Abstract
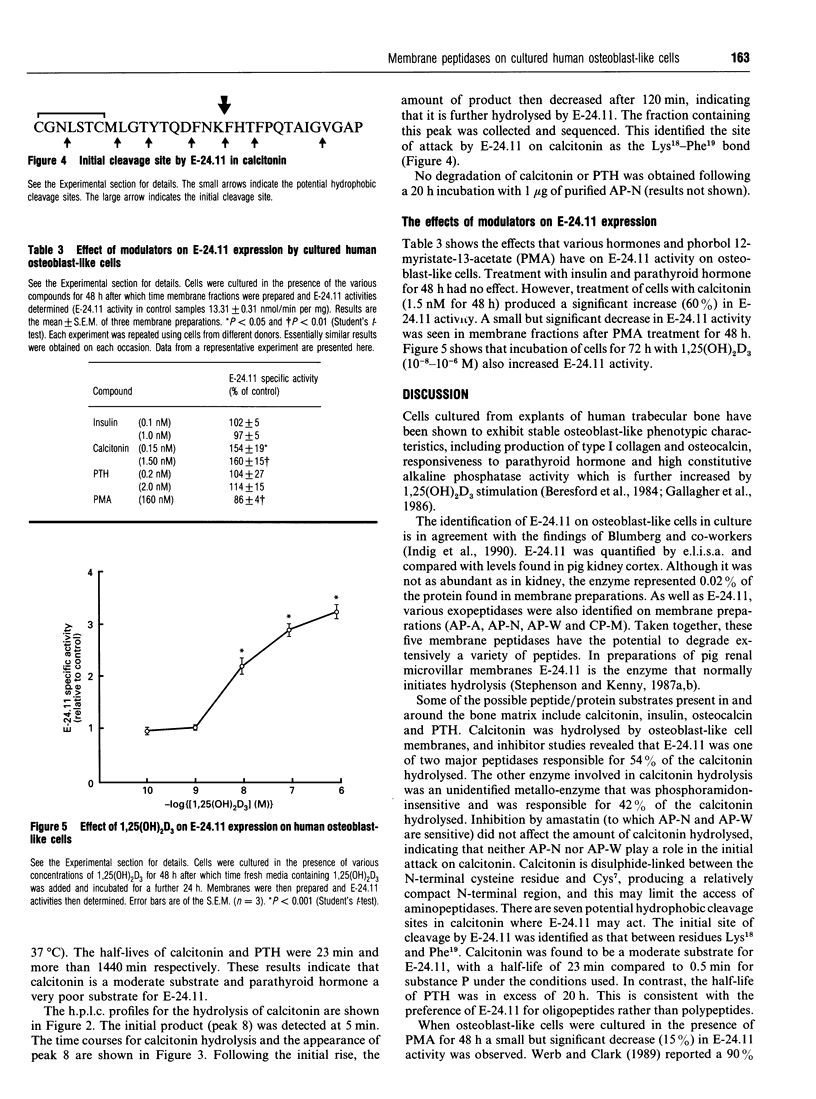
Five membrane peptidase activities have been identified on cultured human osteoblast-like cells. These consisted of the four exopeptidases aminopeptidase-A, aminopeptidase-N, aminopeptidase-W and carboxypeptidase-M, and the endopeptidase, endopeptidase-24.11. The presence of endopeptidase-24.11 was confirmed immunochemically by immunofluorescent staining and by enzyme-linked immunosorbent assay. The inclusion of phosphoramidon partially inhibited the hydrolysis of human calcitonin by a membrane fraction prepared from osteoblast-like cell membranes, thus implicating endopeptidase-24.11 in its inactivation. Another metallopeptidase also contributed substantially to calcitonin hydrolysis. Purified porcine endopeptidase-24.11 (1 microgram) was shown to hydrolyse calcitonin with a half-life of 23 min, which compared to a half-life of 0.5 min for substance P under similar conditions. Sequence data revealed that the initial site of hydrolysis of calcitonin was between residues Lys18 and Phe19. The expression of endopeptidase-24.11 by cultured osteoblast-like cells was shown to be modified by various agents: expression was decreased by phorbol 12-myristate-13-acetate (160 nM for 48 h) and increased in the presence of calcitonin (1.5 nM for 48 h) and 1,25-dihydroxyvitamin D3 (0.01-1 microM for 72 h).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes K., Bourne A., Cook P. A., Turner A. J., Kenny A. J. Membrane peptidases in the peripheral nervous system of the pig: their localization by immunohistochemistry at light and electron microscopic levels. Neuroscience. 1991;44(1):245–261. doi: 10.1016/0306-4522(91)90265-p. [DOI] [PubMed] [Google Scholar]
- Barnes K., Turner A. J., Kenny A. J. Membrane localization of endopeptidase-24.11 and peptidyl dipeptidase A (angiotensin converting enzyme) in the pig brain: a study using subcellular fractionation and electron microscopic immunocytochemistry. J Neurochem. 1992 Jun;58(6):2088–2096. doi: 10.1111/j.1471-4159.1992.tb10950.x. [DOI] [PubMed] [Google Scholar]
- Beresford J. N., Gallagher J. A., Poser J. W., Russell R. G. Production of osteocalcin by human bone cells in vitro. Effects of 1,25(OH)2D3, 24,25(OH)2D3, parathyroid hormone, and glucocorticoids. Metab Bone Dis Relat Res. 1984;5(5):229–234. doi: 10.1016/0221-8747(84)90064-x. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne A., Barnes K., Taylor B. A., Turner A. J., Kenny A. J. Membrane peptidases in the pig choroid plexus and on other cell surfaces in contact with the cerebrospinal fluid. Biochem J. 1989 Apr 1;259(1):69–80. doi: 10.1042/bj2590069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E., McCarthy T., Centrella M. Growth factors and the regulation of bone remodeling. J Clin Invest. 1988 Feb;81(2):277–281. doi: 10.1172/JCI113318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly J. C., Skidgel R. A., Schulz W. W., Johnson A. R., Erdös E. G. Neutral endopeptidase 24.11 in human neutrophils: cleavage of chemotactic peptide. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8737–8741. doi: 10.1073/pnas.82.24.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyen J. H., Elford P., Di Padova F. E., Trechsel U. Interleukin-6 is produced by bone and modulated by parathyroid hormone. J Bone Miner Res. 1989 Aug;4(4):633–638. doi: 10.1002/jbmr.5650040422. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem J. 1983 Jun 1;211(3):743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee N. S., Kenny A. J. Proteins of the kidney microvillar membrane. Enzymic and molecular properties of aminopeptidase W. Biochem J. 1987 Aug 15;246(1):97–102. doi: 10.1042/bj2460097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Hohmann E. L., Elde R. P., Rysavy J. A., Einzig S., Gebhard R. L. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986 May 16;232(4752):868–871. doi: 10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- Hooper N. M., Low M. G., Turner A. J. Renal dipeptidase is one of the membrane proteins released by phosphatidylinositol-specific phospholipase C. Biochem J. 1987 Jun 1;244(2):465–469. doi: 10.1042/bj2440465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Aminopeptidase P is anchored by a glycosyl-phosphatidylinositol moiety. FEBS Lett. 1988 Mar 14;229(2):340–344. doi: 10.1016/0014-5793(88)81152-9. [DOI] [PubMed] [Google Scholar]
- Howell S., Kenny A. J., Turner A. J. A survey of membrane peptidases in two human colonic cell lines, Caco-2 and HT-29. Biochem J. 1992 Jun 1;284(Pt 2):595–601. doi: 10.1042/bj2840595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S., Murray H., Scott C. S., Turner A. J., Kenny A. J. A highly sensitive E.L.I.S.A. for endopeptidase-24.11, the common acute-lymphoblastic-leukaemia antigen (CALLA, CD-10), applicable to material of porcine and human origin. Biochem J. 1991 Sep 1;278(Pt 2):417–421. doi: 10.1042/bj2780417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indig F. E., Ben-Meir D., Spungin A., Blumberg S. Investigation of neutral endopeptidases (EC 3.4.24.11) and of neutral proteinases (EC 3.4.24.4) using a new sensitive two-stage enzymatic reaction. FEBS Lett. 1989 Sep 25;255(2):237–240. doi: 10.1016/0014-5793(89)81098-1. [DOI] [PubMed] [Google Scholar]
- Indig F. E., Benayahu D., Fried A., Wientroub S., Blumberg S. Neutral endopeptidase (EC 3.4.24.11) is highly expressed on osteoblastic cells and other marrow stromal cell types. Biochem Biophys Res Commun. 1990 Oct 30;172(2):620–626. doi: 10.1016/0006-291x(90)90719-4. [DOI] [PubMed] [Google Scholar]
- LeBien T. W., McCormack R. T. The common acute lymphoblastic leukemia antigen (CD10)--emancipation from a functional enigma. Blood. 1989 Feb 15;73(3):625–635. [PubMed] [Google Scholar]
- Lorkowski G., Zijderhand-Bleekemolen J. E., Erdös E. G., von Figura K., Hasilik A. Neutral endopeptidase-24.11 (enkephalinase). Biosynthesis and localization in human fibroblasts. Biochem J. 1987 Dec 1;248(2):345–350. doi: 10.1042/bj2480345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G. Hormonal regulation of bone growth and remodelling. Ciba Found Symp. 1988;136:226–238. doi: 10.1002/9780470513637.ch14. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson S. L., Kenny A. J. Metabolism of neuropeptides. Hydrolysis of the angiotensins, bradykinin, substance P and oxytocin by pig kidney microvillar membranes. Biochem J. 1987 Jan 1;241(1):237–247. doi: 10.1042/bj2410237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson S. L., Kenny A. J. The hydrolysis of alpha-human atrial natriuretic peptide by pig kidney microvillar membranes is initiated by endopeptidase-24.11. Biochem J. 1987 Apr 1;243(1):183–187. doi: 10.1042/bj2430183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Clark E. J. Phorbol diesters regulate expression of the membrane neutral metalloendopeptidase (EC 3.4.24.11) in rabbit synovial fibroblasts and mammary epithelial cells. J Biol Chem. 1989 Jun 5;264(16):9111–9113. [PubMed] [Google Scholar]



