Abstract
Twelve 3,5-disubstituted-thiazolidine-2,4-dione (TZD) hybrids were synthesized using solution phase chemistry. Continuing our previous work, nine O-modified ethyl vanillin (8a–i) derivatives were synthesized and reacted with the TZD core via Knoevenagel condensation under primary reaction conditions to obtain final derivatives 9a–i. Additionally, three isatin–TZD hybrids (11a–c) were synthesized. The intermediates and final derivatives were characterized using 1H and 13C NMR spectroscopy, and the observed chemical shifts agreed with the proposed structures. The in vitro alpha-amylase and alpha-glucosidase inhibitory evaluation of newly synthesized derivatives revealed compounds 9F and 9G as the best dual inhibitors, with IC50 values of 9.8 ± 0.047 μM for alpha-glucosidase (9F) and 5.15 ± 0.0017 μM for alpha-glucosidase (9G), 17.10 ± 0.015 μM for alpha-amylase (9F), and 9.2 ± 0.092 μM for alpha-amylase (9G). The docking analysis of synthesized compounds indicated that compounds have a higher binding affinity for alpha-glucosidase as compared to alpha-amylase, as seen from docking scores ranging from −1.202 to −5.467 (for alpha-amylase) and −4.373 to −7.300 (for alpha-glucosidase). Further, the molecules possess a high LD50 value, typically ranging from 1000 to 1600 mg kg−1 of body weight, and exhibit non-toxic properties. The in vitro cytotoxicity assay results on PANC-1 and INS-1 cells demonstrated that the compounds were devoid of significant toxicity against the tested cells. Compounds 9F and 9G showed high oral absorption, i.e., oral absorption >96%, and their molecular dynamics simulation yielded results closely aligned with the observed docking outcomes. Finally, compounds 9F and 9G were evaluated for in vivo antidiabetic assessment by the induction of diabetes in Wistar rats using streptozotocin. Molecule 9G has been identified as the most effective anti-diabetic molecule due to its ability to modulate several biochemical markers in blood plasma and tissue homogenates. The results were further confirmed by histology investigations conducted on isolated pancreas, liver, and kidney.
1. Introduction
Diabetes mellitus (DM) is a complex metabolic disorder that is expected to affect 700 million people worldwide by the end of 2045.1–4 It is widely regarded as either the inability of the human body to produce sufficient insulin or the inability to utilize produced insulin along with altered protein, fat, and carbohydrate metabolism.5 Type 2 DM is often associated with heart-related complications such as the formation of carotid atherosclerotic plaques and their de-stability.6–8 Moreover, diabetes may lead to pregnancy-related issues, including changes in the size of the placenta.9 Obesity is another major complication related to type 2 DM patients, which further complicates the situation of patients.10 Different treatment approaches are available for the management of diabetes, such as sulfonylureas,11 biguanides,12 PPAR-gamma agonists,13 SGLT-2 inhibitors,14 alpha-amylase and alpha-glucosidase inhibitors,15 DPP-4 inhibitors,16 GLP-1 receptor agonists,17 and insulin therapy.18,19 However, these therapies are successful but often associated with severe side effects on long-term use, like weight gain,20 cardiovascular risk,21 hypoglycemia,22 retinopathy,23 and nephropathy.24–26
One of the most widely accepted therapeutic approaches for managing diabetes is inhibiting the enzymes metabolizing complex polysaccharides to glucose and related products.27 The two enzymes, alpha-amylase and alpha-glucosidase, are the prominent drug targets in such therapy for managing diabetes-associated post-prandial hyperglycemia.28,29 Both of the enzymes are involved in the conversion of complex polysaccharides into absorbable molecules such as glucose. The inhibition of both enzymes prevents the transformation of carbohydrates in the circulatory system, lowering blood glucose levels, especially after a meal containing simple and complex carbohydrates.30,31 Acarbose is a dual inhibitor of alpha-amylase and alpha-glucosidase, while miglitol and voglibose are prominent alpha-glucosidase inhibitors having little affinity for the alpha-amylase enzyme and are associated with severe side effects such as abdominal pain, flatulence, skin allergies, etc. on long-term use.29,31,32
Reactive oxygen species (ROS) play a prominent role in developing neurological and metabolic disorders such as diabetes mellitus by damaging β-cells and interfering with the oxidation of lipids, protein dysfunctions, and insulin resistance. Hyperglycemia may also trigger the oxidative stress pathways to worsen the condition of diabetic patients. The modulation of the antioxidant defense system, i.e., glutathione, catalase, and superoxide dismutase, can benefit patients with type 2 diabetes mellitus.33–35
Thaizolidien-2,4-dione is a privileged heterocyclic scaffold offering broad opportunities for synthetic modification at N-3 and C-5 positions.36 The agents of this class, glitazones, are well known for their PPAR-gamma agonistic activity, which improves insulin secretion from β-cells of the pancreas.37 These agents enhance insulin sensitivity in adipose tissues and the liver and subsequently regulate the metabolism of glucose and other macromolecules. Troglitazone was the first drug of this class that got approval from the USFDA in 1997, while rosiglitazone and pioglitazone were introduced in 1999.38 Troglitazone was recalled from the market in the early 2000s due to hepatotoxicity.39 In 2007, another drug, rosiglitazone, was withdrawn due to the increased risk of myocardial infarction in markets such as South Africa, Europe, India, and Asian markets. However, the drug is still available in the USA market. Pioglitazone is available worldwide for the management of type 2 DM. Another TZD-based drug, a synthetic variant of rosiglitazone, named lobeglitazone, was approved for sale in South Korea in 2013.36,40 In recent years, thiazolidine-2,4-dione-based molecules have been extensively explored as anticancer,41,42 antimicrobial,43 antimalarial,44 antioxidant,43,45 and anti-obesity agents.46,47 Furthermore, thiazolidine-2,4-diones have also been evaluated as potential alpha-amylase and alpha-glucosidase inhibitors for managing type 2 DM-associated risks.48,49
Previously, our group explored the antidiabetic potential of 3,5-disubstituted thiazolidine-2,4-dione hybrids acting via the antagonism of alpha-glucosidase and alpha-amylase (compound A, Fig. 1).50 Kaur et al. described thiazolidinedione–isatin hybrids' synthesis and biological evaluation as potential alpha-glucosidase inhibitors51 (compound B, Fig. 1). In addition, Rasheed et al. synthesized new isatin-based dual inhibitors of alpha-amylase and alpha-glucosidase enzymes (compound C, Fig. 1).32 Furthermore, isatin-based Schiff bases were synthesized and evaluated as alpha-glucosidase inhibitors by Rahim and co-workers.52 Many plant extracts have also been tested for alpha-amylase and alpha-glucosidase inhibitory activity.53 Inspire by cited literature reports and our ongoing work on the identification of novel thiazolidinedione-based lead molecules as dual inhibitors of alpha-glucosidase and alpha-amylase for the management of type 2 DM, two series of the molecule were synthesized, i.e., 9a–i (carrying modified ethylvanillin at the C-5 position of the TZD core) and 11a–c (carrying substituted isatin at C-5 of the TZD core) and evaluated in vitro for alpha-amylase and alpha-glucosidase inhibitory activity. Further, the molecules were subjected to in silico, docking, and molecular dynamics simulation studies. The best two molecules, i.e., 9F and 9G, were subjected to in vivo studies using the streptozotocin-induced diabetes model in Wistar rats. Different parameters such as oral glucose tolerance test, blood glucose, and body weight, as well as various biochemical parameters like blood urea nitrogen, high-density lipoprotein, total cholesterol, serum level of alpha-amylase, PPAR-gamma, etc., were accessed to establish their antidiabetic profile.
Fig. 1. Rationale for designing of proposed molecules.
2. Results and discussion
2.1. Chemistry
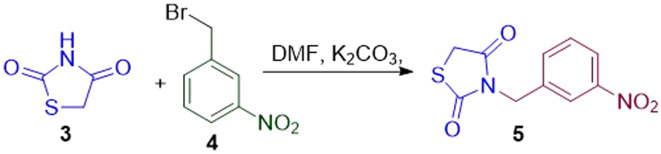
All of the proposed molecules were synthesized using Schemes 1–5 Thiazolidine-2,4-dione (3) was prepared by condensing thiourea (2) with chloroacetic acid (1) in an aqueous-acidic medium (Scheme 1). The 3-(3-nitrobenzyl)-thiazolidine-2,4-dione (5) was prepared by reacting TZD with 3-nitrobenzyl bromide (4, Scheme 2). The required intermediate O-modified ethylvanillin (8a–i) was prepared by treating ethylvanillin (6) with different alkyl/benzyl bromide/chloride moieties (7a–i) in acetone as a solvent and potassium carbonate was used as a base (Scheme 3). The modified aldehyde (8a–i) was used further without any purification. The proposed derivatives 9a–i were synthesized via Knoevenagel condensation of 5 with 8a–i in the presence of piperidine and glacial acetic acid as catalysts (Scheme 4). Molecules 11a–c were synthesized by reacting 5 with substituted isatin derivatives (10a–c) in the presence of 40% aqueous-alcoholic potassium hydroxide (Scheme 5). The final molecules and intermediates were characterized using 1H NMR and 13C NMR.
Scheme 1. Synthesis of thiazolidinedione.
Scheme 2. Synthesis of 3-nitrobenzyl thiazolidinediones.
Scheme 3. Synthesis of O-substituted ethyl vanillin.
Scheme 4. Synthesis of title compounds 9a–i.
Scheme 5. Synthesis of ISATIN–TZD hybrids.
In the 1H NMR spectra of 8a–i, the three protons of the ethoxy side-chain, i.e. (–OCH2CH3) resonate at 1.36–1.56 ppm as a triplet having a coupling constant in the range of 7 and 14 Hz. The –OCH2 group of the ethoxy side chain was observed as a quartet in the 4.02–4.17 ppm range with J values of 7 and 14 Hz, respectively. The –OCH2 of the modified chain of ethylvanillin appeared as a singlet in the 4.88–5.02 ppm range. The carbon spectrum of the synthesized vanillin was in complete agreement with the proposed structure. A peak in the 13.83–14.72 ppm range appeared in the 13C NMR for the terminal ethyl group of the ethoxy side chain, while the carbon of OCH2 of the terminal ethoxy group resonated at 64.58–65.12 ppm. The other alkyl carbons and benzyl carbon were observed at their expected chemical shifts. A similar pattern was also observed in the final compound 9A–I; however, the appearance of N–CH2 protons at 4.81–5.02 ppm in PNMR and nearly at 45 ppm in carbon spectra confirms the presence of the N-substituted TZD ring in the molecule. The physiological constants of the synthesized compounds are summarized in Table 1.
Physiochemical constant of synthesized TZD hybrids.
| Compound | Molecular formula | Molecular mass (g mol−1) | Appearance | % yield | Melting point (°C) | R f (TEF: 4.5 : 0.4 : 0.1) |
|---|---|---|---|---|---|---|
| 9A | C26H22N2O6S | 490 | Yellow solid | 65 | 175–177 | 0.54 |
| 9B | C27H24N2O6S | 504 | Creamy white solid | 74 | 178–179 | 0.48 |
| 9C | C26H21N3O8S | 535 | White flakes | 60 | 170–172 | 0.59 |
| 9D | C26H21N3O8S | 535 | White solid | 62 | 174–176 | 0.57 |
| 9E | C26H21ClN2O6S | 524 | Cream-white solid | 63 | 180–182 | 0.67 |
| 9F | C22H20N2O6S | 440 | Shining white crystals | 74 | 145–147 | 0.45 |
| 9G | C22H18N2O6S | 438 | Yellow solid | 69 | 148–150 | 0.54 |
| 9H | C26H22N2O8S2 | 554 | Yellow solid | 56 | 152–154 | 0.38 |
| 9I | C26H21FN2O6S | 508 | Shining yellow crystals | 69 | 152–154 | 0.45 |
| 11A | C19H13N3O5S | 395 | Brown solid | 45 | 156–158 | 0.35 |
| 11B | C19H12ClN3O5S | 429 | Pale brown | 50 | 159–161 | 0.38 |
| 11C | C19H12BrN3O5S | 472 | Pale brown | 55 | 165–167 | 0.54 |
3. Biological activity
3.1. In vitro evaluation
3.1.1. α-Glucosidase inhibitory assay
The synthesized molecules were tested for in vitro α-glucosidase inhibitory assay using the methodology developed by Singh et al.50 All the molecules displayed moderate to potent activity against the enzyme. Compound 9A (carrying benzyl group at R) showed moderate activity against the enzyme with an IC50 of 31.20 ± 0.0012 μM. Replacing the benzyl group with the 4-Me benzyl group improves the activity profile of compound 9B. Among the 3-NO2 and 4-NO2 groups in compounds 9C and 9D, respectively, 4-NO2 displayed higher activity than the 3-NO2 counterpart. Further, placing the 4-Cl group in compound 9E further lowers the molecule's activity (IC50 = 96.15 ± 0.129 μM). Replacing the aromatic benzyl group with an aliphatic allyl group in compound 9F significantly enhances the molecule's activity, displaying IC50 = 9.8 ± 0.047 μM. Attaching a propargyl group in place of the allyl group in derivative 9G showed the best inhibition of α-glucosidase with an IC50 of 5.15 ± 0.017 μM. Furthermore, compounds 9G and 9I showed moderate inhibition of α-glucosidase. In the isatin-based molecules (11A–C), all three molecules showed weak inhibition of the tested enzyme (Table 2).
In vitro evaluation of the synthesized molecules.
| Compound | R | IC50 α-glucosidase (μM) | IC50 α-amylase (μM) |
|---|---|---|---|
| 9A | –CH2Ph | 31.20 ± 0.012 | 55.58 ± 0.016 |
| 9B | 4-MePhCH2– | 20.31 ± 0.147 | 54.24 ± 0.083 |
| 9C | 3-NO2PhCH2– | 99.10 ± 0.026 | 64.77 ± 0.014 |
| 9D | 4-NO2PhCH2– | 38.33 ± 0.311 | 93.53 ± 0.089 |
| 9E | 4-ClPhCH2– | 96.15 ± 0.129 | 125.77 ± 0.089 |
| 9F | Allyl bromide | 9.8 ± 0.047 | 17.10 ± 0.015 |
| 9G | Propargyl bromide | 5.15 ± 0.017 | 9.2 ± 0.092 |
| 9H | 4-Me-PhSO2– | 42.24 ± 0.010 | 48.72 ± 0.086 |
| 9I | 4-FPhCH2– | 46.51 ± 0.096 | 60.10 ± 0.0163 |
| 11A | H | 58.44 ± 0.075 | 43.99 ± 0.014 |
| 11B | 5-Cl | 53.18 ± 0.174 | 50.76 ± 0.012 |
| 11C | 5-Br | 68.76 ± 0.558 | 60.99 ± 0.011 |
| Acarbose | — | 25.33 ± 1.25 (ref. 50) | 22.57 ± 2.30 (ref. 50) |
3.1.2. α-Amylase inhibitory assay
The molecules were evaluated for α-amylase inhibitory activity using a protocol developed by Singh and coworkers.50 Besides molecules 9F and 9G, molecules were endowed with weak inhibitory activity against the enzyme. The molecule carrying allyl and propargyl groups exhibited the best inhibition of α-amylase having IC50 values of 17.10 ± 0.015 and 9.2 ± 0.092 μM, respectively. Also, the molecules carrying 3-NO2 and 4-NO2 groups in compounds 9C and 9D showed poor inhibition of the tested enzyme. Compound 9E showed the lowest inhibition of the enzyme with an IC50 of 125.77 ± 0.089 μM. Further, compounds 9H and 9I showed poor inhibition of the enzyme. At the same time, the attachment of isatin at the C-5 position of the TZD core did not improve the inhibitory activity of the compounds against α-amylase. In general, the inhibitory activity was poorer than alpha-glucosidase (Table 2).
3.2. Docking studies
All the synthesized molecules were docked within the active site of human pancreatic alpha-amylase (PDB id: 1B2Y) and human pancreatic alpha-glucosidase (UniProt id: P00689) using the Maestro interface of Schrodinger software (2021-3 release). The obtained results are highlighted below.
3.2.1. α-Glucosidase
Docking against human pancreatic alpha-glucosidase
The protein was retrieved from UniProt (UniProt id: P00689), which had an Alpha fold model id: AF-00689-F1 and pLDDT >90%. The protein was prepared using the Schrodinger Maestro interface, and the synthesized molecules were docked within the predicted active site of the protein (predicted using sitemap analysis). The results are summarized in Table S1 (ESI† 1) and Fig. 2. Most of the docked compounds fit well into the active site of protein with a docking score of −4.373 to −6.757. The most active molecules, 9F and 9G, exhibited docking scores of −6.235 and −6.757, respectively. The two oxygen atoms of the 3-NO2 group in 9G form two hydrogen bonds with HIE676 and ASH429, respectively, while the 2-carbonyl of the TZD core forms a hydrogen bond with HIE678. The standard compound exhibited the strongest binding within the protein's active site, with a docking score of −11.690. Four hydrogen bonds were observed between oxygen atoms of different hydroxyl groups and residues, such as ALA32, HIE678, PHE285, ASP283, and ASP34.
Fig. 2. Docking analysis of 9f and 9g within the active site of human pancreatic alpha-glucosidase. For the rest of the poses (see ESI† 1).
3.2.2. α-Amylase
Using the Schrodinger Maestro interface, all the synthesized molecules were docked at the active site of α-amylase (PDB id: 1B2Y). Most of the compounds did not display good binding within the active site of the molecule, with a binding score of −1.202 to −5.476 (Table S1†). Compound 9A binds within the active site of protein by forming two H-bonds, i.e., C2 carbonyl with ASP353 and NO2 with ARG346, while 9B showed weak π–π interaction with HIP305 and salt bridge formation with TRP357. Compounds 9C and 9D formed hydrogen bonds with Arg346 and THR163 & ASP353, respectively, within the protein's active site. π–π interaction with HIP305 was also observed in both of the compounds. Compounds 9E and 9F formed only one π–π interaction with TRP357. In compound 9G, two water bonds with C O and the 3-nitro group were observed, and one π–cation interaction with ASP147 was also observed. The derivative 9H formed a hydrogen bond with GLN63 within the active site of the molecule. Among the isatin derivatives, compound 11A showed π–π interaction with HIP305 and π–cation interaction with ASP147 (Fig. 3 and ESI† 1).
Fig. 3. 2-D interaction pose of 9f and 9g within the active site of 1B2Y.
3.3. In silico toxicity predictions
Pre-ADME prediction and toxicity evaluation were performed using QikProp modules of Schrodinger software. The results are depicted in Table 3. Except for derivatives 9C and 9D, the molecules follow the Lipinski rule of five. The molecules contain the allowed number of rotatable bonds, and the number of H-acceptors ranges from 5 to 8. All the molecules are predicted to have good oil-in-water partition coefficients. The most active derivatives, 9f and 9g, displayed QP log Po/w values of 4.68 and 4.613, respectively, and high human oral absorption of 96%. These results make compounds 9F and 9G suitable for oral administration in vivo studies.
In silico pre-ADME prediction of synthesized thiazolidine-2,4-dione hybrids.
| Compound | No. of rotatable bonds | H-acceptors | H-donors | QP log Po/w | Percent human oral absorption | Lipinski |
|---|---|---|---|---|---|---|
| 9A | 9 | 6 | 0 | 5.713 | 89.32 | Yes, 0 |
| 9B | 9 | 6 | 0 | 6.168 | 91.198 | Yes, 1 |
| 9C | 10 | 8 | 0 | 4.915 | 57.075 | No, 2 |
| 9D | 10 | 8 | 0 | 5.125 | 46.454 | No, 2 |
| 9E | 9 | 6 | 0 | 6.22 | 79.348 | Yes, 1 |
| 9F | 9 | 6 | 0 | 4.68 | 96.235 | Yes, 0 |
| 9G | 8 | 6 | 0 | 4.613 | 96.01 | Yes, 0 |
| 9H | 9 | 8 | 0 | 3.904 | 67.382 | Yes, 0 |
| 9I | 9 | 7 | 0 | 5.956 | 77.79 | Yes, 1 |
| 11A | 4 | 5 | 1 | 2.077 | 72.878 | Yes, 0 |
| 11B | 4 | 5 | 1 | 2.573 | 75.807 | Yes, 0 |
| 11C | 4 | 5 | 1 | 2.648 | 76.247 | Yes, 0 |
3.4. In silico toxicity prediction
The Protox online server performed the in silico toxicity prediction, and the results are highlighted in Table 4. The synthesized molecules did not show any hepatotoxicity or cytotoxicity. All the molecules were predicted to possess high LD50 ranging from 1000–1600 mg kg−1 and belonged to class 4 of toxicity, i.e., harmful if swallowed (300 < LD50 = 2000). The most active molecules, 9F and 9G, showed an LD50 of 1000 mg kg−1. These results further solidified the potential of the above compounds for oral administration.
In silico toxicity prediction of synthesized thiazolidine-2,4-dione hybrids.
| Compound | Protox predicted (LD50 mg kg−1) | Hepatotoxicity | Cytotoxicity | Toxicity class |
|---|---|---|---|---|
| 9A | 1000 | Negative | Negative | 4 |
| 9B | 1000 | Negative | Negative | 4 |
| 9C | 1000 | Negative | Negative | 4 |
| 9D | 1000 | Negative | Negative | 4 |
| 9E | 1000 | Negative | Negative | 4 |
| 9F | 1000 | Negative | Negative | 4 |
| 9G | 1000 | Negative | Negative | 4 |
| 9H | 1000 | Negative | Negative | 4 |
| 9I | 1000 | Negative | Negative | 4 |
| 11A | 1000 | Negative | Negative | 4 |
| 11B | 1000 | Negative | Negative | 4 |
| 11C | 1600 | Negative | Negative | 4 |
3.5. Molecular dynamics simulation studies
Molecular dynamics (MD) simulations were performed on the best molecule that emerged from the in vitro enzymatic assay, i.e., 9G within the active site of human pancreatic alpha-amylase (PDB id: 1B2Y) and human pancreatic alpha-glucosidase (UniProt id: P00689) using the Desmond MD package of Schrodinger software (release 2021-3 (ref. 54)) and Gromacs,55 respectively. The MD simulation studies evaluated used proteins' molecular dynamics and stability in an ambient environment. The obtained results are discussed below.
3.5.1. MD simulation within the active site of human pancreatic alpha-glucosidase
The molecule was simulated for 100 ns using Gromacs55 MD simulation software, and the RMSD, RMSF, and radius of gyration were calculated. The results showed that the ligand and the protein showed high variation in the RMSD and formed a stable complex within the protein's active site after 20 s, which remained stable for 100 ns. The RMSD variation was within 1 to 1.25 nm for 9G within the protein's active site. The radius of gyration ranged from 2.95–3.15 nm. Further, no intramolecular hydrogen bonding was observed. The RMSF value for the different atoms in the ligand was 0.2–1.0 nm (Fig. 4a and b).
Fig. 4. MD simulation of 9G within the active site of human pancreatic alpha-glucosidase (UniProt id: P00689). 4a: RMSD variation during the simulation 4b: RMSF variation of atoms in the ligand.
3.5.2. MD simulation within the active site of human pancreatic alpha-amylase
The MD simulation of 9G within the alpha-amylase active site was conducted using Desmond.54 The simulation was performed for 100 ns, and the results are displayed in Fig. 5a, b and S3 (ESI† 1). The ligand forms a stable complex within the protein's active site throughout the simulation. The RMSD variation for the ligands was 1.5–3 Å throughout the simulation, and the gyration radius was between 5.1–6.0 Å. Further, no intramolecular hydrogen bonding was observed. Water bridges and weak van der Waals forces dominated the bonding between the ligand and protein. Hydrogen bonds were observed with Gln63, Thr163, and Gly306. Few ionic interactions were also observed.
Fig. 5. MD simulation of 9G within the active site of human pancreatic alpha-amylase (PDB id: 1B2Y). 5a: RMSD variation during the simulation 5b: RMSF variation of atoms in the ligand.
3.6. Cell viability assay
a. PANC-1
The cytotoxicity of compounds 9A–I and 11A–C was evaluated using the PANC-1 cell line. Up to 100 μM, none of the synthesized compounds showed notable cytotoxicity in the PANC-1 cell line. A one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests was used to evaluate the cell viability% of 9A–I and 11A–C and the acarbose-treated PANC-1 cell line (at doses of 5, 10, 20, 40, 60, 80, and 100 μM) alongside the control groups (Fig. 6A and ESI† 1).
Fig. 6. A and B: Cytotoxicity evaluation of compounds 9F and 9G.
b. INS-1
The cytotoxic effect of the synthesized compounds and standard drug acarbose on INS1 cells was evaluated using the MTT cell viability assay in cells treated with different concentrations (5, 10, 20, 40, 60, 80, and 100 μM, Fig. 6B and ESI† 1). None of the newly synthesized thiazolidinedione hybrids exhibited toxicity towards INS1 cells. The most active compounds, 9F and 9G, were non-toxic in the tested cell line. Also, acarbose was found non-toxic to the INS1 cell line (Fig. 6B and ESI† 1).
3.7. Anti-oxidant activity of 9a–9i; 11a–11c on high glucose-induced ROS in PANC-1 cells and animal pancreases
a. In vitro ROS assay
To measure the extent of the decrease in high glucose-induced ROS formation, flow cytometry with DCFDA labeling was used. In the control group, the MFI (FITC-A/DCFDA) was initially 57.2 ± 2.41. However, the cells treated with high glucose increased to 4692 ± 254.4, suggesting a significant rise in ROS formation. By simulating a high-glucose diabetic environment, the findings demonstrated that 9g and 9f considerably decreased ROS generation in the PANC-1 cell line. This was compared to the ROS production of the reference medication acarbose (149 ± 14.7), as well as to the ROS production before treatment with 5 μM of 9G and 9F (85.2% ± 8.8 and 97.6% ± 4.88, respectively, Fig. 7).
Fig. 7. In vitro antioxidant evaluation using ROS assay of synthesized compounds.
b. ROS examination in isolated animal pancreases
To analyse the ROS production in the animal pancreases, in the control animal pancreases, the ROS production was found to be around 81.6 ± 2.43, the STZ group increased the production of ROS to 3762 ± 118.14, and the treatment group of STZ + 9G and STZ + 9F decreased the ROS production to 164 ± 4.81 and 674 ± 11.13 (Fig. 8).
Fig. 8. Modulation of ROS production in animal pancreas by test compounds 9F and 9G.
3.8. Effects of 9F and 9G on high glucose-induced α-amylase and α-glucosidase in PANC-1 cells
a. In pancreatic cells
To analyze the alpha-glucosidase and alpha-amylase activity, cells were pre-treated with 9F and 9G for one hour, then incubated with high glucose concentration for 23 hours to examine the inhibitory impact on high glucose-caused intracellular α-amylase and α-glucosidase inhibition. The increased glucose treatment raised the intracellular expression of α-amylase and α-glucosidase enzymes, and the inhibitory activity of 9F and 9G was evaluated using a flow cytometer. Before adding the secondary antibody Alexa Fluor 488, the particular anti-amylase and glucosidase primary antibodies were treated for the whole night. The fluorescence signal was then analyzed using the FITC-A channel. High glucose stimulation increased the expression of α-glucosidase and α-amylase, with MFI values of 4361 ± 257.83 and 2887 ± 95.87, respectively, compared to the control group, where the MFI values were around 70.7 ± 2.88 and 78.5 ± 1.93. The results showed that before 9F and 9G were treated, the levels of high glucose-induced expression decreased in a dose-dependent manner. For α-glucosidase, the MFI was 7109 ± 2.84, and for α-amylase, it was 2242 ± 124.55 and 752 ± 5.47, respectively. The measured minimum fractional index (MFI) for α-glucosidase and α-amylase was 170 ± 3.99 and 114 ± 1.24, respectively, for acarbose. Research in vitro indicated that 9F and 9G reduced the cellular environment of high-glucose-induced diabetes by acting as anti-amylase and anti-glucosidase inhibitors, respectively (Fig. 9).
Fig. 9. Modulation of alpha-amylase and alpha-glucosidase activity by 9F and 9G in pancreatic cells.
b. Intestinal expression
In order to examine the protein-level in vivo activity of 9F and 9G in animal pancreases, the control group had an MFI of 67.8 ± 2.27 for alpha-glucosidase and 83.5 ± 3.55 for alpha-amylase. In contrast, the STZ group showed a rise in both enzymes, with MFIs of 3063 ± 149.2 and 2590 ± 199.74, respectively. The activity of alpha-glucosidase and alpha-amylase was reduced by STZ + 9F with an MFI of 132 ± 5.74 and 676 ± 4.85, respectively. With an MFI of 2539 ± 99.44 and 2248 ± 101.44, respectively, the activity of alpha-glucosidase and alpha-amylase was reduced by STZ + 9G (Fig. 10).
Fig. 10. Modulation of alpha-amylase and alpha-glucosidase activity by 9F and 9G in animal pancreas.
4. Structure–activity relationship
The structurally diverse newly synthesized ethyl vanillin derivatives (9A–I) and isatin derivatives (11A–C) showed varied activity against the tested enzymes. The benzyl group (9A) compound showed poor potency against α-glucosidase and α-amylase. However, replacing the benzyl group with the 4-Me benzyl group (9B) improved the potency of the compound against α-glucosidase while no activity against α-amylase was observed. Among molecules carrying 3-NO2 (9C) and 4-NO2 (9D) groups, derivative 9D exhibited higher activity than 9C against α-glucosidase. Further, replacing the benzyl group with aliphatic groups such as allyl (9F) and propargyl (9G) gave astonishing results. The molecules displayed the best inhibitory activity against both tested enzymes, i.e., nearly two-to-five-times higher inhibitory activity against the enzymes. Furthermore, the replacement of aliphatic groups with tosyl chloride (9H), 4-chloro benzyl (9E), and 4-F benzyl groups (9I) further diminished the activity of molecules. Replacing the substituted vanillin at C-5 with isatin did not improve the activity of derivatives against the tested enzymes. Among isatin derivatives the 5-chloro substituted derivative showed the best inhibitory activity against α-glucosidase. The molecule exhibited a higher activity potential against α-glucosidase than α-amylase. The generalized SAR of synthesized compounds is highlighted below in Fig. 11.
Fig. 11. Structure–activity relationship of synthesized compounds. Compounds were more active against α-glucosidase than α-amylase. Aliphatic groups showed higher activity against both of the tested enzymes. The molecule binds more tightly within the active site of α-glucosidase than α-amylase. Among substituted benzyls, the 4-Me benzyl group displayed the highest activity against α-glucosidase. Electron withdrawing groups showed lower activity than electron donating groups. Isatin derivatives showed weaker inhibition of α-glucosidase than α-amylase. Among isatin, 5-Cl displayed the highest activity against the tested enzymes.
5. In vivo antidiabetic evaluation
The most active compounds from the in vitro assay, i.e., derivatives 9f and 9g, were further subjected to in vivo studies using streptozotocin-induced diabetic animal models in Wistar rats. The rats were obtained and maintained at the Institutional Animal House facility of ISF College of Pharmacy, Moga, Punjab, India, under a 12-hour dark and light cycle and free access to food and water through the protocol. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of IAEC (Institute for Animal Ethics Committee) 816/PO/ReBiBt/S/04/CPCSEA at the Indo-Soviet Friendship College of Pharmacy in Moga, Punjab, India, in accordance with CPCSEA, Government of India and approved by the Animal Ethics Committee of ISFCP/IAEC/CPCSEA/Meeting No: 02/Protocol No. 23; Dated 26/11/2022. The animals were divided into six different groups as follows: (1) normal control (non-diabetic rats, maintained on a commercial pellet diet with free access to water; (2) vehicle control (non-diabetic animals, maintained on 10 mg kg−1 0.5% CMC, given orally and maintained on a commercial pelleted diet with free access to water); (3) STZ (diabetic control rats, maintained on a commercial pelleted diet with free access to water; (4) STZ + 9F (treatment group 1, test compound 9F given orally (10 mg kg−1 of body weight) with free access to food and water); (5) STZ + 9G (treatment group 2, test compound 9G given orally (10 mg kg−1 of body weight) with free access to food and water); (6) STZ + PGZ (treatment group 3, standard drug pioglitazone, given orally (10 mg kg−1 of body weight) with free access to food and water). The obtained results are discussed below.
5.1. Oral glucose tolerance test
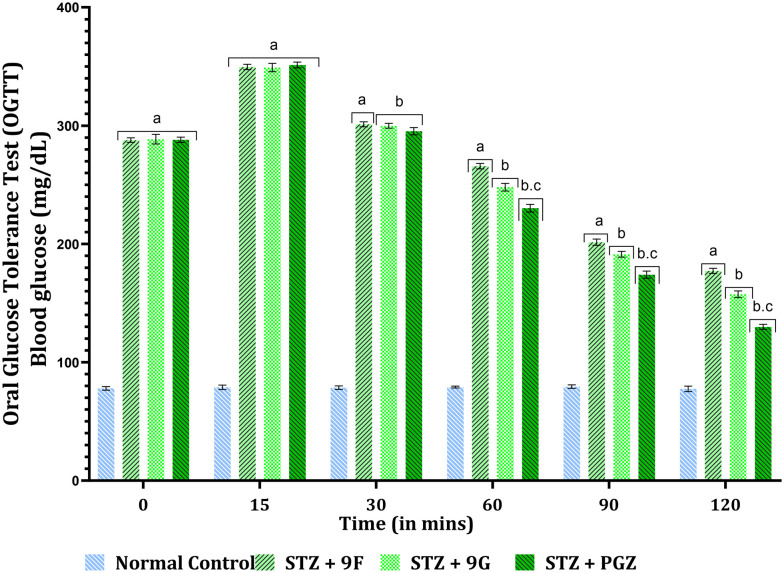
The oral glucose test was performed on the 5th day after animals received a single dose of streptozotocin (i.p., 65 mg kg−1 of body weight). Previously fasted animals for 16 hours were divided into respective groups. The animals were orally fed with a single dose of test compounds and the standard drug (10 mg kg−1 of body weight). Immediately, the animals were orally fed with glucose solution (2 g kg−1 of body weight). The animals' blood glucose levels were measured at 0, 15, 30, 60, 90, and 120 minutes after the glucose administration using the tail puncture method and a commercially available glucose monitoring device. The obtained results are depicted in Fig. 12.
Fig. 12. Oral glucose tolerance test. The data were reported as mean ± standard deviation (SD); p < 0.001 and were evaluated using a two-way analysis of variance (ANOVA) with post hoc Bonferroni's test (n = 6). A, v/s normal control. B, v/s STZ + 9F. c, v/s STZ + 9F, STZ + 9G.
The blood glucose level of animals receiving treatment with test compounds 9F and 9G and the standard drug pioglitazone displayed no statistical difference at 0 min. The normal control animals maintained their normal blood glucose level throughout the experiment. After 15 minutes of glucose administration, the glucose level in the treatment group rose significantly compared to the normal group. After 30 minutes, the blood levels of the treatment group animals began to fall, but no statistical difference was observed. The blood glucose levels showed a significant decrease after 60 minutes of treatment, i.e., there was a statistical difference between the blood glucose levels of animals observed. After 90 min of treatment, the blood glucose levels were significantly reduced, and the same trend was observed after 120 minutes of treatment. The study confirmed that compounds 9F and 9G can effectively reduce the blood glucose level of the animals at the proposed dose (10 mg kg−1 of body weight orally) [F (3, 20) = 26 813, p < 0.001].
5.2. Effect of test compounds on the body weight of animals in the STZ-induced diabetic rat model
The body weight of each group of animals was recorded on days 1, 4, 14, and 35 of the treatment schedules. The animals showed no statistical difference in body weight at day 1. A marginal decrease in the body weight of treatment group animals, i.e., STZ, STZ + 9F, STZ + 9G, and STZ + PGZ, was seen on day 4 of the treatment schedule. A significant decrease in the body weight of animals of the above-cited treatment groups was observed on day 14th of the protocol. On the 14th day, drug treatment was started and continued for the next 21 days.
The animals receiving treatment with the test compounds and standard drug pioglitazone showed a significant increment in body weight on the 35th day of the treatment protocol. In addition, compound 9G showed a significantly higher rise in the body weight of animals than 9F. In addition, the animals of group 3, i.e., STZ-treated, experienced a significant decrease in body weight compared to normal and vehicle control and treatment group animals. The standard drug pioglitazone showed a significant increase in the body weight of animals as compared to STZ + 9F- and STZ + 9G-treated animals [F (5, 30) = 3274, p < 0.001, Fig. 13].
Fig. 13. Effect of the test compounds on body weight in STZ-induced diabetic rats. The data are reported as mean ± standard deviation (SD); p < 0.001 and were evaluated using a two-way analysis of variance (ANOVA) with post hoc Bonferroni's test (n = 6). a v/s normal control; vehicle control. b v/s STZ. c v/s STZ + 9F. d v/s STZ + 9G, STZ + 9F.
5.3. Modulation of blood glucose levels
The blood glucose levels of the experimental animals were measured using the tail puncture method with the help of a commercially available glucometer, i.e., Dr. Morepen's blood glucometer. The change in the blood glucose levels was measured on the treatment days 1, 4, 14, and 35 of the schedules. At the start of the experimental schedule, i.e., day 1 (on which STZ was administrated), no statistical difference was observed between the blood glucose levels of experimental animals. The blood glucose levels of treatment group animals 3–6 significantly rose compared to normal and vehicle control animals on the 4th day of the treatment schedule. On day 14th, a significant increase in the blood glucose level of the groups of animals was observed, and the treatment of animals belonging to groups 4–6 with test compounds, i.e., 9F and 9G, and standard drug pioglitazone was started. On the 35th day of the protocol schedule, the animals treated with test compound 9F showed a significant decrease in their blood glucose levels compared to STZ-treated rats. The 9G-treated experimental animals also experienced a noteworthy reduction in blood glucose levels compared to STZ-treated and STZ + 9F-treated rats. The standard drug, pioglitazone, exhibited the most significant fall in the blood glucose level of the groups of animals as compared to STZ-, STZ + 9F- and STZ + 9G-treated Wistar rats [{F (5, 30) = 63 809, p < 0.001}, Fig. 14].
Fig. 14. Effect of the test compounds on modulation of blood glucose level in STZ-induced diabetic rats. The data are reported as mean ± standard deviation (SD); p < 0.001 and were evaluated using a two-way analysis of variance (ANOVA) with post hoc Bonferroni's test (n = 6). a, v/s normal control; vehicle control. b, v/s STZ. c, v/s STZ + 9F. d, v/s STZ + 9G, STZ + 9F.
5.4. Modulation of the PPAR-gamma level
The pancreatic extract of the experimental animals was taken, and studies were conducted for measuring the levels of PPAR-gamma, a chief protein involved in glucose metabolism. The SZT-treated rats showed a significant decrease in the levels of PPAR-gamma as compared to normal and vehicle control rats. The animals treated with test compounds 9F and 9G showed a considerable improvement in the PPAR-gamma levels compared to STZ-treated rats. The standard drug pioglitazone-treated experimental animals experienced a substantial rise in the PPAR-gamma levels as compared to STZ, STZ + 9F, and STZ + 9G-treated rats [{F (5, 25) = 21 728, p < 0.001}, Fig. 15].
Fig. 15. Effect of the test compounds on PPAR-gamma levels in STZ-induced diabetic rats. The data are reported as mean ± standard deviation (SD); p < 0.001 and were evaluated using a one-way analysis of variance (ANOVA) with post hoc Tukey's test (n = 6). a, v/s normal control, vehicle control; b, v/s STZ; c, v/s STZ + 9F. d, v/s STZ + 9F, STZ + 9G.
5.5. Effect of synthesized compounds on the levels of serum amylase
The modulation of serum amylase levels was determined in experimental groups. The enzyme levels were significantly higher in the STZ-treated rats compared to normal and vehicle control animals. The test compounds 9F and 9G failed to improve the alpha-amylase levels in the serum as no statistical difference between the level of enzyme between STZ-, STZ + 9F, and STZ + 9G-treated rats was observed. However, experimental animals treated with pioglitazone showed significant improvements in the levels of alpha-amylase in the blood of animals [F (5, 25) = 2199, p < 0.001, Fig. 16]. The results affirmed that the synthesized molecules were highly selective towards the alpha-glucosidase enzyme.
Fig. 16. Modulation of serum alpha-amylase levels. The data are reported as mean ± standard deviation (SD); p < 0.001 and were evaluated using a one-way analysis of variance (ANOVA) with post hoc Tukey's test (n = 6). a, v/s normal control, vehicle control; b, v/s STZ; c, v/s STZ + 9F. d, v/s STZ + 9F, STZ + 9G.
5.6. Modulation of the lipid profile
The diabetes-associated obesity is one of the chief reasons for diabetes-related complications. The lipid levels, i.e., total cholesterol, high-density lipoprotein (HDL), and triglycerides, were evaluated in the blood serum of experimental animals. The obtained results are depicted in Fig. 17.
Fig. 17. Effect of the synthesized compounds on the lipid profile in STZ-induced diabetic rats. The data are reported as mean ± standard deviation (SD); p < 0.001 and were evaluated using a one-way analysis of variance (ANOVA) with post hoc Tukey's test (n = 6). a, v/s normal control, vehicle control; b, v/s STZ; c, v/s STZ + 9F. d, v/s STZ + 9F, STZ + 9G.
The total cholesterol levels were significantly higher in the rats who received streptozotocin treatment alone and maintained using a normal pelleted diet throughout the protocol, compared to normal and vehicle control rats. The treatment with test compound 9F showed significant improvement in total cholesterol levels in experimental animals compared to STZ-treated rats. The test compound 9G significantly improved the total cholesterol level in experimental rats' blood. Pioglitazone-treated rats also showed significant improvement in total cholesterol levels [F (5, 25) = 2952, p < 0.001, Fig. 17]. Further, the levels of high-density lipoproteins were also measured in the blood serum of experimental animals. The HDL levels were significantly lower in the STZ-treated rats than in normal and vehicle-control animals. A significant improvement in the HDL levels was seen in the experimental animals receiving treatment with test compounds 9F and 9G, as well as pioglitazone, as compared to STZ-treated rats [F (5, 25) = 279.6, p < 0.001, Fig. 17].
Further, the triglyceride levels were evaluated to study the effect of test compounds on the lipid levels of experimental animals. The triglyceride levels were significantly higher in the STZ-treated experimental animals. A significant reduction in triglyceride levels was observed in the rats treated with the test compounds 9F and 9G, and the standard drug pioglitazone. Among them, pioglitazone showed the most significant improvement, followed by 9G and 9F [F (5, 25) = 951.8, p < 0.001, Fig. 17].
5.7. Effect on kidney functions
The kidney function of the treated experimental animals was determined by measuring blood urea nitrogen (BUN), uric acid, and creatinine levels in the rat serum. The serum BUN levels of STZ-treated rats and vehicle control animals were significantly higher than normal. The test compound 9F significantly improved the BUN levels in the serum. The same results were observed in the animals treated with test compound 9G and pioglitazone; however, the results were significantly improved compared to test compound 9F and STZ-treated rats [F (5, 25) = 1839, p < 0.001, Fig. 18]. Further, the uric acid levels were also measured, and the results highlighted that STZ-treated rats displayed significantly higher levels of uric acid than normal and control animals. The treatment of experimental rats with test compounds 9F and 9G and pioglitazone significantly restored the levels of uric acid in the animals under treatment [F (5, 25) = 6453, p < 0.001, Fig. 18].
Fig. 18. Effect of synthesized compounds on kidney parameters in STZ-induced diabetic rats. The data are reported as mean ± standard deviation (SD); p < 0.001 and were evaluated using a one-way analysis of variance (ANOVA) with post hoc Tukey's test (n = 6). a, v/s normal control, vehicle control; b, v/s STZ; c, v/s STZ + 9F. d, v/s STZ + 9F, STZ + 9G.
A significant rise in the serum creatinine levels of STZ-treated animal subjects was observed compared to normal and vehicle control rats. The test compound 9F significantly restored the serum creatinine as compared to STZ. The test compound 9G improved the levels of creatinine in serum. Eventually, the results were considerably higher than those of test compound 9F and STZ-treated animals. The standard drug pioglitazone also showed significantly better results than 9F, 9G, and STZ-treated animals [F (5, 25) = 15 236, p < 0.001, Fig. 18].
5.8. Effect on liver functions
The liver functioning was evaluated by detecting the levels of serum-glutamic-oxaloacetic transaminase (SGOT), serum-glutamic-pyruvic transaminase (SGPT), and alkaline phosphatase (ALP) in the blood serum of experimental animals. The serum concentration of SGOT [F (5, 25) = 612.1, p < 0.001], SGPT [F (5, 25) = 586.9, p < 0.001], and ALP [F (5, 25) = 520.0, p < 0.001] were significantly higher in the STZ-treated rats as compared to normal and vehicle control group animals. The test compounds 9F and 9G significantly improved the level of all three enzymes in the treated experimental subjects compared to STZ-treated rats. Further, the test derivative 9G exhibited higher activity than test compound 9F. Pioglitazone significantly improved animals' liver health by positively modulating the SGPT, SGOT, and ALP in the rat's serum (Fig. 19).
Fig. 19. Effect of synthesized compounds on liver parameters in STZ-induced diabetic rats. The data are reported as mean ± standard deviation (SD); p < 0.001 and were evaluated using a one-way analysis of variance (ANOVA) with post hoc Tukey's test (n = 6). a, v/s normal control, vehicle control; b, v/s STZ; c, v/s STZ + 9F. d, v/s STZ + 9F, STZ + 9G.
5.9. Evaluation of oxidative stress in the pancreatic tissues
Malondialdehyde (MDA) and superoxide dismutase (SOD) belong to the class of oxidative stress markers, wherein MDA results from the lipid peroxidation of polyunsaturated fatty acids, and SOD is involved in the dismutation of superoxide radicals to molecular oxygen and hydrogen peroxide. The levels of both markers were measured in the pancreatic extract of experimental subjects. The obtained results are highlighted in Fig. 20. A statistically significant higher concentration of MDA was found in the pancreatic homogenate of STZ-treated animals. The levels of MDA were significantly reduced in the pancreatic tissue of rats treated with test compounds 9F and 9G. Pioglitazone-treated experimental animals showed significant modulation in the concertation of MDA in pancreatic tissues as compared to STZ-, STZ + 9F, and STZ + 9G-treated rats [F (5, 25) = 80 180, p < 0.001].
Fig. 20. Effect of synthesized compounds on oxidative stress markers in pancreatic tissue in STZ-induced diabetic rats. The data are reported as mean ± standard deviation (SD); p < 0.001 and were evaluated using a one-way analysis of variance (ANOVA) with post hoc Tukey's test (n = 6). a, v/s normal control, vehicle control; b, v/s STZ; c, v/s STZ + 9F. d, v/s STZ + 9F, STZ + 9G.
Further, the concentration of SOD in the pancreatic tissue was significantly reduced in the diabetic animals (STZ-treated) as compared to normal and vehicle control animals. The test compounds 9F and 9G significantly improved the levels of SOD in the pancreatic tissue as compared to STZ-treated rats. Pioglitazone significantly enhanced the levels of SOD in pancreatic tissues, higher than test compounds 9F and 9G and STZ-treated rats [F (5, 25) = 189.8, p < 0.001].
5.10. Effect on oxidative stress markers in the blood serum
Oxidative stress is one of the primary triggers for type 2 diabetes mellitus in humans. To study the effect of test compounds on the modulation of oxidative stress parameters, the levels of two essential enzymes, i.e., catalase and reduced glutathione (GSH), were detected in the serum of experimental subjects. The treatment of experimental animals with STZ led to a significant fall in the catalase and GSH serum concentration. The test compound 9F exhibited a substantial rise in the serum levels of catalase [F (5, 25) = 269.6, p < 0.001] and GSH [F (5, 25) = 432.0, p < 0.001] and, hence, was able to combat the STZ-induced oxidative stress in Wistar rats. Further, the test compound 9G significantly modulated the serum levels of catalase and GSH in the treated animals, and the activity was higher than that of the test compound 9F and STZ-treated rats. The standard drug pioglitazone also improved the levels of GSH and catalase, higher than the test compounds 9F and 9G and STZ-treated rats (Fig. 21).
Fig. 21. Effect of synthesized compounds on oxidative stress markers in the blood serum in STZ-induced diabetic rats. The data are reported as mean ± standard deviation (SD); p < 0.001 and were evaluated using a one-way analysis of variance (ANOVA) with post hoc Tukey's test (n = 6). a, v/s normal control, vehicle control; b, v/s STZ; c, v/s STZ + 9F. d, v/s STZ + 9F, STZ + 9G.
5.11. Modulation of inflammatory cytokines
5.11.1. Effect on TNF-alpha levels
A low level of TNF alpha is responsible for insulin resistance in patients with type 2 diabetes mellitus. The measurements of TNF-alpha levels in the blood serum and pancreatic tissue were carried out, and the results are depicted in Fig. 22. The obtained results highlighted that the levels of TNF-alpha were significantly reduced in blood serum [F (5, 25) = 26 969, p < 0.001] and pancreatic tissues [F (5, 25) = 12 004, p < 0.001] post-treatment with streptozotocin in diabetic rats. The treatment of animals with the test compounds 9F and 9G showed significant improvement in the levels of TNF-alpha in serum and pancreatic tissues, hence improving insulin resistance in the experimental animals. However, the activity of test compound 9G was higher than that of the test compound 9F and STZ-treated groups. The standard drug also significantly improved the levels of TNF-alpha in both of the isolated samples, higher than the test compounds 9F and 9G and STZ-treated rats.
Fig. 22. Effect of synthesized compounds on TNF-alpha levels in STZ-induced diabetic rats. The data are reported as mean ± standard deviation (SD); p < 0.001 and were evaluated using a one-way analysis of variance (ANOVA) with post hoc Tukey's test (n = 6). a, v/s normal control, vehicle control; b, v/s STZ; c, v/s STZ + 9F. d, v/s STZ + 9F, STZ + 9G.
5.11.2. IL-1beta
A higher level of IL-1beta is responsible for the malfunctioning of β-cells in type 2 diabetes mellitus patients. Hence, restoring the concentration of IL-1beta in type 2 DM could help reduce insulin resistance and improve patients' health conditions. The levels of IL-1beta were determined in the experimental animals' blood serum and pancreatic tissues (Fig. 23). The STZ treatment in the Wistar rats led to a significant rise in the concentration of IL-1beta in the serum [F (5, 25) = 14 174, p < 0.001] and pancreatic tissue [F (5, 25) = 18 029, p < 0.001] of experimental animals. The treatment of experimental animals with test compounds 9F and 9G and pioglitazone significantly improved the levels of IL-1beta in the blood and pancreatic tissues. Among them, pioglitazone displayed the highest activity in modulating IL-1beta levels in the serum and pancreatic tissues.
Fig. 23. Effect of synthesized compounds on IL-1beta levels in STZ-induced diabetic rats. The data are reported as mean ± standard deviation (SD); p < 0.001 and were evaluated using a one-way analysis of variance (ANOVA) with post hoc Tukey's test (n = 6). a, v/s normal control, vehicle control; b, v/s STZ; c, v/s STZ + 9F. d, v/s STZ + 9F, STZ + 9G.
5.12. Effect on blood parameters
The analysis of the whole blood of experimental animals for the examination of various constituents such as basophils, monocytes, eosinophils, lymphocytes, hemoglobin, neutrophils, white blood cells, and total red blood cell count was conducted at the end of the experimental protocol using an autohematoanalyzer (Mindray BC-5000 vet). The obtained results are depicted in Fig. 24. A significant rise in the basophils [F (5, 25) = 324.4, p < 0.001] was observed in the STZ-treated rats, while the levels of eosinophils [F (5, 25) = 61.20, p < 0.001], lymphocytes [F (5, 25) = 14 631, p < 0.001], monocytes [F (5, 25) = 893.9, p < 0.001], hemoglobin [F (5, 25) = 64 585, p < 0.001], neutrophils [F (5, 25) = 6923, p < 0.001], white blood cells [F (5, 25) = 88 592, p < 0.001], and total red blood cells [F (5, 25) = 39 901, p < 0.001] were significantly reduced in the whole blood of animals. The treatment with test compound 9F significantly modulated the levels of blood components, i.e., lowering the level of basophils while raising the others. Derivative 9G also improved the blood component levels of experimental animals, and the activity was higher than compound 9F. The standard drug, pioglitazone, showed the most increased activity compared to 9G, 9F, and STZ-treated rats.
Fig. 24. Effect of synthesized compounds on complete blood count (CBC) parameters in STZ-induced diabetic rats. The data are reported as mean ± standard deviation (SD) and were evaluated using a one-way analysis of variance (ANOVA) with post hoc Tukey's test (n = 6). a, (p < 0.001) v/s normal control, vehicle control; b, (p < 0.001) STZ; c, (p < 0.001) STZ + 9F, STZ; d (p < 0.001) STZ + 9F, STZ + 9G, STZ.
5.13. Histopathological studies
The histology studies of the isolated pancreas, liver, and kidney were performed using H and E staining via a method developed by Gumber et al. and Singh et al. The obtained results are highlighted below.
5.13.1. Effect on histopathology of pancreas
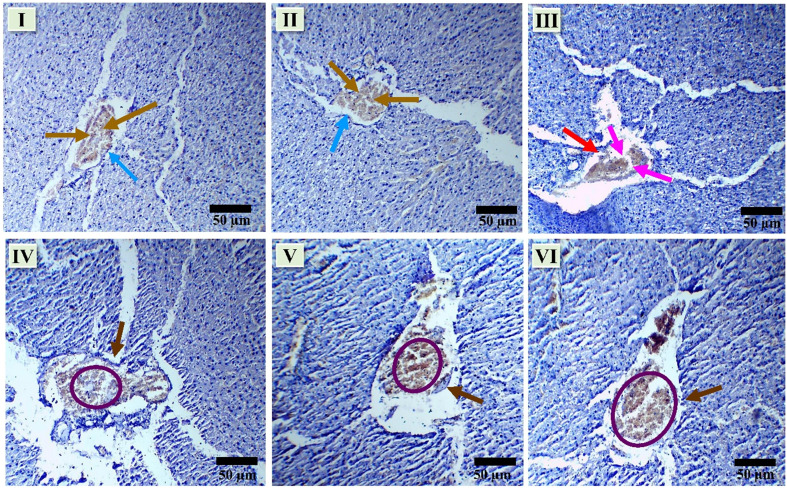
Morphological changes in the pancreas of rats post-treatment with test compounds against streptozotocin (STZ)-induced diabetes were investigated. In sections of the pancreas from normal and vehicle control rats, no morphological changes were observed in the photomicrographic analysis. The pancreas exhibited a regular shape and size, with clearly visible boundary walls and no observable damage to the tissue (indicated by the blue arrow). Insulin-secreting cells, i.e., β-cells, identified by the dark yellow arrow, were present in high numbers and showed no morphological changes in normal cells. Following STZ treatment (III), a significant impact on the islet of Langerhans was observed, marked by pink arrows indicating damage. The boundary cells were destroyed, boundaries were dented (as indicated by red arrows), and the count of β-cells was notably reduced (also indicated by pink arrows). An improvement in pancreatic morphology was evident upon treatment with test compound 9f (IV). The boundaries of the pancreas displayed regularity compared to STZ-treated rats (brown arrow). The number of β-cells increased (highlighted by a purple circle). Similarly, test compound 9g (V) significantly improved pancreatic health in treated animals. Visible boundary cells formed clear boundaries around the pancreatic islet (brown arrow), and the count of β-cells showed a significant increase (purple circle). In comparison, the standard drug pioglitazone (VI) treatment significantly improved the morphology of the pancreatic islet. Clear boundaries were visible (brown arrow), and the pancreas maintained a regular shape and size. The number of β-cells was significantly higher (purple circle) (40× magnification; 50 μm scale bar, Fig. 25).
Fig. 25. Effect of synthesized compounds on histopathology of pancreas in STZ-induced diabetic rats (I – normal control, II – vehicle control, III – STZ, IV – STZ + 9F, V – STZ + 9G and VI – STZ + PGZ).
5.13.2. Effect on histopathology of liver
Morphological changes in the liver of rats post-treatment with test compounds against STZ-induced diabetes are depicted in the figure. The illustration shows a section containing the tubercule, sinusoids, and central vein, stained with hematoxylin and eosin. The image was captured using a digital microscope at a magnification of ×40. The histological analysis of the adult Wistar rat liver was emphasized in response to STZ, test compound, and pioglitazone treatment. The tubercule and sinusoids, two separate layers, surround the central vein. In images I & II, there were no morphological alterations in the tuberculae (indicated by the pink arrow), sinusoids (indicated by the gold arrow), and central vein (indicated by the orange arrow) in the normal and control group rats (Fig. 26). All components were clearly visible and healthy. Animals treated with STZ exhibited disruption in the structural assemblies. The central vein was abruptly damaged (indicated by the orange circle), sinusoids were irregular in shape (indicated by the gold circle), and a reduction in tubercule cells was observed (indicated by the pink circle). In image IV, treatment with test compound 9F slightly improved the network of cells around the central vein. There was less distortion of the central vein (indicated by the orange circle), morphological improvement in sinusoids (indicated by the gold circle), and an increase in the number of trabeculae cells (indicated by the pink circle). Images V & VI show animals treated with test compound 9G and the standard drug pioglitazone, respectively, exhibiting significant improvement in the morphology of the components. The distorted central veins were restored to normal size and shape (indicated by the orange circle), sinusoids were morphologically improved (indicated by the gold circle), and the number of trabeculae cells was higher (indicated by the pink circle) (40× magnification; 50 μm scale bar).
Fig. 26. Effect of synthesized compounds on the histopathology of the liver in STZ-induced diabetic rats (I – normal control, II – vehicle control, III – STZ, IV – STZ + 9F, V – STZ + 9G, and VI – STZ + PGZ).
5.13.3. Effect on histopathology of kidney
Morphological changes in the kidneys of rats were investigated post-treatment with test compounds targeting STZ-induced diabetes. The H & E staining results from photomicrographic sections revealed that the kidneys exhibited regular shapes and sizes in normal (I) and vehicle control animals (II). Bowman's capsule enclosed the glomerulus, and no irregularities in shape and size were observed (indicated by the brown arrow). Pyramidal epithelial cells, characterized by eosinophilic cytoplasm and centrally positioned nuclei, covered the proximal convoluted tubules (highlighted by the pink arrow). Cuboidal cells, aligned with the distal convoluted tubules, were abundant (depicted by the green arrow). In section III, the brown circle illustrates the shrinkage of glomerular cells, and the green circle highlights the necrosis of tubular cells. The pink circle indicates the swallowing of tubular cells, while the aqua circle illustrates the dilation of distal convoluted tubules post-treatment with STZ. Significant alterations in kidney morphology were observed upon administration of test compound 9F (IV) and test compound 9G (V). Necrosis and swelling of tubular cells were significantly reduced, while the glomeruli regained their normal shape and size, and the dilation of distal convoluted tubules was also reduced. In section VI, it is noted that the standard drug minimized the levels of necrosis and swelling in tubular cells while simultaneously improving the morphology of glomeruli and distal convoluted tubules (DCT) (40× magnification; 50 μm scale bar, Fig. 27).
Fig. 27. Effect of synthesized compounds on histopathology of kidney in STZ-induced diabetic rats (I – normal control, II – vehicle control, III – STZ, IV – STZ + 9F, V – STZ + 9G, and VI – STZ + PGZ).
6. Conclusion
Diabetes is a complex metabolic illness that is linked to several risk factors that can be life-threatening. The number of patients affected by diabetes is steadily increasing. Suppressing two metabolic enzymes, α-amylase and α-glucosidase, plays a crucial role in carbohydrate metabolism. Their suppression in individuals diagnosed with type 2 diabetes mellitus has demonstrated advantageous effects. Acarbose, voglibose, and miglitol, which the FDA has approved, are linked to significant adverse effects that persist for a longer duration of treatment. Twelve 3,5-disubstituted thiazolidine-2,4-dione hybrids were synthesized and assessed as potential antidiabetic agents, drawing inspiration from existing literature reports that highlight the potential of thiazolidine-2,4-diones as inhibitors of α-amylase and β-glucosidase, as well as our ongoing research on thiazolidinedione. We produced the derivatives using our standard synthetic processes and thoroughly characterized them using several spectroscopic techniques.
Moreover, the compounds underwent assessment in vitro to determine their inhibitory effects on α-amylase and β-glucosidase. Among them, derivatives 9F and 9G were identified as promising candidates. In addition, molecular docking studies were conducted to understand the binding manner of molecules. The results showed that the synthesized molecules bind more strongly to alpha-glucosidase's active site than alpha-amylase. The synthesized molecules exhibited docking scores ranging from −1.202 to −5.467 against alpha-amylase and from −4.373 to −7.300 against alpha-glucosidase. The compounds exhibited enhanced safety and a substantial LD50 range of 1000–1600 mg kg−1 of body weight, indicating their non-toxic nature. Additionally, they showed a notable degree of oral absorption, rendering them well-suited for in vivo assessment. Molecular dynamics simulations further substantiated the docking analysis. The analysis revealed that both compounds exhibited a strong fit into the binding pockets of alpha-amylase and alpha-glucosidase, with hydrogen bonding and other types of bonding.
Additionally, the in vitro cytotoxicity assay demonstrated that the compounds exhibited no significant cytotoxicity. The analysis of ROS revealed that the derivatives reduced the formation of ROS in PANC-1 cells. Molecules 9F and 9G emerged as the most potent ROS inhibitors. The anti-diabetic properties of compounds 9F and 9G were evaluated in Wistar rats with STZ-induced diabetes. Compound 9G exhibited significant outcomes in the in vivo investigations. It attenuated blood glucose levels, body weight, lipids (HDL, triglycerides, and total cholesterol), renal function such as blood urea nitrogen uric acid, and liver function like SGPT. SGOT and ALP considerably improved post-treatment. Compound 9G significantly reduced diabetes-induced stress by raising the levels of several stress markers such as GSH, catalase, MDA, and SOD in blood serum and pancreatic tissue. The inflammatory cytokines, i.e., TNF-alpha and IL-1beta, were lowered in rats treated with test compound 9G. The test compounds 9F and 9G reduced the ROS production in the pancreas. The results were further verified by blood parameter evaluation and histology of pancreas, liver, and kidney tissues, whereby derivative 9G significantly and modulate the levels of haemoglobin hemoglobin, monocytes, basophils, etc. and improved the damage to the corresponding organ tissues. However, neither tested drug demonstrated any change in serum amylase levels. The activity of molecules was lower than that of conventional medicine pioglitazone.
7. Experimental section
7.1. General experimental information
All the intermediates and title compounds were synthesized using solution-phase chemistry. Thin layer chromatography (TLC) was performed using commercially available pre-coated plates (Merck Kiesel gel 60 F silica). The progress of the reaction was monitored by TLC and visualized by viewing it in the UV and iodine chambers. Different work-up processes were used to purify the reaction products and remove unreacted starting materials and impurities. Recrystallization followed by column chromatography was done using suitable solvents to get a pure sample of title compounds. Melting points and Rf values of all the compounds were determined. The structure and purity of the anticipated compounds were characterized by 1H and 13C NMR, recorded on the Bruker Advance Neo II instrument at 400 MHz frequency using DMSO-d6 or CDCl3 as a solvent, and tetramethylsilane (TMS) (d = 0) as an internal standard. The chemical shifts are reported in parts per million (δ) downfield from the signal of TMS added to the deuterated solvent. Spin multiplicities are given as s (singlet), b (broad), d (doublet), dd (double doublet), t (triplet), q (quartet), or m (multiplet). Melting points were recorded with the decibel digital melting point apparatus.
7.2. Synthesis of title compounds
The synthesis of title and intermediate compounds was undertaken using the following methodology.
Step 1: synthesis of 2,4-thiazolidinedione (3)
Synthesis of thiazolidinedione (3) was carried out using a literature methodology. Chloroacetic acid (1, 5 g, 53.19 mmol) and thiourea (2, 4 gm, 52.63 mmol) were dissolved in two separate beakers in 6 mL of water. Both solutions were mixed, and 4 mL of concentrated hydrochloric acid was added dropwise. The resulting solution was refluxed for 12 h at room temperature. The solution was then cooled to room temperature and refrigerated for 8 h. The obtained white crude precipitates were filtered and washed with 500 mL of chilled distilled water to remove excess acid and then recrystallized from ethanol to get needle-shaped white crystals of thiazolidinedione (Scheme 1).
Step 2: synthesis of N-benzylated thiazolidinedione (5)
The required intermediate, i.e., 3-nitrobenzyl substituted thiazolidinedione derivative (5), was synthesized by condensing thiazolidinedione (3) with 3-nitrobenzyl bromide (4), using anhydrous DMF at room temperature. A solution of 3-nitrobenzyl bromide (4, 922 mg, 4.26 mmol) and thiazolidinedione (3) was prepared by dissolving both in 5 mL dry dimethyl formamide. Potassium carbonate (1178 mg, 8.52 mmol) was added, and the solution was stirred at room temperature for 2–3 h. TLC determined the progress of the reaction. After completion, the reaction mixture was poured over crushed ice with continuous stirring. The obtained solid was filtered, washed with distilled water, and dried. The crude product was then recrystallized using ethanol as a solvent. The final product was obtained after cooling as cream-white fluffy crystals (Scheme 2).
Step 3: modification of ethylvanillin
The modified ethylvanillin derivatives (8a–i) were synthesized by refluxing ethylvanillin (6) with substituted benzyl bromides/chlorides (7a–i) in acetone as a solvent, and anhydrous potassium carbonate was used as a base (Scheme 3). Ethylvanillin (500 mg, 3.012 mmol) was dissolved in previously warmed acetone. Benzyl bromide/chloride (3.012 mmol) was added, and the reaction was stirred under heating in an oil bath. Anhydrous potassium carbonate (3 equivalent) was then added to the clear reaction mixture and stirred to obtain a suspension. The reaction mixture was refluxed under heating in an oil bath for 8 h with continuous stirring. The progress of the reaction was monitored using TLC in a hexane : EtOAc (1 : 1) mixture. Upon completion, the reaction mixture was poured over crushed ice to obtain the precipitate-modified aldehyde. The crude product was washed with cold water to remove excess base and used without any further purification for the synthesis of final derivatives (9a–i).
8a. 4-(Benzyloxy)-3-ethoxybenzaldehyde
1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 1.49–1.53 (t, 3H, J = 6.96 & 13.96 Hz, –OCH2CH3), 4.17–4.22 (q, 2H, J = 7 & 14 Hz, –OCH2CH3), 5.23 (s, 2H, O–CH2), 6.99–7.01 (d, 1H, J = 8.2 Hz, Ar–H), 7.32–7.47 (m, 7H, Ar–H), 9.84 (s, 1H, –CHO); 13C NMR (101 MHz, CDCl3, δ ppm, TMS = 0); 14.72, 64.58, 70.82, 110.79, 112.87, 126.43, 127.04 (2C), 128.10, 128.68 (2C), 130.33, 136.27, 149.46, 153.87, 191.04.
8b. 3-Ethoxy-4-((4-methylbenzyl)oxy)benzaldehyde
1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 1.39–1.43 (t, 3H, J = 7 & 14 Hz, –OCH2CH3), 2.24 (s, 3H, 4-MePH), 4.02–4.08 (q, 2H, J = 6.96 & 13.96 Hz, –OCH2CH3), 5.09 (s, 2H, O–CH2), 6.85–7.31 (m, 7H, Ar–H), 9.70 (s, 1H, –CHO), 13C NMR (101 MHz, CDCl3, δ ppm, TMS = 0); 14.74, 21.24, 64.57, 70.75, 110.73, 112.83, 126.47, 127.19, 127.32, 129.16, 129.35, 130.23, 133.22, 137.86, 149.43, 153.95, 191.04.
8c. 3-Ethoxy-4-((3-nitrobenzyl)oxy)benzaldehyde
1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 1.50–1.53 (t, 3H, J = 6.88 & 13.88 Hz, –OCH2CH3), 4.17–4.22 (q, 2H, J = 6.96 & 13.96 Hz, –OCH2CH3), 5.32 (s, 2H, O–CH2), 7.00–7.02 (d, 1H, J = 8.12 Hz, Ar–H), 7.40–7.45 (m, 2H, Ar–H), 7.53–7.61 (m, 1H, Ar–H), 7.77–7.81 (t, 1H, J = 7.24 & 14.84 Hz, Ar–H), 8.19–8.21 (d, 1H, 8.12 Hz, Ar–H), 8.37 (s, 1H, Ar–H), 9.85 (s, 1H, –CHO); 13C NMR (101 MHz, CDCl3, δ ppm, TMS = 0); 14.69, 64.61, 69.93, 110.97, 113.17, 121.98, 123.09, 126.10, 129.71, 130.98 (2C), 132.85, 138.59, 149.64, 153.09, 190.93.
8d. 3-Ethoxy-4-((4-nitrobenzyl)oxy) benzaldehyde
1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 1.49–1.52 (t, 3H, J = 6.96 & 13.96 Hz, –OCH2CH3), 4.16–4.21 (q, 2H, J = 8.32 & 13.96 Hz, –OCH2CH3), 5.29 (s, 2H, O–CH2), 6.96–6.98 (d, 1H, J = 8.16 Hz, Ar–H), 7.39–7.44 (m, 2H, Ar–H), 7.63–7.65 (d, 2H, J = 9.84 Hz, Ar–H), 8.24–8.26 (d, 2H, J = 6.92 Hz, Ar–H), 9.84 (s, 1H, –CHO); 13C NMR (101 MHz, CDCl3, δ ppm, TMS = 0); 14.73, 64.57, 69.55, 110.85, 112.92, 123.73, 123.92, 126.14, 127.40, 130.91, 143.77, 147.64, 149.51, 153.00, 190.94.
8e. 4-((4-Chlorobenzyl)oxy)-3-ethoxybenzaldehyde
1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 1.36–1.40 (t, 3H, J = 6.92 & 13.92 Hz, –OCH2CH3), 4.03–4.08 (q, 2H, J = 7.04 & 14 Hz, –OCH2CH3), 5.08 (s, 2H, O–CH2), 6.84–6.86 (d, 1H, J = 8.2 Hz, Ar–H), 7.18–7.32 (m, 6H, Ar–H), 9.72 (s, 1H, –CHO); 13C NMR (101 MHz, CDCl3, δ ppm, TMS = 0); 14.72, 64.53, 70.19, 110.75, 112.85, 126.31, 128.46, 128.61, 128.63, 128.85, 130.51, 133.88, 134.78, 149.44, 153.50, 191.
8f. 4-(Allyloxy)-3-ethoxybenzaldehyde
1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 1.40–1.42 (t, 3H, J = 7.02 & 13.98 Hz, –OCH2CH3), 4.02–4.06 (q, 2H, J = 7 & 14 Hz, –OCH2CH3), 4.59–4.60 (t, 2H, J = 5 & 9.86 Hz, CH2–CH CH2), 5.20–5.24 (t, 2H, J = 4.16 & 8.01 Hz, CH2–CH CH2), 5.81–5.94 (m, 1H, CH2–CH CH2), 6.95–6.97 (d, 1H, J = 6 Hz, Ar–H), 7.15–7.18 (m, 2H, Ar–H), 9.86 (s, 1H, –CHO); 13C NMR (101 MHz, CDCl3, δ ppm, TMS = 0); 13.83, 64.52, 71.18, 113.57, 115.56, 117.53, 124.25, 132.67, 134.47, 149.77, 156.42, 191.25.
8g. 3-Ethoxy-4-(prop-2-yn-1-yloxy)benzaldehyde
1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 1.49–1.52 (t, 3H, J = 7 & 13.96 Hz, –OCH2CH3), 2.58 (s, 1H, –CH), 4.15–4.21 (q, 2H, J = 6.96 & 13.96 Hz, –OCH2CH3), 4.88 (s, 2H, O–CH2), 7.16–7.18 (d, 1H, J = 8.12 Hz, Ar–H), 7.43–7.48 (m, 2H, Ar–H), 9.87 (s, 1H, –CHO); 13C NMR (101 MHz, CDCl3, δ ppm, TMS = 0); 14.65, 56.64, 64.54, 76.61, 76.64, 110.60, 112.98, 126.09, 130.91, 149.39, 152.32, 191.05.
8h. 2-Ethoxy-4-formylphenyl 4-methylbenzenesulfonate
1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 1.38–1.42 (t, 3H, J = 6.98 & 13.98 Hz, –OCH2CH3), 2.23 (s, 3H, 4-MePH), 4.04–4.08 (q, 2H, J = 7.02 & 14.02 Hz, –OCH2CH3), 7.01–7.02 (d, 1H, J = 8.3 Hz, Ar–H), 7.31–7.47 (m, 5H, Ar–H), 7.87–7.89 (d, 1H, J = 6 Hz, Ar–H), 9.91 (s, 1H, –CHO); 13C NMR (101 MHz, CDCl3, δ ppm, TMS = 0); 14.39, 21.69, 64.14, 110.79, 117.35, 124.06, 128.57 (2C), 129.40 (2C), 133.37, 137.56, 142.85, 144.99, 151.24, 191.03.
8i. 3-Ethoxy-4-((4-fluorobenzyl)oxy)benzaldehyde
1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 1.46–1.49 (t, 3H, J = 6.96 & 13.96 Hz, –OCH2CH3), 4.13–4.18 (q, 2H, J = 6.96 & 13.96 Hz, –OCH2CH3), 6.97–7.09 (m, 3H, Ar–H), 7.37–7.43 (m, 4H, Ar–H), 9.82 (s, 1H, –CHO), 13C NMR (101 MHz, CDCl3, δ ppm, TMS = 0); 14.69, 64.53, 70.76, 110.77, 112.89, 115.48, 115.69, 126.33, 128.98, 129.06, 130.45, 132.02, 149.45, 153.62, 163.75, 191.02.
Step 4: synthesis of proposed derivatives (9a–i)
The proposed derivatives (9a–i) were synthesized by carrying out Knoevenagel condensation between N-benzyl thiazolidinedione (5) and modified ethyl vanillin (8a–i) in ethyl alcohol under reflux conditions, using piperidine and acetic acid as catalysts. A mixture of 3-(3-nitrobenzyl)thiazolidine-2,4-dione (5, 150 mg, 0.59 mmol) and substituted ethyl vanillin aldehydes (8a–i, 0.59 mmol) was suspended in absolute ethanol. 6–8 drops of piperidine and 3–4 drops of glacial acetic acid were added to the obtained suspension. The mixture was then refluxed at 70 °C for 4–5 h. The progress of the reaction was determined using TLC in TEF (4.5 : 0.4 : 0.1) as a solvent mixture. After cooling, the final products (9a–i) were precipitated from the reaction mixture. The crude product was then filtered and washed with water to remove traces of piperidine and acetic acid. The dried product was then subjected to column chromatography using EtOH : hexane as the solvent, and silica was used as an adsorbent to obtain the desired final compounds in sufficient purity (Scheme 4).
9a. (Z)-5-(4-(Benzyloxy)-3-ethoxybenzylidene)-3-(3-nitrobenzyl)thiazolidine-2,4-dione
C26H22N2O6S, 1H NMR (400 MHz, DMSO-d6, δ ppm, TMS = 0); 1.33–1.37 (t, 3H, J = 6.92 & 13.88, –OCH2CH3), 4.07–4.12 (dd, 2H, J = 6.93 & 13.88 Hz, –OCH2CH3), 4.96 (s, 2H, N–CH2), 5.20 (s, 2H, O–CH2PhR), 7.19–7.25 (m, 2H, Ar–H), 7.32–7.46 (m, 5H, Ar–H), 7.64–7.66 (t, 1H, J = 8.6 & 16.24 Hz, Ar–H), 7.76–7.78 (d, 1H, J = 7.96 Hz, Ar–H), 7.92 (s, 1H, –C CH–), 8.16–8.18 (d, 2H, J = 6.72 Hz, Ar–H); 13C NMR (101 MHz, DMSO-d6, δ ppm, TMS = 0); 15.07, 44.32, 64.49, 70.29, 114.46, 115.15, 117.97, 123.06, 123.28 (2C), 124.36, 124.86, 126.35, 128.17 (2C), 128.98 (2C), 130.76, 134.82 (2C), 136.82, 138.39, 148.33, 148.94, 166.26, 168.47.
9b. (Z)-5-(3-Ethoxy-4-((4-methylbenzyl)oxy)benzylidene)-3-(3-nitrobenzyl)thiazolidine-2,4-dione
C27H24N2O6S; 1H NMR (400 MHz, DMSO-d6, δ ppm, TMS = 0); 1.34–1.37 (t, 3H, J = 6.82 & 13.88, –OCH2CH3), 2.30 (s, 3H, 4-CH3Ph), 4.08–4.13 (dd, 2H, J = 6.93 & 13.88 Hz, –OCH2CH3), 4.98 (s, 2H, N–CH2), 5.20 (s, 2H, O–CH2PhR), 7.19–7.64 (m, 7H, Ar–H), 7.66–7.68 (d, 1H, J = 6.88 Hz, Ar–H), 7.77–7.79 (d, 1H, J = 6.84 Hz, Ar–H), 7.93 (s, 1H, –C CH–), 8.16–8.18 (d, 2H, J = 7.52 Hz, Ar–H); 13C NMR (101 MHz, DMSO-d6, δ ppm, TMS = 0); 15.08, 21.17, 44.32, 64.48, 70.34, 114.46, 115.53, 118.53, 123.07, 123.28, 124.36, 126.31, 128.17, 128.32, 128.46, 128.97, 129.52, 130.76, 134.46, 134.86, 137.10, 138.11, 148.32, 148.94, 150.75, 166.05, 167.99.
9c. (Z)-5-(3-Ethoxy-4-((3-nitrobenzyl)oxy)benzylidene)-3-(3-nitrobenzyl)thiazolidine-2,4-dione
C26H21N3O8S; 1H NMR (400 MHz, DMSO-d6, δ ppm, TMS = 0); 1.41–1.43 (t, 3H, J = 6.96 & 13.96 Hz, –OCH2CH3), 4.05–4.09 (dd, 2H, J = 6.96 & 13.92 Hz, –OCH2CH3), 4.91 (s, 2H, N–CH2), 5.22 (s, 2H, O–CH2), 7.12–7.14 (d, 1H, J = 6 Hz, Ar–H), 7.27–7.30 (2H, M, Ar–H), 7.49–7.54 (m, 3H, Ar–H & –C CH–), 7.60–7.64 (dd, 2H, J = 6.04 & 16.04, Ar–H), 8.08–8.15 (dd, J = 4.64 & 5.98 Hz, Ar–H), 8.23 (s, 1H, Ar–H), 8.33 (s, 1H, Ar–H); 13C NMR (101 MHz, DMSO-d6, δ ppm, TMS = 0); 14.98, 44.12, 64.21, 70.21, 113.93, 115.31, 116.91, 122.91, 124.18, 124.44, 124.83, 126.36, 128.82 (2C), 130.14 (2C), 135.71 (2C), 138.60, 141.64, 148.71, 149.61, 153.04, 163.81, 167.65.
9d. (Z)-5-(3-Ethoxy-4-((4-nitrobenzyl)oxy)benzylidene)-3-(3-nitrobenzyl)thiazolidine-2,4-dione
C26H21N3O8S; 1H NMR (400 MHz, DMSO-d6, δ ppm, TMS = 0); 1.35–1.39 (t, 3H, J = 6.92 & 13.88, –OCH2CH3), 4.11–4.16 (dd, 2H, J = 6.96 & 13.92 Hz, –OCH2CH3), 4.81 (s, 2H, N–CH2), 5.24 (s, 2H, –OCH2), 7.21–7.24 (d, 2H, J = 8.28, Ar–H), 7.43 (d, 1H, J = 1.84 Hz), 7.51–7.53 (dd, 2H, J = 1.86 & 8.24 Hz, Ar–H), 7.63–7.67 (t, 1H, J = 7.8 & 15.68 Hz, Ar–H), 7.71–7.73 (d, 2H, J = 7.44 Hz, Ar–H), 7.93 (1H, –C CH–), 8.14–8.17 (t, 1H, J = 2.46 & 12.8 Hz, Ar–H), 8.26–8.29 (2H, dd, J = 2.32 & 7.04 Hz, Ar–H); 13C NMR (101 MHz, DMSO-d6, δ ppm, TMS = 0); 15.05, 44.04, 64.53, 69.18, 111.61, 113.35, 113.63, 123.04, 123.19, 124.17 (3C), 125.99 (2C), 128.60 (3C), 130.64 (2C), 134.89, 138.19, 144.75, 148.26, 149.12, 172.47, 173.01,
9e. (Z)-5-(4-((4-Chlorobenzyl)oxy)-3-ethoxybenzylidene)-3-(3-nitrobenzyl)thiazolidine-2,4-dione
C26H21ClN2O6S; 1H NMR (400 MHz, DMSO-d6, δ ppm, TMS = 0); 1.50–1.53 (t, 3H, J = 6.96 & 13.96, –OCH2CH3), 4.13–4.19 (dd, 2H, J = 7.00 & 13.96 Hz, –OCH2CH3), 5.01 (s, 2H, N–CH2), 5.19 (s, 2H, O–CH2PhR), 6.93–6.95 (d, 1H J = 8.36 Hz, Ar–H), 7.03–7.08 (m, 2H, Ar–H), 7.28 (s, 3H, Ar–H), 7.38 (s, 3H, Ar–H), 7.53–7.57 (t, 1H, Ar–H, J = 7.92 & 15.88 Hz), 7.8 (s, 1H, –C CH–), 8.19–8.21 (d, 1H, J = 8.24 Hz, Ar–H), 8.31 (s, 1H, Ar–H); 13C NMR (101 MHz, CDCl3, δ ppm, TMS = 0); 14.76 (–OCH2CH3), 44.28 (N–CH2), 64.69 (OCH2CH3), 70.16 (O–CH2PhR), 114.13 (Ar–C), 114.34, 118.21, 123.38, 123.83, 124.65, 126.49, 128.46 (2C), 128.87 (3C, Ar–C & Ph–CH CPh), 129.85, 133.91, 134.90, 134.93, 135.00, 137.03, 149.3, 150.59, 166.00, 167.80.
9f. (Z)-5-(4-(Allyloxy)-3-ethoxybenzylidene)-3-(3-nitrobenzyl)thiazolidine-2,4-dione
C22H20N2O6S, 1H NMR (400 MHz, DMSO-d6, δ ppm, TMS = 0); 1.34–1.38 (t, 3H, J = 6.96 & 13.88, –OCH2CH3), 4.06–4.11 (dd, 2H, J = 6.92 & 13.88 Hz, –OCH2CH3), 4.65–4.67 (d, 2H, J = 5.2 Hz), 4.98 (s, 2H, N–CH2), 5.27–5.30 (dd, 2H, OCH2CH CH2, J = 1.4 & 10.52 Hz), 5.39–5.44 (dd, 2H, OCH2CH CH2, J = 1.56 & 15.62 Hz), 6.00–6.10 (m, 1H, OCH2CH CH2), 7.13–7.23 (m, 2H, Ar–H), 7.64–7.68 (t, 1H, J = 7.72 & 15.52 Hz, Ar–H), 7.77–7.79 (d, 1H, J = 7.68, Ar–H), 7.93 (s, 1H, –C CH–), 8.17–8.19 (d, 2H, J = 8.6 Hz); 13C NMR (101 MHz, DMSO-d6, δ ppm, TMS = 0); 15.07, 44.31, 64.38, 69.30, 114.12, 115.20, 118.43, 123.08, 123.28 (2C), 124.35, 126.16, 130.75, 133.76, 134.86, 138.11, 148.32, 148.81, 150.58, 166.06, 167.99.
9g. (Z)-5-(3-Ethoxy-4-(prop-2-yn-1-yloxy)benzylidene)-3-(3-nitrobenzyl)thiazolidine-2,4-dione
C22H18N2O6S; 1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 1.25–1.28 (t, 3H, J = 6.96 & 13.88, –OCH2CH3), 2.47 (s, 1H, OCH2CCH), 3.85–3.90 (dd, 2H, J = 6.92 & 13.92 Hz, –OCH2CH3), 4.87 (s, 2H, N–CH2), 5.01 (s, 2H, OCH2CCH), 6.94–6.95 (d, 1H, J = 1.82 Hz, Ar–H), 7.05–7.07 (dd, 1H, J = 1.92 & 8.36 Hz, Ar–H), 7.28–7.34 (m, 1H, Ar–H), 7.54–7.58 (t, 1H, J = 7.96 & 15.88 Hz, Ar–H), 7.85 (s, 1H, TZD CH–Ph), 8.19–8.21 (dd, 1H, J = 1.24 & 8.20 Hz, Ar–H), 8.30 (s, 1H, Ar–H); 13C NMR (101 MHz, CDCl3, δ ppm, TMS = 0); 14.28, 21.73, 44.42, 64.48, 114.59, 121.69, 122.82, 123.48, 125.00, 128.56, 129.55, 129.92, 132.75, 133.15, 133.73, 134.97, 136.79, 139.91, 145.39, 148.45, 151.72, 165.66, 167.28.
9h. (Z)-2-Ethoxy-4-((3-(3-nitrobenzyl)-2,4-dioxothiazolidin-5-ylidene)methyl)phenyl 4-methylbenzenesulfonate
C26H22N2O8S2; 1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 1.38–1.38 (t, 3H, J = 6.96 & 13.88 Hz, –OCH2CH3), 2.51 (s, 3H, 4-MePh), 4.05–4.11 (dd, 2H, J = 6.92 & 13.92 Hz), 4.91 (s, 2H, N–CH2), 4.98 (s, 2H, –OCH2–), 7.18–7.24 (m, 3H, Ar–H), 7.64–7.68 (t, 2H, J = 7.8 & 15.68 Hz, Ar–H), 7.77–7.79 (d, 2H, J = 7.76 Hz, Ar–H), 7.92 (s, 1H, TZD CH–Ph), 8.16–8.19 (t, 2H, J = 9.4 & 1.92 Hz, Ar–H); 13C NMR (101 MHz, DMSO-d6, δ ppm, TMS = 0); 15.04, 44.32, 56.38, 64.39, 79.29, 114.45, 115.07, 118.90, 123.10, 123.28 (2C), 123.92, 126.83 (3C), 130.74, 134.32, 134.88 (3C), 138.08, 148.30, 148.92, 149.25, 166.05, 167.99.
9i. (Z)-5-(3-Ethoxy-4-((4-fluorobenzyl)oxy)benzylidene)-3-(3-nitrobenzyl)thiazolidine-2,4-dione
C26H21FN2O6S, 1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 1.49–1.52 (t, 3H, J = 6.96 & 13.92 Hz, –OCH2CH3), 4.13–4.18 (dd, 2H, J = 6.96 & 13.96 Hz, –OCH2CH3), 5.01 (s, 2H, N–CH2), 5.18 (s, 2H, O–CH2), 6.95–6.98 (d, 1H, J = 8.36 Hz), 7.03–7.11 (m, 3H, Ar–H), 7.41–7.44 (dd, 2H, J = 5.36 & 8.52 Hz, Ar–H), 7.53–7.57 (t, 1H, J = 7.96 & 15.92 Hz, Ar–H), 7.77–7.79 (d, 1H, J = 7.68 Hz, Ar–H), 7.87 (s, 1H, TZD CH–Ph), 8.18–8.20 (dd, 1H, J = 1.26 & 8.26 Hz, Ar–H), 8.30 (s, 1H, Ar–H); 1H NMR (101 MHz, CDCl3, δ ppm, TMS = 0); 14.75, 44.27, 54.70, 70.29, 114.16, 114.38, 115.50, 115.72, 118.15, 123.37, 123.83, 124.68, 126.43, 128.99, 129.07, 129.85, 132.12, 132.16, 134.93, 135.02, 137.05, 148.43, 149.33, 150.72, 166.00, 167.81.
Step 5: synthesis of isatin–thiazolidinedione hybrids (11a–c)
A mixture of 3-(3-nitrobenzyl)thiazolidine-2,4-dione (5, 150 mg, 0.59 mmol) and substituted isatin (0.59 mmol) was suspended in 5 ml of absolute ethanol. After obtaining a clear solution, a few drops of 40% aqueous-alcoholic potassium hydroxide solution were added, and the reaction mixture was stirred at room temperature for 6–7 h (Scheme 5). TLC monitored the completion of reaction C. Upon completion, the mixture was poured on crushed ice to obtain crude precipitates. The crude product was then filtered and washed with water to remove the excess base and then purified using column chromatography in EtOAc : hexane; silica gel 60–120 was used as an adsorbent.
11a. (Z)-3-(3-Nitrobenzyl)-5-(3-oxoindolin-2-ylidene)thiazolidine-2,4-dione
C18H11N3O5S, 1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 4.80 (s, 2H, N–CH2), 6.68–7.04 (dd, 1H, J = 1.16 & 5.92 Hz, Ar–H), 6.83–6.86 (m, 1H, Ar–H), 7.23–7.26 (m, 1H, Ar–H), 7.46–7.51 (m, 2H, Ar–H), 7.59–7.62 (m, 1H, Ar–H), 7.91–7.93 (dd, 1H, J = 1.16 & 6 Hz, Ar–H), 8.06 (s, 1H, Ar–H), 9.62 (s, 1H, NH), 13C NMR (101 MHz, DMSO-d6, δ ppm, TMS = 0); 47.89, 104.71, 114.61, 120.32, 121.14, 123.84, 124.82 (2C), 129.47, 130.14, 135.71, 138.59, 144.16, 145.08, 148.70, 165.11, 167.39, 178.75.
11b. (Z)-5-(5-Chloro-3-oxoindolin-2-ylidene)-3-(3-nitrobenzyl)thiazolidine-2,4-dione
C18H10N3O5SCl, 1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 4.82 (s, 2H, N–CH2), 6.67–6.69 (d, 1H, J = 6 Hz, Ar–H), 7.31–7.33 (dd, 1H, J = 1.08 & 8 Hz, Ar–H), 7.48–7.51 (t, 1H, J = 6 & 12 Hz, Ar–H), 7.62–7.64 (m, 2H, Ar–H), 7.91–7.93 (dd, 1H, J = 1.12 & 9.92 Hz, Ar–H), 8.10 (s, 1H, Ar–H), 11.21 (1H, NH); 13C NMR (101 MHz, DMSO-d6, δ ppm, TMS = 0); 44.89, 105.61, 117.36, 119.67, 120.75, 124.83 (2C), 129.62 (2C), 130.14, 135.81, 138.60, 143.30, 145.08, 148.71, 166.11, 168.39, 179.87.
11c. (Z)-5-(5-Bromo-3-oxoindolin-2-ylidene)-3-(3-nitrobenzyl)thiazolidine-2,4-dione
C18H10N3O5SBr, 1H NMR (400 MHz, CDCl3, δ ppm, TMS = 0); 4.91 (s, 2H, N–CH2), 7.01–7.02 (d, 1H, J = 1.12 Hz, Ar–H), 7.05–7.07 (dd, 1H, J = 1.12 & 6 Hz, Ar–H), 7.37–7.39 (d, 1H, J = 6 Hz, Ar–H), 7.48–7.51 (t, 1H, J = 6 & 12 Hz, Ar–H), 7.62–7.64 (d, 1H, J = 6, Ar–H), 7.91–7.93 (d, 1H, J = 3.04, Ar–H), 8.12 (s, 1H, Ar–H), 11.01 (1H, NH); 13C NMR (101 MHz, DMSO-d6, δ ppm, TMS = 0); 44.68, 104.12, 118.67, 123.31 (2C), 124.44, 124.89, 126.79, 130.45, 136.12, 138.74, 145.10, 145.65, 147.98, 165.67, 168.12, 180.12.
7.3. Biological evaluation of synthesized compounds
7.3.1. In vitro α-glucosidase inhibitory activity
a. Preparation of test compounds and enzymes
α-Glucosidase (maltase) ex. yeast and substrate 4-nitrophenyl-α-d-glucopyranoside and acarbose were purchased from Sisco Research Lab. The enzyme dilution (1 U mL−1) and substrate (5 mM) were prepared in phosphate buffer (pH 6.8). The different concentrations of test compounds 9a–i, 11a–c, i.e., 1000, 500, 200, 100, and 10 μM, were prepared by dissolving a calculated amount of the test compound in DMSO to obtain a stock solution of 1000 μM. The other dilutions were prepared using the serial dilution method with DMSO as a diluent. Similarly, the dilution of the standard drug acarbose was prepared. The final or working dilutions of test compounds and standard drugs, i.e., 100, 50, 20, 10, and 1 μM, were adjusted in the microwell plates.
b. Assay
In a 96-well plate, each well was charged with 70 μL of phosphate buffer and 10 μL of enzyme solution.56 Then, 10 μL of the standard and test compounds were added to respective wells. A blank solution was prepared in the well by adding 70 μL of buffer, 20 μL of distilled water, and 10 μL of DMSO. In comparison, a control solution was prepared by adding 70 μL of buffer, 10 μL of enzyme solution, 10 μL of DMSO, and 10 μL of distilled water to the microwell. Finally, the reaction was started by adding 10 μL of substrate into the wells of test compounds. The plates were then incubated at 37.5 ± 2 °C for 15–20 minutes. All the samples were tested in triplicate. The absorbance was read at 405 nm using a Bio-Rad Microplate Reader. The obtained data was processed using MS Excel. The % inhibition of the test compounds and the standard drug was calculated using the formula below:% inhibition = 100 × [AT − AB/AC − AB]AT = absorbance of the test compound; AB = absorbance of the blank; AC = absorbance of the control.
The obtained % inhibition was plotted against the concentration of compounds, and straight-line equation y = mx + C was used to calculate the IC50 value, taking y intercept = 50.
7.3.2. In vitro α-amylase inhibitory activity
a. Preparation of test compounds and enzymes
α-Amylase (porcine) and substrate starch, standard drug acarbose, and dinitrosalicylic acid reagent (DNS) were purchased from Sisco Research Lab. The enzyme dilution (1 U mL−1) and substrate (1%) were prepared in phosphate buffer (pH 6.8). The different concentrations of test compounds 9a–i, 11a–c, i.e., 1000, 500, 200, 100, and 10 μM, were prepared. A calculated amount of the test compound was dissolved in DMSO to obtain a stock solution of 1000 μM. The other dilutions were prepared using the serial dilution method with DMSO as a diluent. Similarly, a dilution of the standard drug acarbose was prepared. The final or working dilutions of the test compounds and standard drug, i.e., 100, 50, 20, 10, and 1 μM, were adjusted in the well plates as described below.
b. Assay
In a 96-well plate, 10 μL of enzyme solution and 10 μL of the standard and test compounds were added to respective wells. A blank solution was prepared in the well by adding 70 μL of buffer, 20 μL of DNS reagent, and 10 μL of DMSO, while a control solution was prepared by adding 70 μL of buffer, 10 μL of enzyme solution, 10 μL of DMSO, and 10 μL of DNS to the microwell. The test compounds and enzyme solution were pre-incubated at 25 °C for 10 min. After 10 min, 10 μL of starch solution was added to start the reaction and incubated at 37.5 ± 2 °C for 15 min. The reaction was quenched by adding 20 μL of DNS reagent, followed by incubation at 70 °C for 20 min to stop the reaction altogether. The solution was diluted by adding 50 μL of buffer solution. All the samples were tested in triplicate. The absorbance was read at 540 nm using a Bio-Rad Microplate Reader. The obtained data was processed using MS Excel. The % inhibition of the test compounds and the standard drug was calculated using the formula below:% inhibition = 100 × [AT − AB/AC − AB]AT = absorbance of the test compound; AB = absorbance of the blank; AC = absorbance of the control.
The obtained % inhibition was plotted against the concentration of compounds, and the straight-line equation y = mx + C was used to calculate the IC50 value, taking y intercept = 50.
7.3.3. MTT assay
a. PANC-1
The MTT test on PANC-1 cells was used to measure the cytotoxicity of the reference medication (acarbose) and the compounds we made at different doses (9a–9i & 11a–11c). In a 96-well plate, 9 × 103 PANC-1 cells were added to each well in triplicate. A twenty-four-hour incubation period was allowed after administration to the cells (n = 3) at 37 °C with 5% CO2. After that, we cultured the PANC-1 cells at 37 °C for another day after treating them with 5, 10, 20, 40, 60, 80, and 100 μM of 9a–9i & 11a–11c. After that, 10 μL of MTT with a concentration of 5 mg mL−1 was added, and the cells were left to grow for another three hours at 37 °C. The liquid on top of the solid was removed after the plates were centrifuged at 2100 × g for 15 minutes. The formazan crystals were mixed with 200 μL of DMSO and left to rest in darkness for half an hour. The data were obtained at 570 nm using the iMARk™ microplate absorbance reader manufactured by Biorad.57,58
b. INS-1
The MTT test on INS-1 cells was used to measure the cytotoxicity of the reference medication (acarbose) and the compounds we made at different doses (9a–9i, 11a–11c). In a 96-well plate, 20 × 103 PANC-1 cells were added to each well in triplicate. A twenty-four-hour incubation period was allowed after administration to the cells (n = 3) at 37 °C with 5% CO2. After that, we cultured the PANC-1 cells at 37 °C for another day after treating them with 5, 10, 20, 40, 60, 80, and 100 μM of 9a–9i & 11a–11c. After that, 10 μL of MTT with a concentration of 5 mg mL−1 was added, and the cells were left to grow for another three hours at 37 °C. The liquid on top of the solid was removed after the plates were centrifuged at 2100 × g for 15 minutes. The formazan crystals were mixed with 200 μL of DMSO and left to rest in darkness for half an hour. The data were obtained at 570 nm using the iMARk™ microplate absorbance reader manufactured by Biorad.59
7.3.4. ROS measurement with DCFDA
a. In vitro ROS evaluation in PANC-1 cells
The anti-oxidant effect of inhibitors on total ROS generation caused by high glucose was determined by using 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA), a fluorescein isothiocyanate (FITC) active dye. Before being incubated at 37 °C with 5% CO2 for 24 hours, 1 × 105 PANC-1 cells were seeded into each well of a 12-well plate. The cells were then pre-treated with the exact dosage, 5 μM, of all the synthesized compounds, 9a–9i, 11a–11c, and acarbose, for 1 hour. Afterward, PANC-1 cells were exposed to 10 μM glucose and incubated at 37 °C with 5% CO2 for 23 hours. Each well was incubated at 37 °C for 30 minutes after adding 10 μM of the sensitive fluorescent probe DCFH-DA. The cells were stained and rinsed before ROS expression measurement using a BD Accuri™ C6 Plus (San Jose, CA) cell analyzer with FlowJo V10.60
b. ROS evaluation in isolated pancreas
In order to examine the formation of reactive oxygen species (ROS) in the pancreas, tissue samples were collected after being gently mashed through a 70 μm cell strainer using the rubber tip of a 3 mL syringe plunger. The samples were then rinsed with FACS buffer. The mixture was spun in a centrifuge at 300 × g for 3 minutes at 21 °C. Centrifugation was performed at 800 × g for 20 minutes at 21 °C after removing the supernatant. Then, the tissue remnants were re-suspended in 5 mL of 42% percoil. After removing the top layer and supernatant, the remaining tissue was treated in 5 ml of RBC lysis buffer for 5 minutes at 21 °C. The next step is a 3-minute centrifugation run at 21 °C using 300 × g. After the supernatant was discarded, the cells were re-suspended in 5 ml of FACS buffer. To quantify the expression of reactive oxygen species (ROS), the cells were washed three times with 300 μL of wash buffer, stained with DCFH-DA, and then processed through a BD Accuri™ C6 plus flow cytometer.
7.3.5. Estimation of α-amylase and α-glucosidase expression
a. Expression of enzymes in PNAC-1 cells
1 × 105 PANC-1 cells were seeded into each well of a 12-well plate. Following an hour of pre-treatment with 5 μM of 9F and 9G, and 20 μM of acarbose, the cells were subjected to a 23-hour treatment with 10 μM of high glucose. After 24 hours, the cells were collected, ground into pellets, and the liquid was drained. Once the cell pellet had been chilled on ice for fifteen minutes, 100 μL of 4% formaldehyde was added to fix it. Centrifuging the pellet and rinsing it with 1× PBS removed all traces of formaldehyde. A 100% cooled methanol permeabilization step was performed on the cells before centrifugation, and two washes were done with 1× PBS. Later, the cells were remixed with 100 μL of primary anti-α amylase (cell signaling #3796) and anti-α glucosidase (ab133720) antibodies, diluted and left overnight at 4 °C. A second cycle of centrifugation at 1500 × g for 5 minutes was performed on the samples after they had been washed three times with 1× PBS. Then, 100 μl of a fluorochrome-conjugated secondary antibody, Alexa Fluor 488, which was diluted, was added and allowed to incubate at room temperature for 30 minutes. The cells were centrifuged and rinsed with 1× PBS before the supernatant was discarded. The samples were examined using a flow cytometer called the BD Accuri™ C6 Plus in San Jose, CA, after being resuspended in 200–500 μl of 1× PBS. FlowJo V10 software was used to analyze the data.61
b. Expression of enzymes in rat intestine
In order to examine the activity of alpha-glucosidase and alpha-amylase in the pancreas of animals, tissues were collected after animals were sacrificed. Then, using the rubber end of a 3 ml syringe plunger, the tissue was mashed through a 70 μm cell strainer, and finally, it was rinsed with FACS buffer. The mixture was spun in a centrifuge at 300 × g for 3 minutes at 21 °C. Centrifugation was performed at 800 × g for 20 minutes at 21 °C after removing the supernatant. Then, the tissue remnants were resuspended in 5 ml of 42% percoil. After removing the top layer and supernatant, the remaining tissue was treated in 5 ml of RBC lysis buffer for 5 minutes at 21 °C. The next step is a 3-minute centrifugation run at 21 °C using 300 × g. After the supernatant was discarded, the cells were resuspended in 5 ml of FACS buffer. After that, the cells were stained by re-suspending them in 100 μL of diluted primary anti-α amylase (cell signalling #3796) and anti-α glucosidase (ab133720) antibodies and left at 4 °C overnight. A second cycle of centrifugation at 1500 × g for 5 minutes was performed on the samples after they had been washed three times with 1× PBS. Then, 100 μL of a fluorochrome-conjugated secondary antibody, Alexa Fluor 488, which was diluted, was added and allowed to incubate at room temperature for 30 minutes. The cells were centrifuged and rinsed with 1× PBS before the supernatant was discarded. The samples were examined using a flow cytometer called the BD Accuri™ C6 Plus in San Jose, CA, after being resuspended in 200–500 μL of 1× PBS. FlowJo V10 software was used for the analysis of the data.
7.4. In silico studies
7.4.1. Docking studies
The molecular docking study of the synthesized analogs was performed using the Schrodinger Glide docking protocol using the Maestro interface (Version 12.9.123, Release 2021-3) to analyze the interactions of the ligand with active site amino acids of alpha-amylase and alpha-glucosidase enzymes. The synthesized analogues were prepared using ChemDraw 16.0 software, and energies were minimized using the molecular mechanics 2 (MM2) force field of Chem3D (PerkinElmer, USA). Further, ligand preparation was performed using the ligand preparation wizard of Maestro using OPLS2 force fields. The crystal structure of human pancreatic alpha-amylase (PDB id: 1B2Y) and alpha-glucosidase enzymes (UniProt id: P00689) was retrieved from RCSB PDB and UniProt, respectively. The proteins were prepared accordingly using the protein preparation wizard of Maestro, and all of the test compounds were docked at the active site, using grid parameters: X = 18.984, Y = 5.764, Z = 47.008 for alpha-amylase and alpha-glucosidase enzymes. All the docked complexes were visualized using Maestro.62
7.4.2. In silico drug-likeness predictions
The drug-likeness predicts the oral bioavailability of the derivatives based on various structural and physicochemical properties. In the present study, the drug-likeness of the synthesized derivatives was predicted using Lipinski's rule of five, which describes that for a molecule to act as a therapeutic candidate, it must possess five characteristics which are (a) the number of hydrogen bond donors should not be more than 5, (b) the number of hydrogen bond acceptors should not be more than 10, (c) the molecular mass should be less than 500 Da, and (d) log P (octanol–water partition coefficient) should not be greater than 5. Drug likeness was predicted by QikProp tools [Schrödinger Release 2021-3: QikProp, Schrödinger, LLC, New York, NY, 2021 (ref. 63)].
7.4.3. In silico toxicity prediction
The synthesized derivatives' toxicity analysis was done using ProTox online software to predict the synthesized compounds' LD50 dose, toxicity class, hepatotoxicity, and cytotoxicity.64
7.4.4. Molecular dynamics simulations
7.4.4.1. MD simulation within the active site of pancreatic alpha-amylase
The molecular dynamics (MD) simulations were performed on the best molecule that emerged from the in vitro enzymatic assay, i.e., 9G within the active site of human pancreatic alpha-amylase (PDB id: 1B2Y) using the Desmond MD package of Schrodinger software (release 2021-3). The MD simulation studies evaluated used proteins' molecular dynamics and stability in an ambient environment. The proteins and ligands were prepared using the Maestro interface using prep wizards for the ligand and protein, respectively. The prepared protein was immersed in an orthorhombic box of the simple point charge SPC water, extending at least 10 Å from the solute. After that, the system was equilibrated with a constant volume for one ns. Finally, the production simulations were performed with continuous pressure for 100 ns. The root-mean-square deviation (RMSD), the root mean square fluctuation (RMSF), and the ligand properties were calculated to determine the structural changes.54
7.4.4.2. Simulation within the active site of human pancreatic alpha-glucosidase
The molecular dynamics (MD) simulations were performed on the best molecule that emerged from the in vitro enzymatic assay, i.e., 9G within the active site of human pancreatic alpha-glucosidase (UniProt id: P00689) using Gromacs software with the standard protocol developed by Bowers et al.54
7.5. In vivo studies
The test compound was evaluated using an STZ-induced diabetic model in Wister rats employing the previously developed methodology.50
7.5.1. Induction of diabetes
Streptozotocin was procured from SRL Chemical Ltd. Adult Wistar rats of either sex were used for the in vivo studies. The animals were procured and maintained under light and dark conditions at the institutional animal house facility of ISF College of Pharmacy. Diabetes was induced by injecting streptozotocin (65 mg kg−1 of body weight) in freshly prepared 0.1 M citrate buffer solution (pH 4.5) intraperitoneally into the previously fasted rats. To overcome the hypoglycemic condition due to the administration of streptozotocin, the rats were allowed to drink a 5% glucose solution throughout the night, and their blood glucose levels were measured. Animals whose blood glucose levels will be 250 mg dl−1 or beyond this after 72 hours of STZ administration were considered diabetic. The blood glucose levels were measured using the tail puncture method using a commercial Dr. Morepen blood glucometer. The animals were then put on a pelleted diet with free access to tap water throughout the protocol. The research technique followed institutional norms for animal care and management in order to reduce suffering and maximize the utilization of animals. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of IAEC (Institute for Animal Ethics Committee) 816/PO/ReBiBt/S/04/CPCSEA at the Indo-Soviet Friendship College of Pharmacy in Moga, Punjab, India, in accordance with CPCSEA, Government of India and approved by the Animal Ethics Committee of ISFCP/IAEC/CPCSEA/Meeting No: 02/Protocol No. 23; Dated 26/11/2022.
7.5.2. Treatment of animals with the test compounds and pioglitazone
The animals were divided into different groups, i.e., normal control, vehicle control, STZ-treated, STZ + 9F, STZ + 9G, and STZ + pioglitazone on a random basis. The test compounds and the standard drug were given orally for 21 days in 0.5% sodium CMC suspension at a dose of 10 mg kg−1 of body weight. The animals were fed a regular pelleted diet with free tap water access. The blood glucose levels and body weight were checked on the 1st, 4th, 14th, and 35th day of the treatment protocol.
7.5.3. Scarification of animals
After the completion of the protocol, the animals were sacrificed post-euthanizing using thiopental sodium and cervical dislocation. Their organs, such as kidneys, liver, and pancreas, were collected and weighed for fresh organ weights. The pancreatic tissues of animals were crushed to prepare tissue homogenate. Histopathology of pancreatic, kidney, and liver tissues was also performed. The whole blood of the animals was collected through the retro-orbital vein, and various blood parameters were analyzed using a five-part veterinary autoanalyzer (Mindray). The blood was centrifuged immediately to obtain fresh serum plasma. The serum levels of amylase, lipid profile, kidney function test, oxidative stress parameters, etc., were analyzed using different assay methods.
7.5.4. Preparation of tissue homogenate
Fresh pancreases of the rats were collected and washed with phosphate buffer (pH 8) to remove blood stains and cell debris. 10 mL of freshly prepared phosphate buffer was taken in an organ tube, and freshly isolated pancreases were placed in it and homogenized using a tissue homogenizer. The homogenate was then centrifuged to obtain a clear supernatant, which was collected and immediately used to analyze different parameters.
7.5.5. Measurement of the serum amylase level
The serum amylase levels of the animals were determined using the rat amylase ELISA kit assay (E-EL-R2544; Elabscience, Wuhan, China).65
7.5.6. Measurement of the PPAR-gamma level
The levels of PPAR-gamma in pancreatic tissue were determined using the rat PPAR-gamma ELISA kit assay (E-EL-R0724; Elabscience, Wuhan, China).66
7.5.7. Measurement of oxidative stress
7.5.7.1. Estimation of malondialdehyde (MDA)
Malondialdehyde was estimated in the pancreatic tissue homogenate using the methodology developed by Mehan et al.67 and Gumbar et al.68 The results were expressed as μM mg−1 protein.
7.5.7.2. Measurement of superoxide dismutase (SOD) levels
The levels of SOD in pancreatic tissue homogenate were determined by UV-spectroscopy (Shimadzu double beam spectrophotometer, UV-1900i) via the autoxidation of epinephrine at pH 10.4. 200 μL of tissue homogenate and 0.8 mL of glycine buffer (pH 10.4) were mixed and incubated at room temperature for 10 min. After incubation, the absorbance of the resulting solution was read at 480 nm, and results were expressed in μM mg−1 protein.68
7.5.7.3. Measurement of the serum GSH level
The levels of GSH in the rat serum were determined using the Ellman method. 1 mL of blood serum was mixed with 10% w/v trichloroacetic acid (1 : 1), and the mixture was centrifuged for 10 min at 5000 rpm at 4 °C. 0.5 mL of resultant supernatant was diluted with 0.4 mL of distilled water, and 2 mL of freshly prepared phosphate buffer solution (pH 8) was added. 0.2 mL of freshly prepared 0.001 M DTNB [5,5′-dithiobis (2-nitrobenzoic acid) blended in 1% w/v sodium citrate] was then added, and the solution was incubated for ten minutes. After 10 min, the absorbance of the resulting yellow complex was measured spectrophotometrically at 412 nm. The data were plotted on a standard curve using the reduced form of glutathione and reported in IU mL−1.68,69
7.5.7.4. Measurement of the serum catalase level
Freshly isolated serum (0.2 mL) was mixed with 1.2 mL phosphate buffer (pH 8). The enzymatic reaction was initiated by adding 1 ml of 0.03 M hydrogen peroxide solution. Distilled water was used as a blank for comparison. The absorbance of the resulting solution was read at 240 nm spectrophotometrically. The enzyme activity was computed based on the quantity of hydrogen peroxide oxidized/mg of protein and expressed in IU mL−1.50,68,70
7.6. Measurement of the lipid profile, liver function test, and kidney function test
The blood was collected through retro-orbital vein puncture in an EDTA-impregnated tube and analyzed at Diksha Diagnostic Lab Ltd, Moga (Registration Number-UANPB14D00002352) for the quantification of serum triglycerides, total cholesterol, and high-density lipoproteins. At the same time, SGOT, SGPT, ALP, blood urea nitrogen, creatinine, and uric acid levels were also measured to establish the liver and kidney functioning of the treated rats.50
7.7. Measurement of inflammatory markers
7.7.1. Measurement of TNF-alpha
The levels of TNF-alpha were quantified in both serum and pancreatic tissue homogenate using the ELISA kit assay [KB1145; Krishgen Biosystem, Mumbai, India].65,71
7.7.2. Measurement of IL-1beta
The levels of TNF-alpha were quantified in both serum and pancreatic tissue homogenate using the ELISA kit assay [KLR0119; Krishgen Biosystem, Mumbai, India].71
7.8. Measurement of blood parameters
The blood of animals was collected using the retro-orbital vein puncture method in an EDTA tube, and immediately 10 μL of the blood was fed to the inlet of the autohematoanalyzer (Mindray BC-5000 vet) for quantification of various complete blood counts (CBC) such as haemoglobin, lymphocytes, monocytes, etc.
7.9. Histopathology of pancreas, kidney and liver
The histology of the removed organs was performed using the process described by Gumbar et al.64,68,72
7.10. Statistical analysis
All results were statistically analysed using Graph Pad Prism version 8.0 (Graph Pad software, INC, La Jolla, CA, United States) and expressed as mean ± standard deviation (SD). Results were analysed using one-way ANOVA and two-way ANOVA followed by post hoc Turkey's and Bonferroni's tests, respectively, with p < 0.001 statistical significance.
Ethical statement
The work was approved by the Institutional Animal Ethics Committee (IAEC) under the registration number of ISFCP/IAEC/CPCSEA/Meeting No: 02/Protocol No. 23 dated 26/11/2022. The approval letter is attached in ESI† 1.
Data availability
The datasets supporting this article have been uploaded as part of the ESI.†
Author contributions
Gurpreet Singh: design and synthesis of title molecules, spectral characterization, in vitro and in vivo experimentation, compilation of results, and manuscript preparation. Rajveer Singh: software, resources, mechanistic studies, in vitro cytotoxicity. Vikramdeep Monga: design of molecules, docking analysis, MD simulations, spectral data and manuscript verification. Sidharth Mehan: design in vivo experimentation, supervise, verify statistical analysis, and prepare the manuscript.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Material
Acknowledgments
This work was supported by the DST-SERB Govt. of India (Grant Number: CRG/2021/001009) for whole blood CBC and biochemical analysis. The authors thank Chairman Mr. Parveen Garg, ISF College of Pharmacy, Moga; Director-cum-Principal, Prof. (Dr.) G. D. Gupta, ISF College of Pharmacy, Moga and Principal, Prof. (Dr.) R. K. Narang, ISFCPR, Ratian Road, Moga (Punjab), India, for their excellent vision and support. Gurpreet Singh also thanks IK Gujral Punjab Technical University, Kapurthala, Punjab, for providing the necessary support. The authors also thank DST-FIST Lab, ISFCP, for providing FACS analysis. The author, Vikramdeep Monga, acknowledges the Central University of Punjab, Bathinda, for providing the necessary infrastructure and laboratory facilities.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4md00199k
References
- Kaur N. Kumar V. Nayak S. K. Wadhwa P. Kaur P. Sahu S. K. Alpha-amylase as molecular target for treatment of diabetes mellitus: A comprehensive review. Chem. Biol. Drug Des. 2021;98(4):539–560. doi: 10.1111/cbdd.13909. [DOI] [PubMed] [Google Scholar]
- Yang Y.-Y. Chen Z. Yang X.-D. Deng R.-R. Shi L.-X. Yao L.-Y. et al., Piperazine ferulate prevents high-glucose-induced filtration barrier injury of glomerular endothelial cells. Exp. Ther. Med. 2021;22(4):1–10. doi: 10.1111/cbdd.13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D. Cai X. Guan Q. Ou Y. Zheng X. Lin X. Burden of type 1 and type 2 diabetes and high fasting plasma glucose in Europe, 1990-2019: a comprehensive analysis from the global burden of disease study 2019. Front. Endocrinol. 2023;14:1307432. doi: 10.3389/fendo.2023.1307432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. Zhang Y. Yang Y. Pan J. Diabetes-related avoidable hospitalisations and its relationship with primary healthcare resourcing in China: A cross-sectional study from Sichuan Province. Health Soc. Care Community. 2022;30(4):e1143–e1156. doi: 10.1111/hsc.13522. [DOI] [PubMed] [Google Scholar]
- Li J.-M. Li X. Chan L. W. Hu R. Zheng T. Li H. et al., Lipotoxicity-polarised macrophage-derived exosomes regulate mitochondrial fitness through Miro1-mediated mitophagy inhibition and contribute to type 2 diabetes development in mice. Diabetologia. 2023;66(12):2368–2386. doi: 10.1007/s00125-023-05992-7. [DOI] [PubMed] [Google Scholar]
- Yu T. Xu B. Bao M. Gao Y. Zhang Q. Zhang X. et al., Identification of potential biomarkers and pathways associated with carotid atherosclerotic plaques in type 2 diabetes mellitus: A transcriptomics study. Front. Endocrinol. 2022;13:981100. doi: 10.3389/fendo.2022.981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Jiang Z. Yang L. Fang Y. Lu S. Akakuru O. U. et al., HPDA/Zn as a CREB Inhibitor for Ultrasound Imaging and Stabilization of Atherosclerosis Plaque. Chin. J. Chem. 2023;41(2):199–206. doi: 10.1002/cjoc.202200406. [DOI] [Google Scholar]
- Wang C. Wang S. Wang Z. Han J. Jiang N. Qu L. et al., Andrographolide regulates H3 histone lactylation by interfering with p300 to alleviate aortic valve calcification. Br. J. Pharmacol. 2024;181(12):1843–1856. doi: 10.1111/bph.16332. [DOI] [PubMed] [Google Scholar]
- Liang X. Zhang J. Wang Y. Wu Y. Liu H. Feng W. et al., Comparative study of microvascular structural changes in the gestational diabetic placenta. Diabetes Vasc. Dis. Res. 2023;20(3):14791641231173627. doi: 10.1177/14791641231173627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W. Zhang Z. Li L. Liang X. Wu Y. Wang X. et al., Gut microbiota induces DNA methylation via SCFAs predisposing obesity-prone individuals to diabetes. Pharmacol. Res. 2022;182:106355. doi: 10.1016/j.phrs.2022.106355. [DOI] [PubMed] [Google Scholar]
- Tomlinson B. Patil N. G. Fok M. Chan P. Lam C. W. K. The role of sulfonylureas in the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2022;23(3):387–403. doi: 10.1080/14656566.2021.1999413. [DOI] [PubMed] [Google Scholar]
- Xu Z. Zhang P. Chen Y. Jiang J. Zhou Z. Zhu H. Comparing SARC-CalF with SARC-F for screening sarcopenia in adults with type 2 diabetes mellitus. Front. Nutr. 2022;9:803924. doi: 10.3389/fnut.2022.803924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldi S. Costa V. Ciccodicola A. Aprile M. PPARγ and diabetes: beyond the genome and towards personalized medicine. Curr. Diabetes Rep. 2021;21:1–15. doi: 10.1007/s11892-020-01370-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez D. E. Foresto R. D. Ribeiro A. B. SGLT-2 inhibitors in diabetes: a focus on renoprotection. Rev. Assoc. Med. Bras. 2020;66:s17–s24. doi: 10.1590/1806-9282.66.s1.17. [DOI] [PubMed] [Google Scholar]
- Proença C. Ribeiro D. Freitas M. Fernandes E. Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity: A review. Crit. Rev. Food Sci. Nutr. 2022;62(12):3137–3207. doi: 10.1080/10408398.2020.1862755. [DOI] [PubMed] [Google Scholar]
- Gilbert M. P. Pratley R. E. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front. Endocrinol. 2020;11:178. doi: 10.3389/fendo.2020.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H. Zhang A. Li D. Wu Y. Wang C.-Z. Wan J.-Y. et al., Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis. BMJ. 2024;384:e076410. doi: 10.1136/bmj-2023-076410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberi Z. Sauli E. Cun L. Deng J. Li W.-J. He X.-L. et al., Insulin-delivery methods for children and adolescents with type 1 diabetes. Ther. Adv. Endocrinol. Metab. 2020;11:2042018820906016. doi: 10.1177/2042018820906016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G. Zhao K. You K. Gao Z. Kakuchi T. Feng B. et al., Synthesis and characterization of phenylboronic acid-containing polymer for glucose-triggered drug delivery+ Sci. Technol. Adv. Mater. 2020;21(1):1–10. doi: 10.1080/14686996.2019.1700394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A. Kaur P. Sahu S. K. Mittal A. A new insight into the treatment of diabetes by means of pan PPAR agonists. Chem. Biol. Drug Des. 2022;100(6):947–967. doi: 10.1111/cbdd.14020. [DOI] [PubMed] [Google Scholar]
- Montaigne D. Butruille L. Staels B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021;18(12):809–823. doi: 10.1038/s41569-021-00569-6. [DOI] [PubMed] [Google Scholar]
- Hidayat K. Fang Q.-L. Shi B.-M. Qin L.-Q. Influence of glycemic control and hypoglycemia on the risk of fracture in patients with diabetes mellitus: a systematic review and meta-analysis of observational studies. Osteoporosis Int. 2021;32:1693–1704. doi: 10.1007/s00198-021-05934-2. [DOI] [PubMed] [Google Scholar]
- Yin L. Zhang D. Ren Q. Su X. Sun Z. Prevalence and risk factors of diabetic retinopathy in diabetic patients: A community based cross-sectional study. Medicine. 2020;99(9):e19236. doi: 10.1097/MD.0000000000019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Y. Shi L. X. Li J. H. Yao L. Y. Xiang D. X. Piperazine ferulate ameliorates the development of diabetic nephropathy by regulating endothelial nitric oxide synthase. Mol. Med. Rep. 2019;19(3):2245–2253. doi: 10.3892/mmr.2019.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. Zhou Y. Zhao X. Yang X. Wan X. An Z. et al., Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Alleviate Diabetic Kidney Disease in Rats by Inhibiting Apoptosis and Inflammation. Front. Biosci.-Landmark. 2023;28(9):203. doi: 10.31083/j.fbl2809203. [DOI] [PubMed] [Google Scholar]
- Xiong D. Hu W. Han X. Cai Y. Rhein inhibited ferroptosis and EMT to attenuate diabetic nephropathy by regulating the rac1/NOX1/β-catenin Axis. Front. Biosci.-Landmark. 2023;28(5):100. doi: 10.31083/j.fbl2805100. [DOI] [PubMed] [Google Scholar]
- Mushtaq A. Azam U. Mehreen S. Naseer M. M. Synthetic α-glucosidase inhibitors as promising anti-diabetic agents: Recent developments and future challenges. Eur. J. Med. Chem. 2023;249:115119. doi: 10.1016/j.ejmech.2023.115119. [DOI] [PubMed] [Google Scholar]
- Oboh G. Ogunsuyi O. B. Ogunbadejo M. D. Adefegha S. A. Influence of gallic acid on α-amylase and α-glucosidase inhibitory properties of acarbose. J. Food Drug Anal. 2016;24(3):627–634. doi: 10.1016/j.jfda.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. Iqbal S. Rahim F. Shah M. Hussain R. Alrbyawi H. et al., New biologically hybrid pharmacophore thiazolidinone-based indole derivatives: synthesis, in vitro alpha-amylase and alpha-glucosidase along with molecular docking investigations. Molecules. 2022;27(19):6564. doi: 10.3390/molecules27196564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J. Singh A. Singh G. Verma R. K. Mall R. Novel indolyl linked para-substituted benzylidene-based phenyl containing thiazolidienediones and their analogs as α-glucosidase inhibitors: synthesis, in vitro, and molecular docking studies. Med. Chem. Res. 2018;27:903–914. doi: 10.1007/s00044-017-2112-6. [DOI] [Google Scholar]
- Dileep K. Nithiyanandan K. Remya C. Binding of acarbose, an anti-diabetic drug to lysozyme: a combined structural and thermodynamic study. J. Biomol. Struct. Dyn. 2018;36(13):3354–3361. doi: 10.1080/07391102.2017.1388283. [DOI] [PubMed] [Google Scholar]
- Rasheed L. Rehman W. Rahim F. Ali Z. Alanazi A. S. Hussain R. et al., Molecular Modeling and Synthesis of Indoline-2, 3-dione-Based Benzene Sulfonamide Derivatives and Their Inhibitory Activity against α-Glucosidase and α-Amylase Enzymes. ACS Omega. 2023;8(17):15660–15672. doi: 10.1021/acsomega.3c01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad M. Chaudhary A. R. Mumtaz M. W. Raza S. A. Ahmad M. Mukhtar H. et al., Polyphenol fingerprinting and hypoglycemic attributes of optimized Cycas circinalis leaf extracts. J. Sci. Food Agric. 2021;101(4):1530–1537. doi: 10.1002/jsfa.10771. [DOI] [PubMed] [Google Scholar]
- Lei X. G. Zhu J.-H. Cheng W.-H. Bao Y. Ho Y.-S. Reddi A. R. et al., Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiol. Rev. 2016;96(1):307–364. doi: 10.1152/physrev.00010.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H. Katakami N. Matsuhisa M. Matsuoka T.-a. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflammation. 2010;2010:453892. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajal K. Singh G. Pradhan T. Bhurta D. Monga V. The medicinal perspective of 2, 4-thiazolidinediones based ligands as antimicrobial, antitumor and antidiabetic agents: A review. Arch. Pharm. 2022;355(9):2100517. doi: 10.1002/ardp.202100517. [DOI] [PubMed] [Google Scholar]
- Cheng H. S. Tan W. R. Low Z. S. Marvalim C. Lee J. Y. H. Tan N. S. Exploration and development of PPAR modulators in health and disease: an update of clinical evidence. Int. J. Mol. Sci. 2019;20(20):5055. doi: 10.3390/ijms20205055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Järvinen H. Thiazolidinediones. N. Engl. J. Med. 2004;351(11):1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- Kohlroser J. Mathai J. Reichheld J. Banner B. F. Bonkovsky H. L. Hepatotoxicity due to troglitazone: report of two cases and review of adverse events reported to the United States Food and Drug Administration. Am. J. Gastroenterol. 2000;95(1):272–276. doi: 10.1111/j.1572-0241.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- Dutta D. Bhattacharya S. Kumar M. Datta P. K. Mohindra R. Sharma M. Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis. Diabetes Metab. Syndr. 2023;17(1):102697. doi: 10.1016/j.dsx.2022.102697. [DOI] [PubMed] [Google Scholar]
- Tilekar K. Shelke O. Upadhyay N. Lavecchia A. Ramaa C. Current status and future prospects of molecular hybrids with thiazolidinedione (TZD) scaffold in anticancer drug discovery. J. Mol. Struct. 2022;1250:131767. doi: 10.1016/j.molstruc.2021.131767. [DOI] [Google Scholar]
- Sethi N. S. Prasad D. N. Singh R. K. Synthesis, anticancer, and antibacterial studies of benzylidene bearing 5-substituted and 3, 5-disubstituted-2, 4-thiazolidinedione derivatives. Med. Chem. 2021;17(4):369–379. doi: 10.2174/1573406416666200512073640. [DOI] [PubMed] [Google Scholar]
- Kumar H. Aggarwal N. Marwaha M. G. Deep A. Chopra H. Matin M. M. et al., Thiazolidin-2, 4-Dione Scaffold: An Insight into Recent Advances as Antimicrobial, Antioxidant, and Hypoglycemic Agents. Molecules. 2022;27(19):6763. doi: 10.3390/molecules27196763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay D. B. Mokariya J. A. Patel P. J. Patel S. G. Das A. Nandi A. et al., Indole clubbed 2, 4-thiazolidinedione linked 1, 2, 3-triazole as a potent antimalarial and antibacterial agent against drug-resistant strain and molecular modeling studies. Arch. Pharm. 2024:e2300673. doi: 10.1002/ardp.202300673. [DOI] [PubMed] [Google Scholar]
- Sameeh M. Y. Khowdiary M. M. Nassar H. S. Abdelall M. M. Amer H. H. Hamed A. et al., Thiazolidinedione Derivatives: In Silico, In Vitro, In Vivo, Antioxidant and Anti-Diabetic Evaluation. Molecules. 2022;27(3):830. doi: 10.3390/molecules27030830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman P. Yadav N. Auti P. S. Jaswal S. Singh G. Mehan S. et al., Discovery of thiazolidinedione-based pancreatic lipase inhibitors as anti-obesity agents: synthesis, in silico studies and pharmacological investigations. J. Biomol. Struct. Dyn. 2024:1–23. doi: 10.1080/07391102.2024.2310799. [DOI] [PubMed] [Google Scholar]
- George G. Auti P. S. Sengupta P. Yadav N. Paul A. T. Synthesis, molecular modelling and pharmacological evaluation of novel indole-thiazolidinedione based hybrid analogues as potential pancreatic lipase inhibitors. J. Biomol. Struct. Dyn. 2023:1–20. doi: 10.1080/07391102.2023.2293255. [DOI] [PubMed] [Google Scholar]
- Fettach S. Thari F. Z. Hafidi Z. Tachallait H. Karrouchi K. El Achouri M. et al., Synthesis, α-glucosidase and α-amylase inhibitory activities, acute toxicity and molecular docking studies of thiazolidine-2, 4-diones derivatives. J. Biomol. Struct. Dyn. 2022;40(18):8340–8351. doi: 10.1080/07391102.2021.1911854. [DOI] [PubMed] [Google Scholar]
- Sameeh M. Y. Khowdiary M. M. Nassar H. S. Abdelall M. M. Alderhami S. A. Elhenawy A. A. Discovery potent of thiazolidinedione derivatives as antioxidant, α-amylase inhibitor, and antidiabetic agent. Biomedicines. 2021;10(1):24. doi: 10.3390/biomedicines10010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G. Singh R. Monga V. Mehan S. 3, 5-Disubstituted-thizolidine-2, 4-dione hybrids as antidiabetic agents: Design, synthesis, in-vitro and In vivo evaluation. Eur. J. Med. Chem. 2024:116139. doi: 10.1016/j.ejmech.2024.116139. [DOI] [PubMed] [Google Scholar]
- Kaur R. Kumar R. Dogra N. Kumar A. Yadav A. K. Kumar M. Synthesis and studies of thiazolidinedione–isatin hybrids as α-glucosidase inhibitors for management of diabetes. Future Med. Chem. 2021;13(05):457–485. doi: 10.4155/fmc-2020-0022. [DOI] [PubMed] [Google Scholar]
- Rahim F. Malik F. Ullah H. Wadood A. Khan F. Javid M. T. et al., Isatin based Schiff bases as inhibitors of α-glucosidase: Synthesis, characterization, in vitro evaluation and molecular docking studies. Bioorg. Chem. 2015;60:42–48. doi: 10.1016/j.bioorg.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Dirir A. M. Daou M. Yousef A. F. Yousef L. F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. 2022;21(4):1049–1079. doi: 10.1007/s11101-021-09773-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers K. J., Chow E., Xu H., Dror R. O., Eastwood M. P. and Gregersen B. A., et al., Scalable algorithms for molecular dynamics simulations on commodity clusters, Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, 2006
- Van Der Spoel D. Lindahl E. Hess B. Groenhof G. Mark A. E. Berendsen H. J. GROMACS: fast, flexible, and free. J. Comput. Chem. 2005;26(16):1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- Rahim F. Taha M. Iqbal N. Hayat S. Qureshi F. Uddin I. et al., Isatin based thiosemicarbazide derivatives as potential inhibitor of α-glucosidase, synthesis and their molecular docking study. J. Mol. Struct. 2020;1222:128922. doi: 10.1016/j.molstruc.2020.128922. [DOI] [Google Scholar]
- Chandel S. Singh R. Gautam A. Ravichandiran V. Screening of Azadirachta indica phytoconstituents as GSK-3β inhibitor and its implication in neuroblastoma: Molecular docking, molecular dynamics, MM-PBSA binding energy, and in-vitro study. J. Biomol. Struct. Dyn. 2022;40(23):12827–12840. doi: 10.1080/07391102.2021.1977705. [DOI] [PubMed] [Google Scholar]
- Chandel S. Bhattacharya A. Gautam A. Zeng W. Alka O. Sachsenberg T. et al., Investigation of the anti-cancer potential of epoxyazadiradione in neuroblastoma: experimental assays and molecular analysis. J. Biomol. Struct. Dyn. 2023:1–19. doi: 10.1080/07391102.2023.2262593. [DOI] [PubMed] [Google Scholar]
- El-Huneidi W. Anjum S. Bajbouj K. Abu-Gharbieh E. Taneera J. The coffee diterpene, kahweol, ameliorates pancreatic β-cell function in streptozotocin (STZ)-treated rat INS-1 cells through NF-kB and p-AKT/Bcl-2 pathways. Molecules. 2021;26(17):5167. doi: 10.3390/molecules26175167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Singh R. Dutta D. Chandel S. Bhattacharya A. Ravichandiran V. et al., In Vitro Anticancer Activity of Methanolic Extract of Justicia adhatoda Leaves with Special Emphasis on Human Breast Cancer Cell Line. Molecules. 2022;27(23):8222. doi: 10.3390/molecules27238222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. Chandel S. Ghosh A. Matta T. Gautam A. Bhattacharya A. et al., Glucogallin Attenuates the LPS-Induced Signaling in Macrophages and Protects Mice against Sepsis. Int. J. Mol. Sci. 2022;23(19):11254. doi: 10.3390/ijms231911254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesner R. A. Murphy R. B. Repasky M. P. Frye L. L. Greenwood J. R. Halgren T. A. et al., Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein–ligand complexes. J. Med. Chem. 2006;49(21):6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Alzain A. A. Ismail A. Fadlelmola M. Mohamed M. A. Mahjoub M. Makki A. A. et al., De novo design of novel spike glycoprotein inhibitors using e-pharmacophore modeling, molecular hybridization, ADMET, quantum mechanics and molecular dynamics studies for COVID-19. Pak. J. Pharm. Sci. 2022;35(1):313–321. [PubMed] [Google Scholar]
- Banerjee P. Eckert A. O. Schrey A. K. Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018;46(W1):W257–W263. doi: 10.1093/nar/gky318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ul Haq M. E. Akash M. S. H. Rehman K. Mahmood M. H. Chronic exposure of bisphenol A impairs carbohydrate and lipid metabolism by altering corresponding enzymatic and metabolic pathways. Environ. Toxicol. Pharmacol. 2020;78:103387. doi: 10.1016/j.etap.2020.103387. [DOI] [PubMed] [Google Scholar]
- Olaniyi K. S. Akintayo C. O. Oniyide A. A. Omoaghe A. O. Oyeleke M. B. Fafure A. A. Acetate supplementation restores testicular function by modulating Nrf2/PPAR-γ in high fat diet-induced obesity in Wistar rats. J. Diabetes Metab. Disord. 2021;20:1685–1696. doi: 10.1007/s40200-021-00924-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehan S. Meena H. Sharma D. Sankhla R. JNK: a stress-activated protein kinase therapeutic strategies and involvement in Alzheimer's and various neurodegenerative abnormalities. J. Mol. Neurosci. 2011;43(3):376–390. doi: 10.1007/s12031-010-9454-6. [DOI] [PubMed] [Google Scholar]
- Gumbar S. Bhardwaj S. Mehan S. Khan Z. Narula A. S. Kalfin R. et al., Renal mitochondrial restoration by gymnemic acid in gentamicin-mediated experimental nephrotoxicity: Evidence from serum, kidney and histopathological alterations. Front. Pharmacol. 2023;14:1218506. doi: 10.3389/fphar.2023.1218506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niknahad H. Heidari R. Mohammadzadeh R. Ommati M. M. Khodaei F. Azarpira N. et al., Sulfasalazine induces mitochondrial dysfunction and renal injury. Renal Failure. 2017;39(1):745–753. doi: 10.1080/0886022X.2017.1399908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. Rahi S. Mehan S. Neuroprotective potential of solanesol in intracerebroventricular propionic acid induced experimental model of autism: Insights from behavioral and biochemical evidence. Toxicol. Rep. 2019;6:1164–1175. doi: 10.1016/j.toxrep.2019.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra S. Mehan S. Khan Z. Gupta G. D. Narula A. S. Matrine mediated neuroprotective potential in experimental multiple sclerosis: Evidence from CSF, blood markers, brain samples and in-silico investigations. J. Neuroimmunol. 2023;384:578200. doi: 10.1016/j.jneuroim.2023.578200. [DOI] [PubMed] [Google Scholar]
- Singh L. Rana S. Mehan S. Role of adenylyl cyclase activator in controlling experimental diabetic nephropathy in rats. Int. J. Physiol., Pathophysiol. Pharmacol. 2018;10(5):144. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the ESI.†