Abstract
Safer, less-reactive superacid processing enables printing and coating of carbon nanotubes into films, fibers, and fabrics.
Carbon nanotubes (CNTs) have the potential to serve as building blocks for commercial and industrial products (1). Their unique features make them ideal for use in applications such as electrical cables, cell phone screens, and smart fabrics.
One reason is size. With diameters as minute as 1 nm and lengths stretching to centimeters, the ratio of width to height is similar to that of a human hair stretched to the tip of the Empire State Building. Known for their extreme mechanical and electrical properties, CNTs have mainly been used as high-performance additives in conventional composite materials (2).
However, despite advances in fabrication and processing over the past 20 years, CNTs still face obstacles when it comes to scaling up processing for real-world applications. Transforming CNTs into fibers and aerogels using solvent-free methods (3) has resulted in a number of remarkable materials, but this type of processing is not easily scalable. Processing CNTs using liquids is notoriously difficult because CNTs tend to bundle and agglomerate in almost all solvents.
Nanotube processing
Engineers can consider CNTs as a macroscale rigid-rod polymer system, similar to the aramid polymers used to create Kevlar (4). Much like CNTs, these rigid-rod polymers are valued because they can form fibers with an extremely high strength-to-weight ratio. The most industrially relevant of these rigid-rod polymers are para-aramids. These fibers are famed for their applications including ballistic protection. Para-aramids are typically processed in fuming sulfuric acid; this acid contains additional reagents to prevent dilution if the acid is exposed to small quantities of water. The fibers are processed at (i) high concentrations, (ii) high elongational flow, and/or (iii) high extensional drawing to increase alignment and strength. Aramid fibers are limited by molecular weight and misalignment at the fiber spinning nozzle.
Despite the increased complexity of synthesizing CNTs (compared to para-aramid synthesis via simple condensation polymerization), using CNT-based structures in premium applications is within reach by using acid-based processing techniques similar to those used for para-aramid fibers.
A promising approach to CNT processing is the use of superacids. In this case, CNTs are not merely “suspended” in the superacid but truly dissolved as individual nanoparticles. The acid protonates the surface of the CNTs, causing them to electrostatically repel one another. The most effective of these superacids is chlorosulfonic acid. Highly aligned CNT-based fibers (or films) can be spun from high-concentration CNT/superacid slurries before removing the acid to yield a solid CNT-only material, mimicking the processing of para-aramids. These fibers are both strong and electrically conductive, allowing for structurally sound electrical components such as light-emitting diodes and electron-emitting cathodes (5, 6).
Unfortunately, industrial processing using superacids is challenging. Superacids are dangerous and corrosive, especially chlorosulfonic acid. Counterintuitively, the addition of water to chlorosulfonic acid will not lead to immediate dilution but, instead, to the production of hydrochloric and sulfuric acid. This makes the extraction of superacids from the CNTs difficult. Chlorosulfonic acid also reacts with water in the air, which means that environmentally controlled spaces are needed. In addition, the chemical reactivity of the acid also prevents recovery and recycling of the solvent for subsequent processing, drastically limiting economic feasibility beyond the laboratory scale. Thus, the key challenge for CNT-acid systems is this: Can these processes shift to a different solvent system that would allow for scalable, safer processing at volumes that are relevant to practical applications?
An alternate approach
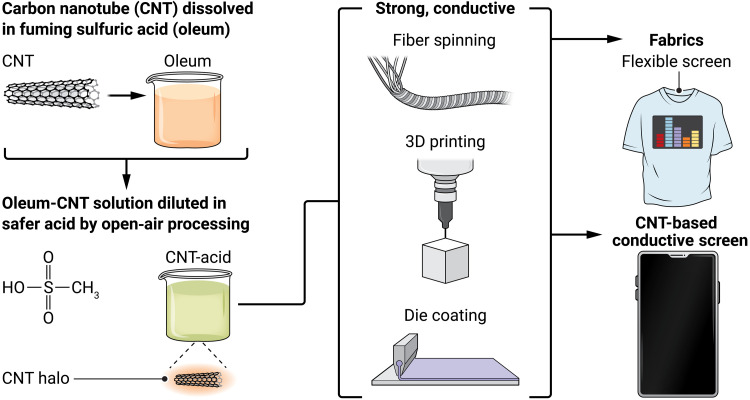
In this issue of Science Advances, Headrick et al. (7) describe previously unknown acid solvent systems with low corrosivity that can still form CNT solutions (Fig. 1). What is unique about the approach is that the CNTs are first dissolved in fuming sulfuric acid (“oleum”) at high concentrations and then the solutions are diluted in related, safer acids, such as methanesulfonic acid (MSA) and p-toluenesulfonic acid (tPoS), which do not have violent reactions with water or burn through everyday materials such as latex gloves. Even after the oleum-CNT solution is diluted in the weaker acid, a halo of oleum stays around the CNT, allowing CNTs to remain well dispersed.
Fig. 1. Fabrication of CNTs with less-hazardous acids enables safe, open-air processing of CNTs into strong conductive films, fibers, fabrics, and 3D-printed structures.
Credit: Ashley Mastin/Science Advances.
The solvents used by Headrick et al. (7) are substantially safer and easier to use in open-air environments without the need for industrial ventilation. They are compatible with a number of plastic substrates, allowing for carrier films or materials consumed during processing. Larger-scale equipment can also be built without the specialty metals required for superacids. Interestingly, tPoS forms a completely solid phase at room temperature before solvent removal. This feature may allow for unique and simplified solvent recovery systems.
Using this system, conventional liquid phase processing methodologies [such as fiber spinning, blade coating, and even three-dimensional (3D) printing] become possible. CNTs can be dispersed at high concentrations and processed at high shear rates and draw rates, all of which allow for dense structures with a high degree of alignment but not a high degree of agglomeration.
For instance, Headrick et al. (7) showed that the CNT-MSA solution can be extruded through a notch die onto a plastic substrate to form a thin film. Because the CNTs are dispersed as individual tubules, the record-setting figure of merit of 143 (for high conductivity and high transparency at the same time) is remarkable. Usually, these two properties are inversely proportional, with either high CNT coverage and low transparency or low CNT coverage and high transparency.
The ability to coat individual CNTs onto a wide range of surfaces in high-concentration slurries makes these materials possible. CNT inks could be used in applications such as flexible screens and wearable batteries or even as replacements for indium tin oxide (ITO). Substituting CNTs for ITO, which is currently used in smartphone screens and other electronics, would allow for flexible and durable transparent conductors. Die-based coatings can be cross-applied to a wide range of substrates (including fabrics) and can even be applied using low-cost screen printing.
In addition to high-performance fiber spinning, CNT-acid solutions can also be used for direct-ink-write (DIW) 3D printing. Solidified tPoS allows for self-supporting 3D-printed structures without complex chemical reactions or transport limitations. Other DIW systems require drying or cross-linking to maintain stability. The self-supporting feature provides for direct printing of CNT-based aerogels, avoiding the typical sol-gel chemistry and complex drying processes associated with typical aerogel production.
CNT-acid processing solutions may enable a range of consumer products by providing a substantial avenue for the expansion of CNTs beyond the benchtop and into major industrial processes. Overcoming the industrial safety, solvent, equipment, and economic limitations of laboratory scale, the superacid processing is another step in that journey.
REFERENCES
- 1.Takakura A., Beppu K., Nishihara T., Fukui A., Kozeki T., Namazu T., Miyauchi Y., Itami K., Strength of carbon nanotubes depends on their chemical structures. Nat. Commun. 10, 3040 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green M. J., Behabtu N., Pasquali M., Adams W. W., Nanotubes as polymers. Polymer 50, 4979–4997 (2009). [Google Scholar]
- 3.Zhang M., Atkinson Ken K. R., Baughman R. H., Multifunctional carbon nanotube yarns by downsizing an ancient technology. Science 306, 1358–1361 (2004). [DOI] [PubMed] [Google Scholar]
- 4.H. Yang, Kevlar Aramid Fiber (Wiley, 1993). [Google Scholar]
- 5.Davis V. A., Parra-Vasquez A. N. G., Green M. J., Rai P. K., Behabtu N., Prieto V., Booker R. D., Schmidt J., Kesselman E., Zhou W., Fan H., Adams W. W., Hauge R. H., Fischer J. E., Cohen Y., Talmon Y., Smalley R. E., Pasquali M., True solutions of single-walled carbon nanotubes for assembly into macroscopic materials. Nat. Nanotechnol. 4, 830–834 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Behabtu N., Young Colin C., Tsentalovich Dmitri E., Kleinerman O., Wang X., Ma Anson W. K., Bengio E. A., ter Waarbeek Ron F., de Jong Jorrit J., Hoogerwerf Ron E., Fairchild Steven B., Ferguson John B., Maruyama B., Kono J., Talmon Y., Cohen Y., Otto Marcin J., Pasquali M., Strong, light, multifunctional fibers of carbon nanotubes with ultrahigh conductivity. Science 339, 182–186 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Headrick R. J., Williams S. M., Owens C. E., Taylor L. W., Dewey O. S., Ginestra C. J., Liberman L., Ya’akobi A. M., Talmon Y., Maruyama B., Mc Kinley G. H., Hart A. J., Pasquali M., Versatile acid solvents for pristine carbon nanotube assembly. Sci. Adv. 8, eabm3285 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]