investigator’s blinding and didn’t analyze and consider the risk of publication bias in the much-hyped ESP block. They also didn’t analyze the time to rescue analgesia, no. of demands in studies where PCA was used, and pain scores at movement, which is crucial for early ambulation.
Spine fracture surgeries are associated with moderate to severe postoperative pain. Thus, it carries a higher incidence of opioid consumption and postoperative nausea and vomiting (PONV). We in our study also observed for PONV and other complications. None of our patients had any episodes of PONV or any other complications. This may be because we administered prophylactically dexamethasone and ondansetron to all the patients. Additionally, we didn’t have a relevant sample size to find or comment on any complications.
Limitations
Our study was conducted in a trauma center, with the population exclusively being traumatic spine injury patients, including mostly young males. Hence our data can’t be extrapolated to other spine surgeries like scoliosis and populations of other age groups. Another major limitation of our study was the small sample size.
Conclusion
ESP block, a relatively safe and easy-to-perform regional technique, has shown promising results for various surgeries. There is a dearth of well-blinded quality literature on ESP block in painful, traumatic spine surgery. We found that an ESP block provided better pain control than conventional local infiltration for these surgeries. We also concluded that a rather multimodal approach of ESP block with opioids might be needed to get the desired pain control and early recovery. However, our study was severely limited by sample size. Further, more prospective, randomized clinical studies are needed to investigate the role of ESP block for traumatic spine surgeries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to express our deep gratitude to Dr Kamran Farooque, Professor, Department of Orthopedics, for his valuable and constructive suggestions during the planning and development of this research work. Without his coherent and illuminating instructions, this paper would not have reached its present form. His generosity has been one of the valuable contributions to this paper.
References
- 1.World Health Organisation Spinal cord injury. 2013 Available from: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury . [Google Scholar]
- 2.Bajwa SJS, Haldar R. Pain management following spinal surgeries: An appraisal of the available options. J Craniovertebr Junction Spine. 2015;6:105–10. doi: 10.4103/0974-8237.161589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avis G, Gricourt Y, Vialatte PB, Meunier V, Perin M, Simon N, et al. Analgesic efficacy of erector spinae plane blocks for lumbar spine surgery: A randomized double-blind controlled clinical trial. Reg Anesth Pain Med. 2022;47:610–6. doi: 10.1136/rapm-2022-103737. [DOI] [PubMed] [Google Scholar]
- 4.Melvin JP, Schrot RJ, Chu GM, Chin KJ. Low thoracic erector spinae plane block for perioperative analgesia in lumbosacral spine surgery: A case series. Can J Anaesth 65, 2018;9:1057–65. doi: 10.1007/s12630-018-1145-8. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Chaudhary N. Bilateral ultasound guided erector spinae plane block for postoperative pain management in lumbar spine surgery: A case series. J Neurosurg Anesthesiol. 2019;31:354. doi: 10.1097/ANA.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 6.Restrepo-Garces CE, Chin KJ, Suarez P, Diaz A. Bilateral continuous erector spinae plane block contributes to effective postoperative analgesia after major open abdominal surgery: A case report. A A Case Rep. 2017;9:319–21. doi: 10.1213/XAA.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 7.Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: A novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41:621–7. doi: 10.1097/AAP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 8.Dewinter G, Moens P, Fieuws S, Vanaudenaerde B, Van de Velde M, Rex S. Systemic lidocaine fails to improve postoperative morphine consumption, postoperative recovery and quality of life in patients undergoing posterior spinal arthrodesis. A double-blind, randomized, placebo-controlled trial. Br J Anaesth. 2017;118:576–85. doi: 10.1093/bja/aex038. [DOI] [PubMed] [Google Scholar]
- 9.Global Burden of Disease 2016 Neurology Collaborators Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–80. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Spinal Cord Injury (National Spinal Cord Injury Statistical Centre (online Database) Cited 2010 November 01. [Google Scholar]
- 11.Fehlings MG, Vaccaro A, Wilson JR. Early versus delayed decompression for traumatic cervical spinal cord injury: Results of the surgical timing in acute spinal cord injury study (STASCIS) PLoS One. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J-M, Long X-H, Zhou Y, Peng H-W, Liu Z-L, Huang S-H. Is urgent decompression superior to delayed surgery for traumatic spinal cord injury? a meta-analysis. World Neurosurg. 2016;87:124–31. doi: 10.1016/j.wneu.2015.11.098. [DOI] [PubMed] [Google Scholar]
- 13.Chin KJ, El-Boghdadly Mechanisms of action of the erector spinae plane (ESP) block: A narrative review. Can J Anaesth. 2021;68:387–408. doi: 10.1007/s12630-020-01875-2. [DOI] [PubMed] [Google Scholar]
- 14.Malawat A, Verma K, Jethava D, Jethava DD. Erector spinae plane block for complete surgical anesthesia and postoperative analgesia for breast surgeries: A prospective feasibility study of 30 cases. Indian J Anaesth. 2020;64:118–24. doi: 10.4103/ija.IJA_639_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W, Wang W, Xie W, Chen Z, Liu Y. Erector spinae plane block for postoperative analgesia in breast and thoracic surgery: A systematic review and meta-analysis. J Clin Anesth. 2020;66:109900. doi: 10.1016/j.jclinane.2020.109900. doi: 10.1016/j.jclinane.2020.109900. [DOI] [PubMed] [Google Scholar]
- 16.Tulgar S, Kose HC, Selvi O, Senturk O, Thomas DT, Ermis MN, et al. Comparison of ultrasound-guided lumbar erector spinae plane block and transmuscular quadratus lumborum block for postoperative analgesia in hip and proximal femur surgery: A prospective randomized feasibility study. Anesth Essays Res. 2018;12:825–31. doi: 10.4103/aer.AER_142_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozdemir H, Araz C, Karaca O, Turk E. Comparison of ultrasound-guided erector spinae plane block and subcostal transversus abdominis plane block forpostoperative analgesia after laparoscopic cholecystectomy: A randomized, controlled trial. J Investigative Surg. 2022;35:870–7. doi: 10.1080/08941939.2021.1931574. [DOI] [PubMed] [Google Scholar]
- 18.Altıparmak B, Korkmaz Toker M, Uysal AI, Kuşçu Y, GümüşDemirbilek S. Ultrasound-guided erector spinae plane block versus oblique subcostal transversus abdominis plane block for postoperative analgesia of adult patients undergoing laparoscopic cholecystectomy: Randomized, controlled trial. J Clin Anesth. 2019;57:31–6. doi: 10.1016/j.jclinane.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Tulgar S, Kose HC, Selvi O, Senturk O, Thomas DT, Ermis MN, et al. Comparison of ultrasound-guided lumbar erector spinae plane block and transmuscular quadratus lumborum block for postoperative analgesia in hip and proximal femur surgery: A prospective randomized feasibility study. Anesth Essays Res. 2018;12:825–31. doi: 10.4103/aer.AER_142_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu MJ, Zhou XY, Yao YB, Shen X, Wang R, Shen QH. Postoperative analgesic efficacy of erector spinae plane block in patients undergoing lumbar spinal surgery: A systematic review and meta-analysis. Pain Ther. 2021;10:333–47. doi: 10.1007/s40122-021-00256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


