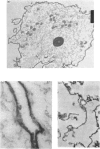
Abstract
A method has been developed for the separation of the major membrane fractions of Acanthamoeba castellanii after growth at different temperatures. The acyl-lipid compositions of individual membrane fractions, microsomal membranes, plasma membrane and mitochondria were analysed after a shift in culture temperature from 30 degrees C to 15 degrees C. The major change in lipid composition observed was an alteration in the relative proportions of oleate and linoleate. This reciprocal change was seen in all the membrane fractions, but occurred most rapidly in the phosphatidylcholine of the microsomal fraction. Thus, there appears to be a rapid induction of delta 12-desaturase activity in A. castellanii after a downward shift in growth temperature. Changes were also seen in the proportions of the n-6 C20 fatty acids, with a decrease in the proportions of icosadienoate and increases of icosatrienoate and arachidonate. However, unlike the alteration in oleate/linoleate ratios, this change was not seen in all the individual lipids of each membrane fraction.
Full text
PDF

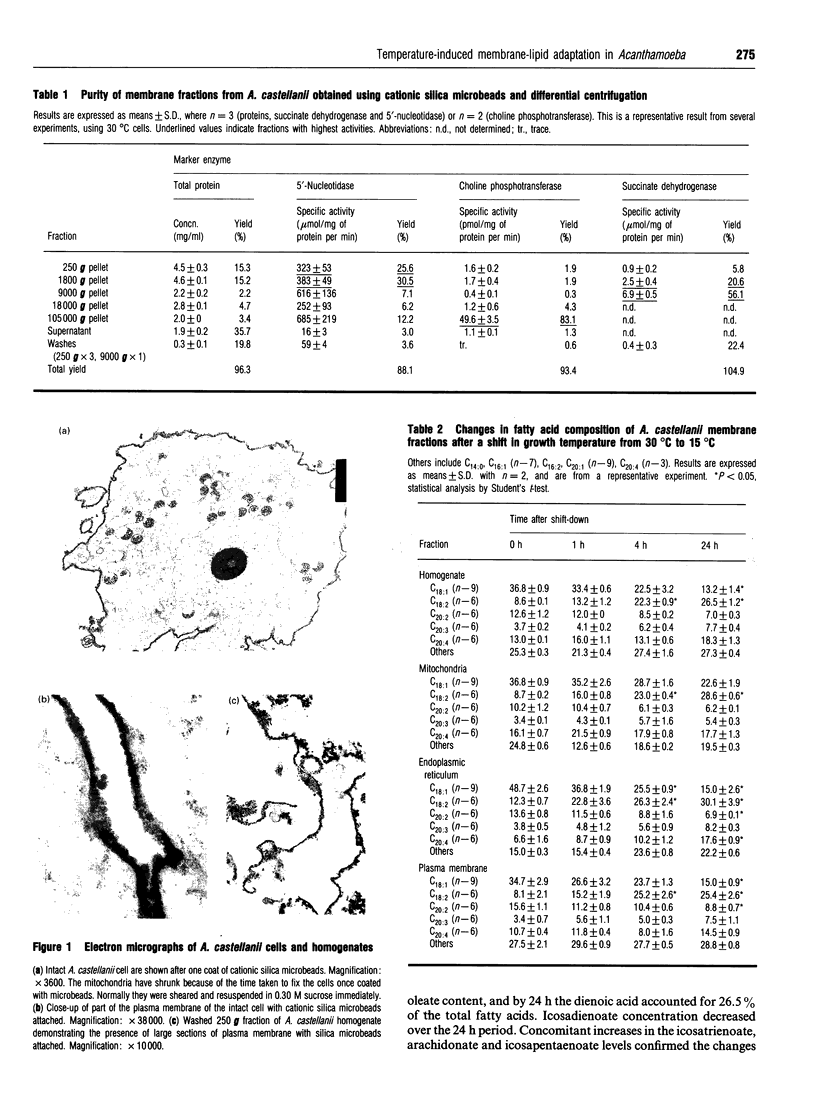
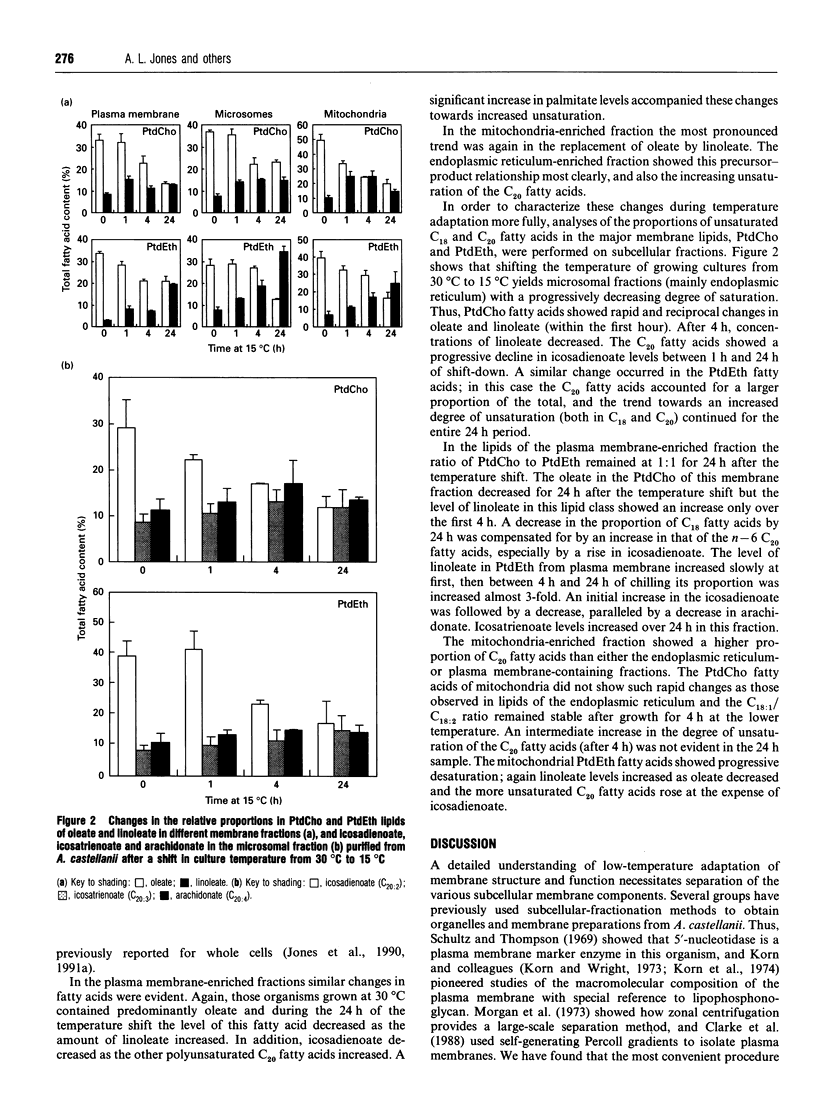


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Assembly of phospholipids into cellular membranes: biosynthesis, transmembrane movement and intracellular translocation. Annu Rev Cell Biol. 1988;4:579–610. doi: 10.1146/annurev.cb.04.110188.003051. [DOI] [PubMed] [Google Scholar]
- Bowers B., Korn E. D. Cytochemical identification of phosphatase activity in the contractile vacuole of Acanthamoeba castellanii. J Cell Biol. 1973 Dec;59(3):784–791. doi: 10.1083/jcb.59.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARROLL K. K. Separation of lipid classes by chromatography on Florisil. J Lipid Res. 1961 Apr;2:135–141. [PubMed] [Google Scholar]
- Chagla A. H., Griffiths A. J. Growth and encystation of Acanthamoeba castellanii. J Gen Microbiol. 1974 Nov;85(1):139–145. doi: 10.1099/00221287-85-1-139. [DOI] [PubMed] [Google Scholar]
- Chaney L. K., Jacobson B. S. Coating cells with colloidal silica for high yield isolation of plasma membrane sheets and identification of transmembrane proteins. J Biol Chem. 1983 Aug 25;258(16):10062–10072. [PubMed] [Google Scholar]
- Clarke B. J., Hohman T. C., Bowers B. Purification of plasma membrane from Acanthamoeba castellanii. J Protozool. 1988 Aug;35(3):408–413. doi: 10.1111/j.1550-7408.1988.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Cossins A. R., Raynard R. S. Adaptive responses of animal cell membranes to temperature. Symp Soc Exp Biol. 1987;41:95–111. [PubMed] [Google Scholar]
- GARBUS J., DELUCA H. F., LOOMANS M. E., STRONG F. M. The rapid incorporation of phosphate into mitochondrial lipids. J Biol Chem. 1963 Jan;238:59–63. [PubMed] [Google Scholar]
- Harwood J. Strategies for coping with low environmental temperatures. Trends Biochem Sci. 1991 Apr;16(4):126–127. doi: 10.1016/0968-0004(91)90052-w. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Lloyd D., Hann A. C., Harwood J. L. Lipid changes in individual membranes of Acanthamoeba castellanii during temperature adaptation. Biochem Soc Trans. 1991 Aug;19(3):318S–318S. doi: 10.1042/bst019318s. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Pruitt N. L., Lloyd D., Harwood J. L. Effect of growth temperature on fatty acid biosynthesis in Acanthamoeba castellanii. Biochem Soc Trans. 1990 Aug;18(4):627–627. doi: 10.1042/bst0180627. [DOI] [PubMed] [Google Scholar]
- KORN E. D. BIOSYNTHESIS OF UNSATURATED FATTY ACIDS IN ACANTHAMOEBA SP. J Biol Chem. 1964 Feb;239:396–400. [PubMed] [Google Scholar]
- KORN E. D. FATTY ACIDS OF ACANTHAMOEBA SP. J Biol Chem. 1963 Nov;238:3584–3587. [PubMed] [Google Scholar]
- Korn E. D., Dearborn D. G., Wright P. L. Lipophosphonoglycan of the plasma membrance of Acanthamoeba castellanii. Isolation from whole amoebae and identification of the water-soluble products of acid hydrolysis. J Biol Chem. 1974 Jun 10;249(11):3335–3341. [PubMed] [Google Scholar]
- Korn E. D., Wright P. L. Macromolecular composition of an amoeba plasma membrane. J Biol Chem. 1973 Jan 25;248(2):439–447. [PubMed] [Google Scholar]
- Koudelka A. P., Bradley D. K., Kambadur N., Ferguson K. A. Oleic acid desaturation in Tetrahymena pyriformis. Biochim Biophys Acta. 1983 Apr 13;751(2):129–137. doi: 10.1016/0005-2760(83)90166-2. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. V., Thompson G. A. Microsomal Phospholipid Molecular Species Alterations during Low Temperature Acclimation in Dunaliella. Plant Physiol. 1984 Feb;74(2):193–197. doi: 10.1104/pp.74.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. T., Furlong S. T., Dawidowicz E. A. Plasma membrane biogenesis in eukaryotic cells: translocation of newly synthesized lipid. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1385–1388. doi: 10.1073/pnas.81.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PESCE A., MCKAY R. H., STOLZENBACH F., CAHN R. D., KAPLAN N. O. THE COMPARATIVE ENZYMOLOGY OF LACTIC DEHYDROGENASES. I. PROPERTIES OF THE CRYSTALLINE BEEF AND CHICKEN ENZYMES. J Biol Chem. 1964 Jun;239:1753–1761. [PubMed] [Google Scholar]
- Pugh E. L., Kates M. Desaturation of phosphatidylcholine and phosphatidylethanolamine by a microsomal enzyme system from Candida lipolytica. Biochim Biophys Acta. 1973 Sep 25;316(3):305–316. doi: 10.1016/0005-2760(73)90071-4. [DOI] [PubMed] [Google Scholar]
- Schmidt R., Ackermann R., Kratky Z., Wasserman B., Jacobson B. Fast and efficient purification of yeast plasma membranes using cationic silica microbeads. Biochim Biophys Acta. 1983 Jul 27;732(2):421–427. doi: 10.1016/0005-2736(83)90059-7. [DOI] [PubMed] [Google Scholar]
- Schultz T. M., Thompson J. E. Enrichment of 5'-nucleotidase in membrane fragments isolated from Acanthamoeba sp. Biochim Biophys Acta. 1969 Oct 14;193(1):203–211. doi: 10.1016/0005-2736(69)90073-x. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Horowitz M. I. Separation of polar lipids by column chromatography on hydroxylapatite. J Chromatogr. 1970 Jun 24;49(3):455–461. doi: 10.1016/s0021-9673(00)93659-8. [DOI] [PubMed] [Google Scholar]
- Stymne S., Appelqvist L. A. The biosynthesis of linoleate from oleoyl-CoA via oleoyl-phosphatidylcholine in microsomes of developing safflower seeds. Eur J Biochem. 1978 Oct;90(2):223–229. doi: 10.1111/j.1432-1033.1978.tb12594.x. [DOI] [PubMed] [Google Scholar]
- Wilson A. C., Adams W. C., Miller R. W. Lipid involvement in oleoyl CoA desaturase activity of Fusarium oxysporum microsomes. Can J Biochem. 1980 Feb;58(2):97–102. doi: 10.1139/o80-014. [DOI] [PubMed] [Google Scholar]