Abstract
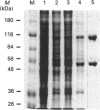
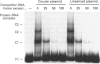
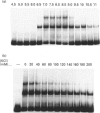
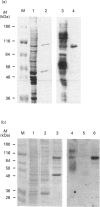
In studies of protein binding to the upstream region of the human proliferation-associated antigen p120 gene, a heterodimer of 52 and 100 kDa proteins was purified from HeLa cells. A 1:1 ratio of p52 and p100 was constant throughout the purification. The heterodimer was localized to cell nuclei, as shown by immunofluorescence. The pI values of the p52 and p100 were 7.8 and 8.6 respectively. The peptide sequences obtained for p52 (QSNKTFNLEKQNHTPRKKHQ and PLRGKQLRVRFAAHSASLTVR) and for p100 (PGGPKPGGGPGLSTPGGHPKPPHRGGGEPPRGRQ and GPGPGQSGPKPPIPPPPPHQQ) were not found in the computer databanks. One p52 peptide sequence, PLRGKQLRVRFA, shows considerable sequence similarity to a conserved motif in topoisomerase II of multiple species. The p52/100 heterodimer bound to different DNA probes. The binding was competed by poly(dI-dC), sonicated salmon sperm DNA, and circular or linearized plasmid DNA. The optimal DNA binding for the heterodimer was at pH 7-9 with low salt. The DNA-binding subunit of the heterodimer was the p100 polypeptide, as shown by u.v.-cross-linking assays and Southwestern blots.
Full text
PDF
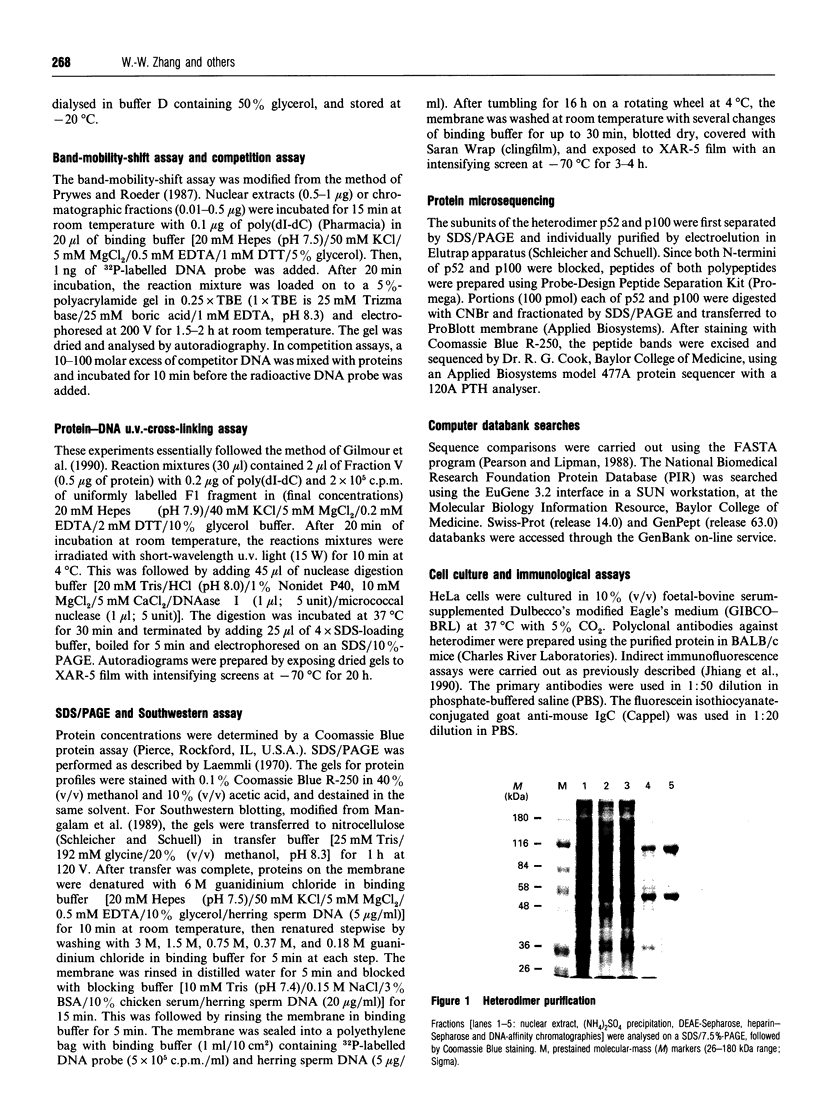
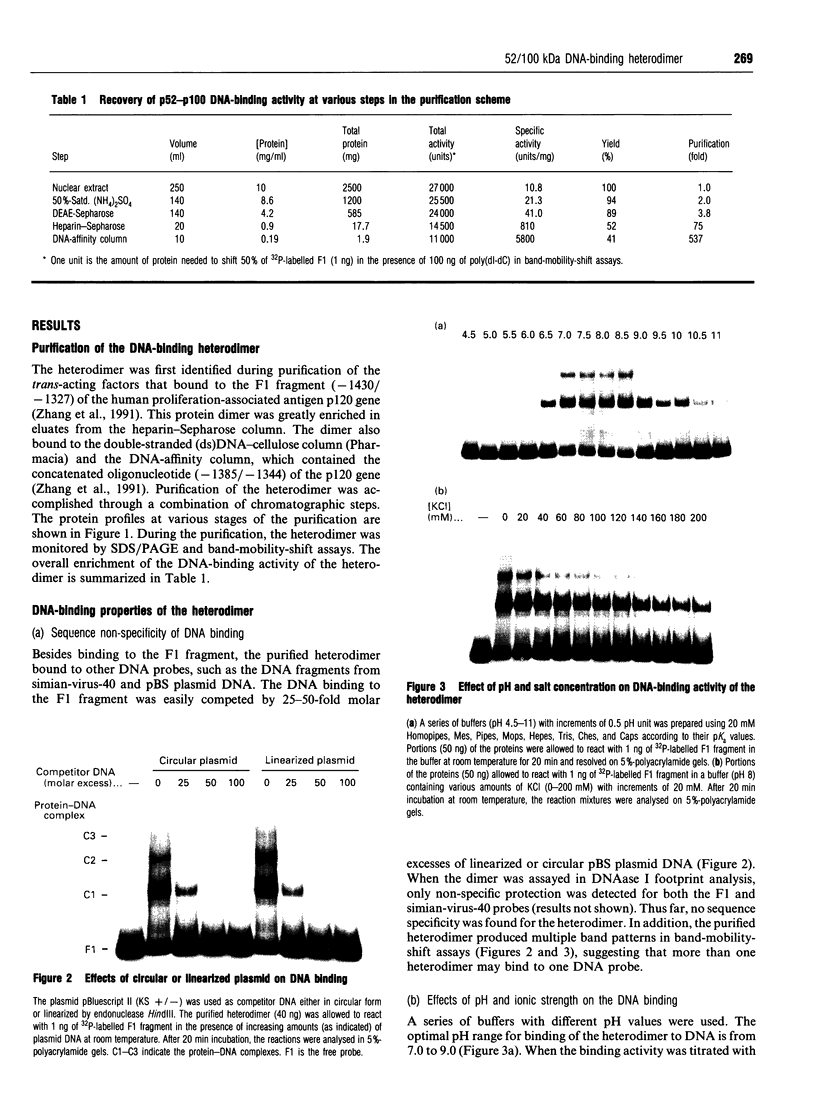

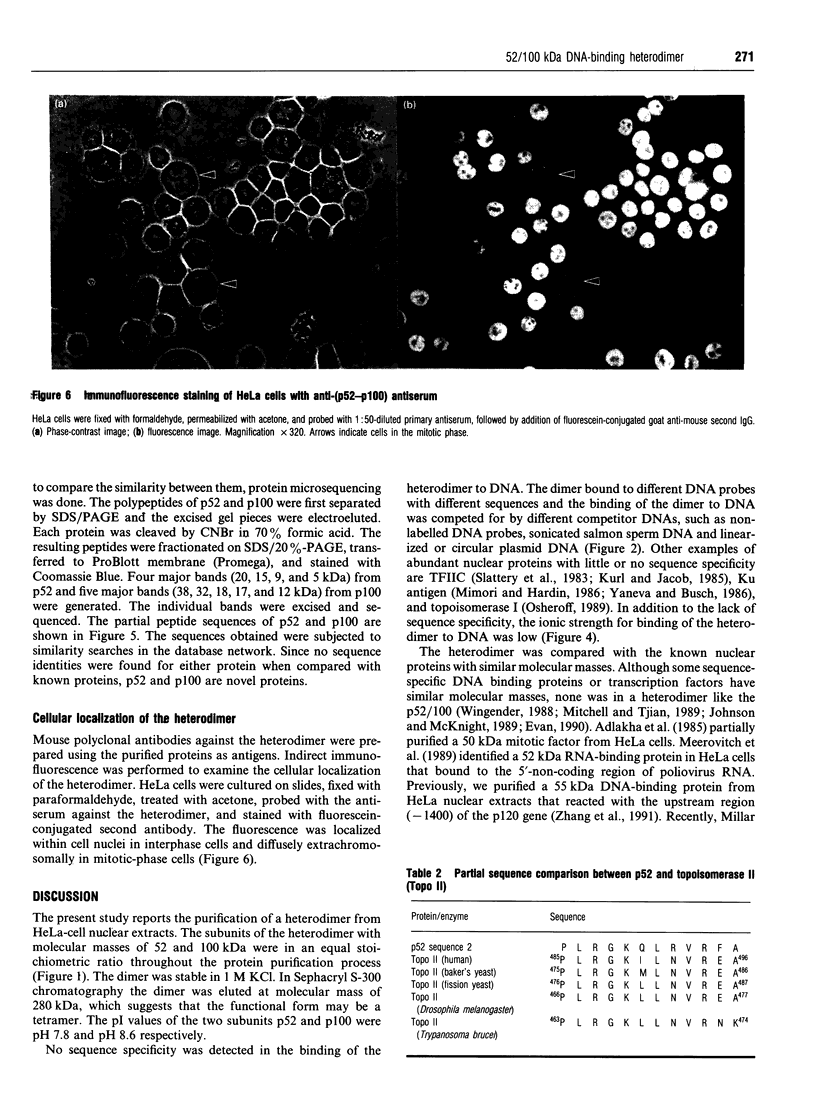

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlakha R. C., Wright D. A., Sahasrabuddhe C. G., Davis F. M., Prashad N., Bigo H., Rao P. N. Partial purification and characterization of mitotic factors from HeLa cells. Exp Cell Res. 1985 Oct;160(2):471–482. doi: 10.1016/0014-4827(85)90194-6. [DOI] [PubMed] [Google Scholar]
- Auborn K., Guo M., Prives C. Helicase, DNA-binding, and immunological properties of replication-defective simian virus 40 mutant T antigens. J Virol. 1989 Feb;63(2):912–918. doi: 10.1128/jvi.63.2.912-918.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch H. The final common pathway of cancer. Cancer Res. 1990 Aug 15;50(16):4830–4838. [PubMed] [Google Scholar]
- Chatterjee A., Busch R. K., Jung D., Zhang W. W., Busch H. Purification of a group of HeLa nuclear proteins that bind to a regulatory element (-1430/-1327) of the human proliferating cell nucleolar protein P120 gene. Biochem Biophys Res Commun. 1991 Oct 31;180(2):805–812. doi: 10.1016/s0006-291x(05)81136-2. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Tachibana C. Y., Abrams H. D., Hann S. R. V-myc- and c-myc-encoded proteins are associated with the nuclear matrix. Mol Cell Biol. 1985 Jan;5(1):114–126. doi: 10.1128/mcb.5.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I. Nuclear oncoproteins. Introduction: the great nuclear debate. Semin Cancer Biol. 1990 Dec;1(6):351–358. [PubMed] [Google Scholar]
- Gilmour D. S., Dietz T. J., Elgin S. C. UV cross-linking identifies four polypeptides that require the TATA box to bind to the Drosophila hsp70 promoter. Mol Cell Biol. 1990 Aug;10(8):4233–4238. doi: 10.1128/mcb.10.8.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhiang S. M., Yaneva M., Busch H. Expression of human proliferation-associated nucleolar antigen p120. Cell Growth Differ. 1990 Jul;1(7):319–324. [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurl R. N., Jacob S. T. Characterization of a factor that can prevent random transcription of cloned rDNA and its probable relationship to poly(ADP-ribose) polymerase. Nucleic Acids Res. 1985 Jan 11;13(1):89–101. doi: 10.1093/nar/13.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latchman D. S. Eukaryotic transcription factors. Biochem J. 1990 Sep 1;270(2):281–289. doi: 10.1042/bj2700281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalam H. J., Albert V. R., Ingraham H. A., Kapiloff M., Wilson L., Nelson C., Elsholtz H., Rosenfeld M. G. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev. 1989 Jul;3(7):946–958. doi: 10.1101/gad.3.7.946. [DOI] [PubMed] [Google Scholar]
- Meerovitch K., Pelletier J., Sonenberg N. A cellular protein that binds to the 5'-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev. 1989 Jul;3(7):1026–1034. doi: 10.1101/gad.3.7.1026. [DOI] [PubMed] [Google Scholar]
- Millar J. B., Blevitt J., Gerace L., Sadhu K., Featherstone C., Russell P. p55CDC25 is a nuclear protein required for the initiation of mitosis in human cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10500–10504. doi: 10.1073/pnas.88.23.10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Hardin J. A. Mechanism of interaction between Ku protein and DNA. J Biol Chem. 1986 Aug 5;261(22):10375–10379. [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Osheroff N. Biochemical basis for the interactions of type I and type II topoisomerases with DNA. Pharmacol Ther. 1989;41(1-2):223–241. doi: 10.1016/0163-7258(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Patton J. G., Mayer S. A., Tempst P., Nadal-Ginard B. Characterization and molecular cloning of polypyrimidine tract-binding protein: a component of a complex necessary for pre-mRNA splicing. Genes Dev. 1991 Jul;5(7):1237–1251. doi: 10.1101/gad.5.7.1237. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Beck Y., Shure H. DNA binding properties of simian virus 40 T-antigens synthesized in vivo and in vitro. J Virol. 1980 Feb;33(2):689–696. doi: 10.1128/jvi.33.2.689-696.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Purification of the c-fos enhancer-binding protein. Mol Cell Biol. 1987 Oct;7(10):3482–3489. doi: 10.1128/mcb.7.10.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery E., Dignam J. D., Matsui T., Roeder R. G. Purification and analysis of a factor which suppresses nick-induced transcription by RNA polymerase II and its identity with poly(ADP-ribose) polymerase. J Biol Chem. 1983 May 10;258(9):5955–5959. [PubMed] [Google Scholar]
- Wang N. P., Chen P. L., Huang S., Donoso L. A., Lee W. H., Lee E. Y. DNA-binding activity of retinoblastoma protein is intrinsic to its carboxyl-terminal region. Cell Growth Differ. 1990 May;1(5):233–239. [PubMed] [Google Scholar]
- Watt R. A., Shatzman A. R., Rosenberg M. Expression and characterization of the human c-myc DNA-binding protein. Mol Cell Biol. 1985 Mar;5(3):448–456. doi: 10.1128/mcb.5.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender E. Compilation of transcription regulating proteins. Nucleic Acids Res. 1988 Mar 25;16(5):1879–1902. doi: 10.1093/nar/16.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki L., Kanda P., Lanford R. E. Identification of four nuclear transport signal-binding proteins that interact with diverse transport signals. Mol Cell Biol. 1989 Jul;9(7):3028–3036. doi: 10.1128/mcb.9.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaneva M., Busch H. A 10S particle released from deoxyribonuclease-sensitive regions of HeLa cell nuclei contains the 86-kilodalton-70-kilodalton protein complex. Biochemistry. 1986 Sep 9;25(18):5057–5063. doi: 10.1021/bi00366a013. [DOI] [PubMed] [Google Scholar]
- Zhang W. W., Farrés J., Busch H. Purification of a novel 55 kDa HeLa cell nuclear DNA-binding protein. Biochem Biophys Res Commun. 1991 Jan 31;174(2):542–548. doi: 10.1016/0006-291x(91)91451-h. [DOI] [PubMed] [Google Scholar]