Abstract
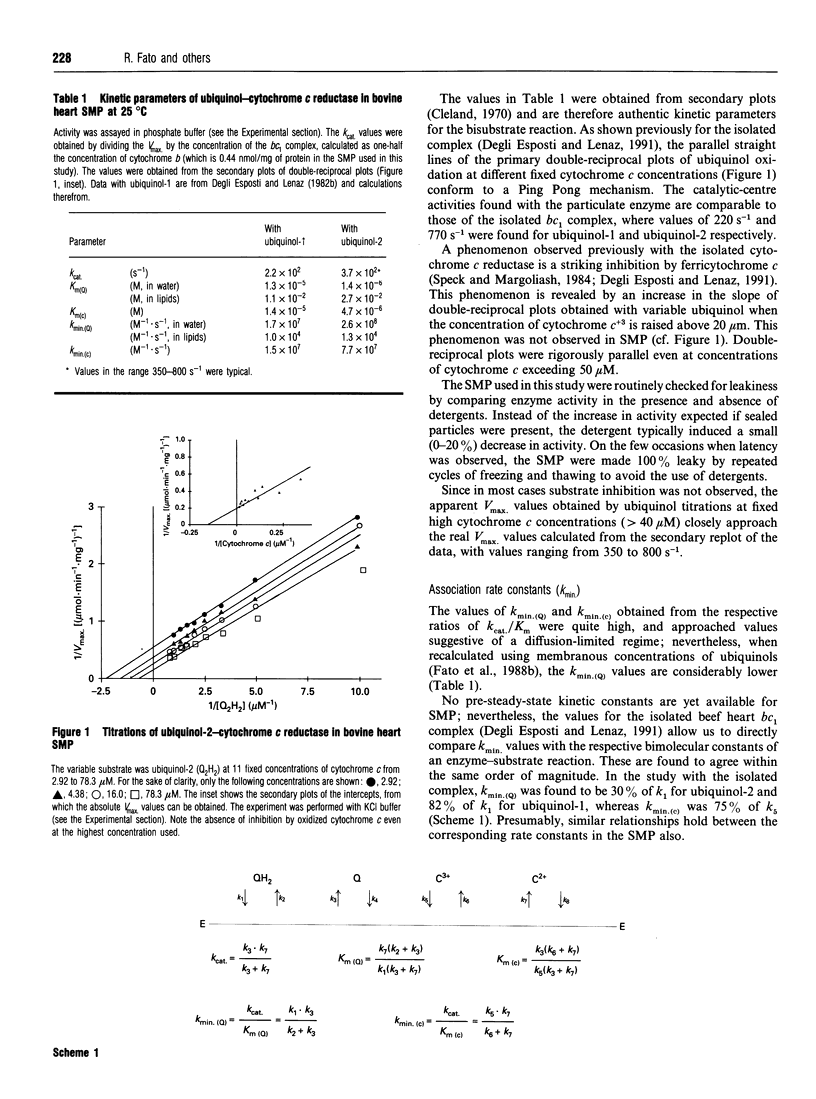
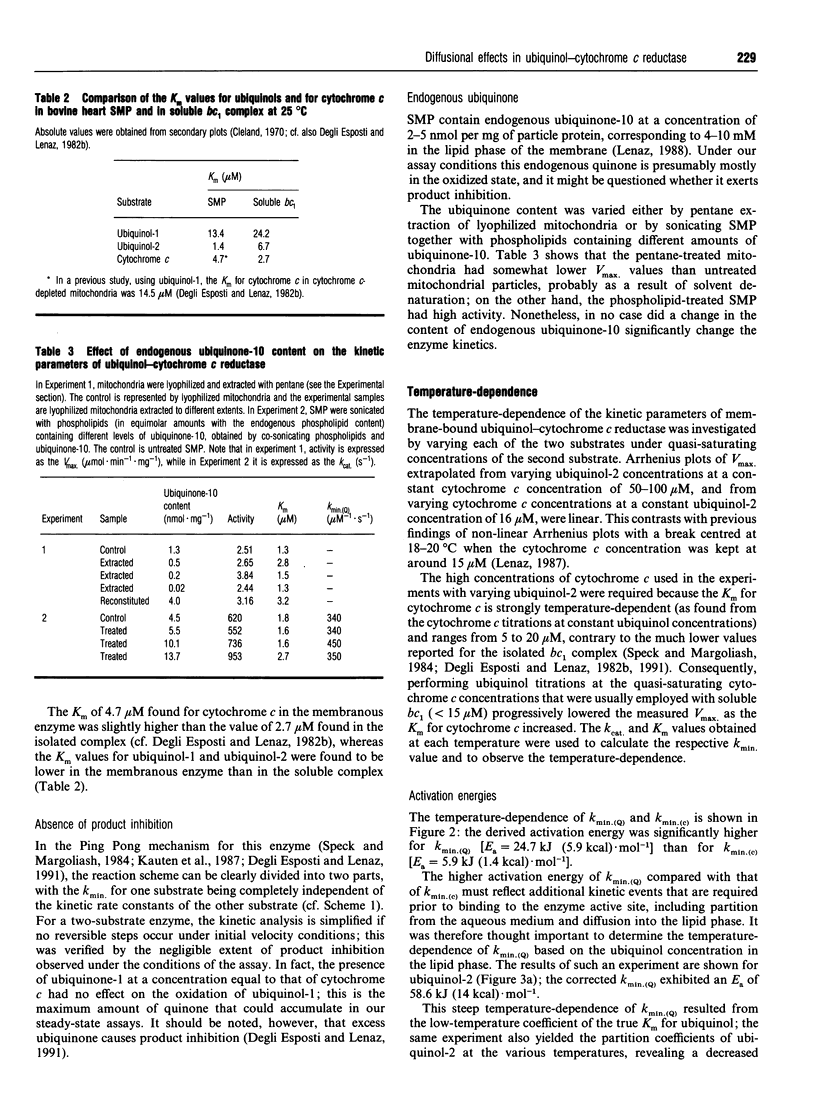
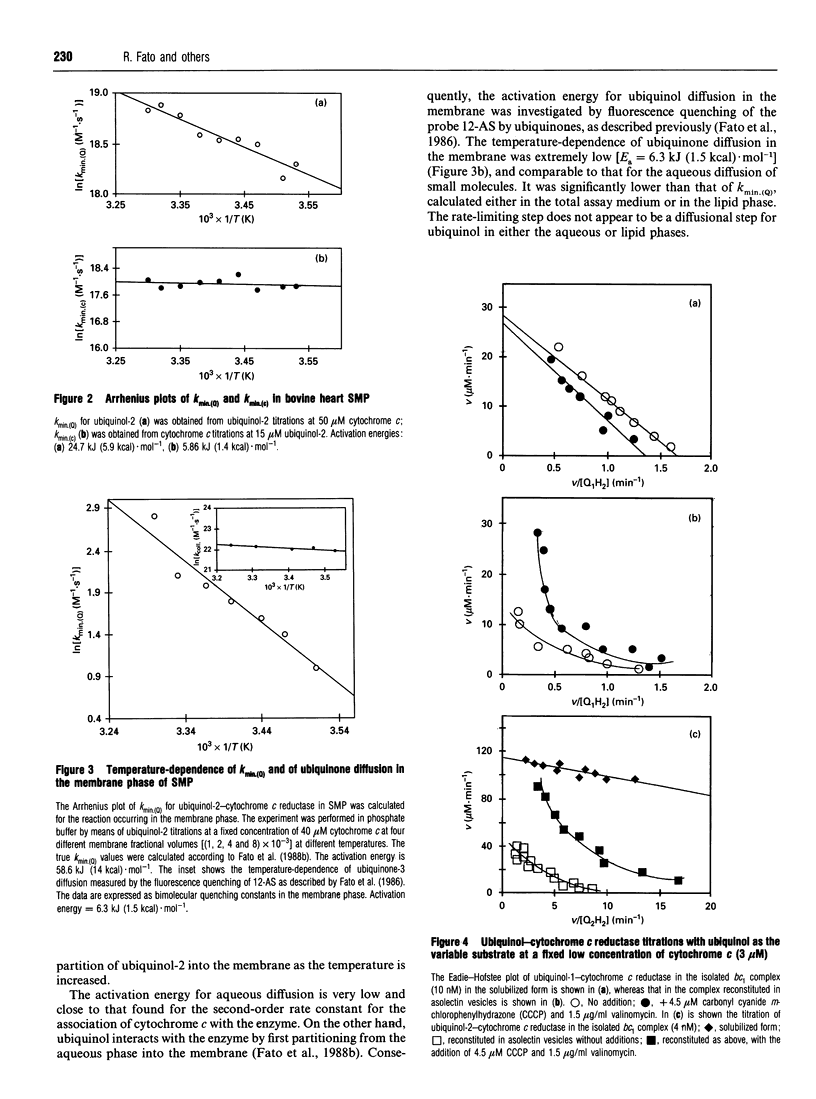
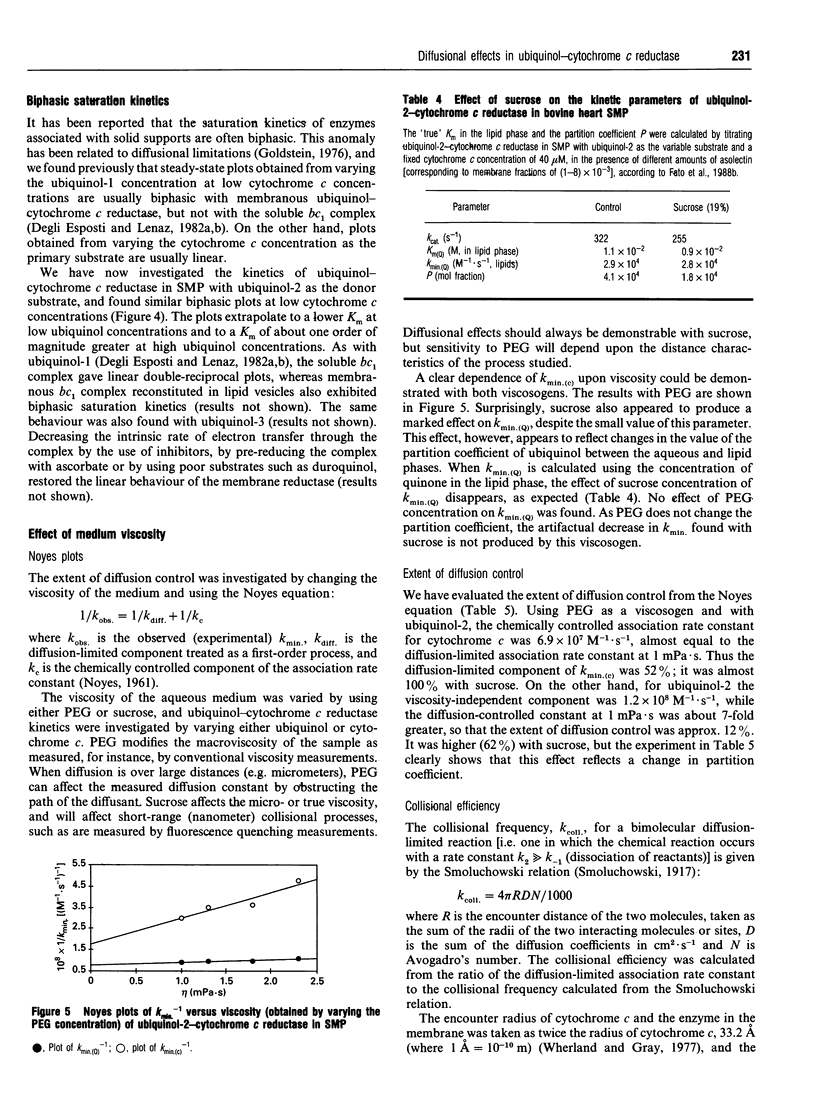
In an attempt to establish the relative importance of diffusional and chemical control in the reactivity of the two of the two substrates, ubiquinol and cytochrome c, we have undertaken as extensive characterization of the steady-state kinetics of ubiquinol-cytochrome c reductase (EC 1.10.2.2) when present in open submitochondrial particles from bovine heart. The kinetic pattern follows a Ping Pong mechanism; contrary to the situation found with the isolated enzyme [Speck and Margoliash (1984) J. Biol. Chem. 259, 1064-1072, and confirmed in our laboratory], no substrate inhibition by oxidized cytochrome c was observed with the membrane-bound enzyme. Endogenous oxidized ubiquinone-10 is unable to exert product inhibition under the conditions employed. In the Ping Pong mechanism for this enzyme, the reaction scheme can be clearly divided into two parts, and the Kmin. (kcat./km) value for one substrate is independent of the rate constant for the second substrate. Both ubiquinol-1 and ubiquinol-2 can be used as electron donors reacting with the enzyme from within the lipid bilayer [Fato, Castelluccio, Palmer and Lenaz (1988) Biochim. Biophys. Acta 932, 216-222]; the kmin. values for ubiquinols, when calculated on the basis of their membranous concentrations, are significantly lower than the kmin. for cytochrome c. The temperature-dependence of the kinetic parameters was investigated by titrating each of the substrates under quasi-saturating concentrations of the second substrate. Arrhenius plots of Vmax. extrapolated from both cytochrome c and ubiquinol titrations were linear, when care was taken to verify the quasi-saturating concentrations of the fixed co-substrate. The Arrhenius plots for the kmin. values for both ubiquinol and cytochrome c were linear, but the activation energy was much higher for the former, particularly when calculated for ubiquinol dissolved in the lipid phase; the very low value of activation energy of the kmin. for cytochrome c is strong support for diffusion control being present in the reaction of cytochrome c with the membranous enzyme. In contrast to the soluble enzyme, ubiquinone titrations of submitochondrial particles at low cytochrome c concentrations deviated from hyperbolic behaviour. Changing the medium viscosity with either poly(ethylene glycol) or sucrose had a strong effect on the cytochrome c kmin., whereas the change in the ubiquinol kmin. was much smaller. From the viscosity studies the extent of diffusional control could be calculated, revealing that the reaction with cytochrome c was mostly diffusion-limited. The viscosity of the membrane was changed by incorporating cholesterol; no significant effect on the ubiquinol kmin. ascribable to diffusion control could be recognized.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold K., Zschoernig O., Barthel D., Herold W. Exclusion of poly(ethylene glycol) from liposome surfaces. Biochim Biophys Acta. 1990 Mar;1022(3):303–310. doi: 10.1016/0005-2736(90)90278-v. [DOI] [PubMed] [Google Scholar]
- Berg O. G., von Hippel P. H. Diffusion-controlled macromolecular interactions. Annu Rev Biophys Biophys Chem. 1985;14:131–160. doi: 10.1146/annurev.bb.14.060185.001023. [DOI] [PubMed] [Google Scholar]
- Blackwell M. F., Gounaris K., Zara S. J., Barber J. A method for estimating lateral diffusion coefficients in membranes from steady-state fluorescence quenching studies. Biophys J. 1987 May;51(5):735–744. doi: 10.1016/S0006-3495(87)83400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J., Hornby W. E. Immobilized glutamate oxaloacetate transaminase. Steady state kinetic analysis and stability studies. Biochim Biophys Acta. 1975 Sep 22;403(1):79–88. doi: 10.1016/0005-2744(75)90010-8. [DOI] [PubMed] [Google Scholar]
- Casadio R., Baccarini-Melandri A., Melandri B. A. On the determination of the transmembrane pH difference in bacterial chromatophores using 9-aminoacridine. Eur J Biochem. 1974 Aug 15;47(1):121–128. doi: 10.1111/j.1432-1033.1974.tb03675.x. [DOI] [PubMed] [Google Scholar]
- Chazotte B., Hackenbrock C. R. The multicollisional, obstructed, long-range diffusional nature of mitochondrial electron transport. J Biol Chem. 1988 Oct 5;263(28):14359–14367. [PubMed] [Google Scholar]
- Coleman P. S., Lavietes B., Born R., Weg A. Cholesterol enrichment of normal mitochondria in vitro: a model system with properties of hepatoma mitochondria. Biochem Biophys Res Commun. 1978 Sep 14;84(1):202–207. doi: 10.1016/0006-291x(78)90282-6. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M., Bertoli E., Parenti-Castelli G., Fato R., Mascarello S., Lenaz G. Incorporation of ubiquinone homologs into lipid vesicles and mitochondrial membranes. Arch Biochem Biophys. 1981 Aug;210(1):21–32. doi: 10.1016/0003-9861(81)90159-4. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M., Lenaz G. Kinetics of ubiquinol-1-cytochrome c reductase in bovine heart mitochondria and submitochondrial particles. Biochim Biophys Acta. 1982 Nov 15;682(2):189–200. doi: 10.1016/0005-2728(82)90098-6. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M., Meier E. M., Timoneda J., Lenaz G. Modification of the catalytic function of the mitochondrial cytochrome b-c1 complex by dicyclohexylcarbodiimide. Biochim Biophys Acta. 1983 Nov 30;725(2):349–360. doi: 10.1016/0005-2728(83)90209-8. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M., Palmer G., Lenaz G. Circular dichroic spectroscopy of membrane haemoproteins. The molecular determinants of the dichroic properties of the b cytochromes in various ubiquinol:cytochrome c reductases. Eur J Biochem. 1989 Jun 1;182(1):27–36. doi: 10.1111/j.1432-1033.1989.tb14796.x. [DOI] [PubMed] [Google Scholar]
- Engasser J. M., Hisland P. Diffusional increase and decrease in half-maximal-activity substrate concentrations with two-substrate enzymic reactions. Biochem J. 1978 Jul 1;173(1):341–343. doi: 10.1042/bj1730341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engasser J. M. Kinetics of soluble and collagen-bound aspartate aminotransferase: diffusional effects with a two-substrate enzymatic reaction. J Biol Chem. 1977 Nov 25;252(22):7919–7922. [PubMed] [Google Scholar]
- Engel W. D., Schägger H., von Jagow G. Ubiquinol-cytochrome c reductase (EC 1.10.2.2). Isolation in triton X-100 by hydroxyapatite and gel chromatography. Structural and functional properties. Biochim Biophys Acta. 1980 Sep 5;592(2):211–222. doi: 10.1016/0005-2728(80)90182-6. [DOI] [PubMed] [Google Scholar]
- Fato R., Battino M., Degli Esposti M., Parenti Castelli G., Lenaz G. Determination of partition and lateral diffusion coefficients of ubiquinones by fluorescence quenching of n-(9-anthroyloxy)stearic acids in phospholipid vesicles and mitochondrial membranes. Biochemistry. 1986 Jun 3;25(11):3378–3390. doi: 10.1021/bi00359a043. [DOI] [PubMed] [Google Scholar]
- Fato R., Castelluccio C., Armaroli S., Contarini A., Parenti Castelli G., Lenaz G. Diffusional effects in the steady state kinetics of ubiquinol cytochrome c reductase in bovine heart submitochondrial particles. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1145–1153. doi: 10.1016/s0006-291x(88)81260-9. [DOI] [PubMed] [Google Scholar]
- Fato R., Castelluccio C., Palmer G., Lenaz G. A simple method for the determination of the kinetic constants of membrane enzymes utilizing hydrophobic substrates: ubiquinol cytochrome c reductase. Biochim Biophys Acta. 1988 Feb 11;932(2):216–222. doi: 10.1016/0005-2728(88)90158-2. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Kinetic behavior of immobilized enzyme systems. Methods Enzymol. 1976;44:397–443. doi: 10.1016/s0076-6879(76)44031-4. [DOI] [PubMed] [Google Scholar]
- Gupte S. S., Hackenbrock C. R. Multidimensional diffusion modes and collision frequencies of cytochrome c with its redox partners. J Biol Chem. 1988 Apr 15;263(11):5241–5247. [PubMed] [Google Scholar]
- Gupte S. S., Hackenbrock C. R. The role of cytochrome c diffusion in mitochondrial electron transport. J Biol Chem. 1988 Apr 15;263(11):5248–5253. [PubMed] [Google Scholar]
- Gupte S., Wu E. S., Hoechli L., Hoechli M., Jacobson K., Sowers A. E., Hackenbrock C. R. Relationship between lateral diffusion, collision frequency, and electron transfer of mitochondrial inner membrane oxidation-reduction components. Proc Natl Acad Sci U S A. 1984 May;81(9):2606–2610. doi: 10.1073/pnas.81.9.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R., Chazotte B., Gupte S. S. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J Bioenerg Biomembr. 1986 Oct;18(5):331–368. doi: 10.1007/BF00743010. [DOI] [PubMed] [Google Scholar]
- Hardy L. W., Kirsch J. F. Diffusion-limited component of reactions catalyzed by Bacillus cereus beta-lactamase I. Biochemistry. 1984 Mar;23(6):1275–1282. doi: 10.1021/bi00301a040. [DOI] [PubMed] [Google Scholar]
- Hasinoff B. B., Davey J. P. The kinetics of the aerobic oxidation of ferrocytochrome c by cytochrome c oxidase in solvents of increased viscosity are partially diffusion controlled. Biochim Biophys Acta. 1987 Jun 9;892(1):1–9. doi: 10.1016/0005-2728(87)90241-6. [DOI] [PubMed] [Google Scholar]
- Hasinoff B. B. Kinetics of acetylthiocholine binding to electric eel acetylcholinesterase in glycerol/water solvents of increased viscosity. Evidence for a diffusion-controlled reaction. Biochim Biophys Acta. 1982 May 21;704(1):52–58. doi: 10.1016/0167-4838(82)90131-5. [DOI] [PubMed] [Google Scholar]
- Kauten R., Tsai A. L., Palmer G. The kinetics of reduction of yeast complex III by a substrate analog. J Biol Chem. 1987 Jun 25;262(18):8658–8667. [PubMed] [Google Scholar]
- Kim C. H., Balny C., King T. E. Role of the hinge protein in the electron transfer between cardiac cytochrome c1 and c. Equilibrium constants and kinetic probes. J Biol Chem. 1987 Jun 15;262(17):8103–8108. [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. Further evidence for the pool function of ubiquinone as derived from the inhibition of the electron transport by antimycin. Eur J Biochem. 1973 Nov 15;39(2):313–323. doi: 10.1111/j.1432-1033.1973.tb03129.x. [DOI] [PubMed] [Google Scholar]
- Lenaz G. Role of mobility of redox components in the inner mitochondrial membrane. J Membr Biol. 1988 Sep;104(3):193–209. doi: 10.1007/BF01872322. [DOI] [PubMed] [Google Scholar]
- Lluis C. Lactate dehydrogenase associated with the mitochondrial fraction and with a mitochondrial inhibitor--I. Enzyme binding to the mitochondrial fraction. Int J Biochem. 1984;16(9):997–1004. doi: 10.1016/0020-711x(84)90117-4. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., LUSTGARTEN J. Interconversion of horse heart cytochrome C monomer and polymers. J Biol Chem. 1962 Nov;237:3397–3405. [PubMed] [Google Scholar]
- McCloskey M., Poo M. M. Protein diffusion in cell membranes: some biological implications. Int Rev Cytol. 1984;87:19–81. doi: 10.1016/s0074-7696(08)62439-0. [DOI] [PubMed] [Google Scholar]
- Meier P., Blume A., Ohmes E., Neugebauer F. A., Kothe G. Structure and dynamics of phospholipid membranes: an electron spin resonance study employing biradical probes. Biochemistry. 1982 Feb 2;21(3):526–534. doi: 10.1021/bi00532a018. [DOI] [PubMed] [Google Scholar]
- Nałecz M. J., Azzi A. Functional characterization of the mitochondrial cytochrome b-c1 complex: steady-state kinetics of the monomeric and dimeric forms. Arch Biochem Biophys. 1985 Aug 1;240(2):921–931. doi: 10.1016/0003-9861(85)90101-8. [DOI] [PubMed] [Google Scholar]
- Nicholls P. Catalytic activity of cytochromes c and c1 in mitochondria and submitochondrial particles. Biochim Biophys Acta. 1976 Apr 9;430(1):30–45. doi: 10.1016/0005-2728(76)90219-x. [DOI] [PubMed] [Google Scholar]
- Norling B., Glazek E., Nelson B. D., Ernster L. Studies with ubiquinone-depleted submitochondrial particles. Quantitative incorporation of small amounts of ubiquinone and its effects on the NADH and succinate oxidase activities. Eur J Biochem. 1974 Sep 16;47(3):475–482. doi: 10.1111/j.1432-1033.1974.tb03715.x. [DOI] [PubMed] [Google Scholar]
- Ragan C. I., Cottingham I. R. The kinetics of quinone pools in electron transport. Biochim Biophys Acta. 1985 Apr 8;811(1):13–31. doi: 10.1016/0304-4173(85)90003-5. [DOI] [PubMed] [Google Scholar]
- Ramasarma T. Lipid quinones. Adv Lipid Res. 1968;6:107–180. doi: 10.1016/b978-1-4831-9942-9.50010-3. [DOI] [PubMed] [Google Scholar]
- Saraste M. Location of haem-binding sites in the mitochondrial cytochrome b. FEBS Lett. 1984 Jan 30;166(2):367–372. doi: 10.1016/0014-5793(84)80114-3. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Skornick Y., Haran-Ghera N. Effective tumor immunization induced by cells of elevated membrane-lipid microviscosity. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5313–5316. doi: 10.1073/pnas.76.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snozzi M., Crofts A. R. Electron transport in chromatophores from Rhodopseudomonas sphaeroides GA fused with liposomes. Biochim Biophys Acta. 1984 Aug 31;766(2):451–463. doi: 10.1016/0005-2728(84)90261-5. [DOI] [PubMed] [Google Scholar]
- Speck S. H., Ferguson-Miller S., Osheroff N., Margoliash E. Definition of cytochrome c binding domains by chemical modification: kinetics of reaction with beef mitochondrial reductase and functional organization of the respiratory chain. Proc Natl Acad Sci U S A. 1979 Jan;76(1):155–159. doi: 10.1073/pnas.76.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck S. H., Margoliash E. Characterization of the interaction of cytochrome c and mitochondrial ubiquinol-cytochrome c reductase. J Biol Chem. 1984 Jan 25;259(2):1064–1072. [PubMed] [Google Scholar]
- Szarkowska L. The restoration of DPNH oxidase activity by coenzyme Q (ubiquinone). Arch Biochem Biophys. 1966 Mar;113(3):519–525. doi: 10.1016/0003-9861(66)90228-1. [DOI] [PubMed] [Google Scholar]
- Tsai A. L., Kauten R., Palmer G. Redox changes in coenzyme Q in the millisecond time range: an approach using rapid quenching and high-performance liquid chromatography. Anal Biochem. 1985 Nov 15;151(1):131–136. doi: 10.1016/0003-2697(85)90062-4. [DOI] [PubMed] [Google Scholar]
- WEBSTER D. The determination of total and ester cholesterol in whole blood, serum or plasma. Clin Chim Acta. 1962 Mar;7:277–284. doi: 10.1016/0009-8981(62)90021-9. [DOI] [PubMed] [Google Scholar]
- Weiss H., Wingfield P. Enzymology of ubiquinone-utilizing electron transfer complexes in nonionic detergent. Eur J Biochem. 1979 Aug 15;99(1):151–160. doi: 10.1111/j.1432-1033.1979.tb13241.x. [DOI] [PubMed] [Google Scholar]
- Yu C. A., Yu L., King T. E. Kinetics of electron transfer between cardiac cytochrome c 1 and c. J Biol Chem. 1973 Jan 25;248(2):528–533. [PubMed] [Google Scholar]
- Zhu Q. S., Berden J. A., De Vries S., Slater E. C. On the role of ubiquinone in the respiratory chain. Biochim Biophys Acta. 1982 Apr 19;680(1):69–79. doi: 10.1016/0005-2728(82)90317-6. [DOI] [PubMed] [Google Scholar]


