Abstract
Objective
We sought to evaluate changes in microbiome biodiversity and physical properties of the skin after eight weeks of once-daily topical microencapsulated benzoyl peroxide (E-BPO) compared to vehicle cream in participants with rosacea.
Methods
This was a randomized, double-blind, crossover, single-center, vehicle-controlled evaluation of E-BPO on the skin microbiome in rosacea. Participants had facial rosacea with global severity of 3 or 4 on the Investigator Global Assessment (IGA) scale. In the Treatment 1-2 group, participants received E-BPO for eight weeks then switched to vehicle cream for four weeks. In the Treatment 2-1 group, participants received vehicle cream for eight weeks, then E-BPO for four weeks.
Results
Thirty-one participants were enrolled and randomly assigned to either group. Demographic characteristics were comparable between the treatment groups. After eight weeks of E-BPO treatment, there was a marked reduction in the relative abundance of Staphylococcus accompanied by an increase in Cutibacterium. At the species level, there was an increase in the relative abundance of C. acnes and a decrease in abundance of S. epidermidis. No noticeable difference was detected at the genus or species level at Week 8 in the 2-1 group. Sebum level, IGA, lesion counts, facial erythema, and inflammatory scores were improved with E-BPO versus vehicle cream. Adverse events were mild or moderate in severity.
Limitations
The study included a small number of subjects and only surface-swabs were used for microbiome sampling.
Conclusion
E-BPO shifted the skin microbiome in rosacea and demonstrated improvements in clinical symptoms and skin physical properties and a well-tolerated safety profile. US National Library of Medicine; Trial ID: NCT05675501]; URL: clinicaltrials.gov
Keywords: Benzoyl peroxide, BPO, rosacea, microbiome, topicals, skin microbiome
Rosacea is a prevalent, chronic, relapsing skin disease associated with a substantial burden.1,2 Its pathogenesis is unknown, but recent advances point to the importance of skin-environmental interactions involving physical, chemical, and microbial factors.3 Imbalances in cutaneous organisms (e.g., Staphylococcus epidermidis, Cutibacterium acnes, Demodex folliculorum, and Bacillus oleronius) have been implicated in the pathogenesis of rosacea.4
Further evidence supports an altered immune response to environmental triggers as a potential cause.5 An increase in S. epidermidis abundance has been detected in rosacea pustules exhibiting an altered secretory profile.6,7 Also, rosacea severity has been correlated with relative abundance of S. epidermidis.8 Linear regression analysis performed showed a weak negative correlation between C. acnes abundance and rosacea severity but a positive correlation between Corynebacterium kroppenstedtii relative abundance and rosacea severity.9 Loss of C. acnes abundance and loss of Cutibacterium diversity have been associated with skin disorders, including rosacea, and have been inversely correlated with sebum quantity.8–10 C. acnes has been suspected of protecting healthy skin by breaking down sebum into free fatty acids, which can prevent pathogen growth.11,12 The sebaceous fatty acids found in sebum support the maintenance of skin barrier integrity, and altered sebum quality has been associated with rosacea.12
Although rosacea occurs primarily in sebaceous gland-rich skin, molecular analysis of skin barrier alterations in rosacea demonstrated significant alterations in barrier integrity compared with non-rosacea sebaceous gland-rich skin.13 Demodex mites, a skin commensal, are found in rosacea-affected skin. Whether they cause rosacea or are secondary to it, Demodex mites stimulate toll-like receptor 2, increasing LL-37, which triggers angiogenesis and inflammation in rosacea.14 Demodex mites have a microbiome, including B. oleronius, whose antigens may induce proliferation of peripheral blood mononuclear cells in patients with rosacea15 and stimulate production of LL-37, matrix metalloproteinase-9, tumor necrosis factor, and interleukin 8 in healthy individuals.6
Microencapsulated benzoyl peroxide (E-BPO) is approved for treating rosacea. The use of unencapsulated BPO, although effective,16 has largely been abandoned due to poor tolerability on the facial skin of rosacea patients. After application, unencapsulated BPO can cause local skin irritation (e.g., erythema, burning, stinging).17 Phase 3 trials showed high E-BPO efficacy with good safety and tolerability parameters in participants with rosacea.18 The mechanism of action of BPO in rosacea is unknown, but its antimicrobial properties may contribute to its efficacy.
This study aimed to evaluate changes in skin microbiome biodiversity and skin physical properties after eight weeks of once-daily topical E-BPO versus vehicle cream in participants with rosacea.
METHODS
Study design. This was a double-blind, vehicle-controlled, crossover study starting with eight weeks of active versus vehicle treatment conducted at a single center between February 2020 and July 2021 (NCT05675501). Study groups were crossed over after eight weeks for an additional four weeks of the alternate therapy plus four weeks of observation to detect any durable changes in the microbiome (Figure 1). A pea-sized amount of E-BPO or vehicle was applied as a thin coating to each facial area (forehead, nose, chin, and each cheek) once daily at approximately the same time after using an approved cleanser. Safety was assessed by monitoring the incidence of adverse events (AEs) and the results of cutaneous safety and local tolerability assessments at baseline and all postbaseline study visits.
FIGURE 1.
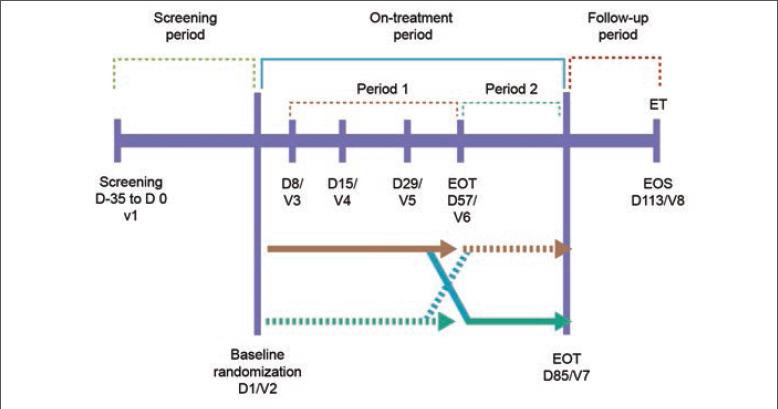
Study design D: day; EOS: end of study; EOT: end of treatment; V: visit
The protocol was approved by an institutional review board (IntegReview, IORG0000689). The study (SGT-54-08) complied with the United States (US) Food and Drug Administration regulations, the Declaration of Helsinki, and the International Council for Harmonisation Good Clinical Practice guidelines. Participants signed written informed consent before enrollment.
Participants. Participants with facial rosacea who had an IGA global severity score of 3 (moderate) or 4 (severe) were eligible. Key exclusion criteria included the presence of more than two facial nodules or any nodule larger than 1cm, ocular rosacea, or any other facial skin condition that interfered with rosacea diagnosis and/or assessment.
Assessments. Clinical assessments for IGA, inflammatory lesion counts, rosacea inflammatory grade, and erythema were performed at baseline and Weeks 1, 2, 4, 8, 12, and 16. All scales used in this study have previously been described and validated in the literature.19–21 IGA was scored from 0 (clear: skin clear of inflammatory papules or pustules, no erythema present) to 4 (severe: numerous small and/or larger papules or pustules and severe, bright to deep red erythema). For inflammatory lesion counts, a papule was defined as a solid, elevated inflammatory lesion measuring 5mm or smaller in diameter, and a pustule as an elevated inflammatory lesion measuring 5mm or smaller in diameter containing pus (i.e., yellow-white exudate). Erythema was scored from 0 (none: no visible erythema) to 3 (severe: severe, bright to deep red erythema, either centrofacial or generalized to whole face). The inflammatory grade was scored from 0 (none: skin clear of inflammatory papules or pustules) to 3 (severe: numerous small and/or larger papules or pustules). Skin biophysical properties were assessed using common, noninvasive methods with assessment of skin sebum excretion rate performed at all clinical visits.
Sebum excretion rate assessments. The facial sebum excretion rate was measured with the use of a sebumeter (Courage + Khazaka, Köln, Germany). The measurement is based on the principle of grease spot photometry. The tape of the sebumeter is placed in contact with the skin for a set amount of time and becomes transparent according to the sebum content on the surface of the skin. Then the transparency is measured by a photocell represents the sebum content.
Skin microbiome. Shotgun metagenomic sequencing of swabs and follicular samples from all participants was performed. The change in skin microbiome (including the mycobiome) was measured based on facial swab sampling from three locations (glabella, left medial cheek, right medial cheek) obtained at all clinical visits. A control swab with only ambient air exposure was collected at each visit.
Bacterial identification was made by DNA extraction from skin swabs using DNeasy PowerSoil Pro Kit (QIAGEN) according to the manufacturer’s protocol. The DNA libraries were prepared using the Nextera XT DNA Library Preparation Kit and IDT Unique Dual Indexes (Illumina) with total DNA input of 1ng. Taxonomic results were checked to ensure no contamination or barcoding issues. Controls were reviewed to confirm taxonomic output as expected for positive and negative control compositions.
For taxonomic bioinformatics analyses, unassembled sequencing reads were directly analyzed by CosmosID-HUB Microbiome Platform (CosmosID) for multikingdom microbiome analysis and profiling of antibiotic resistance and virulence genes, plus quantification of organisms’ relative abundance. The system uses curated genome databases and a data-mining algorithm that analyzes hundreds of millions of metagenomic sequence reads into discrete microorganisms.
For functional bioinformatics analyses, initial quality control was performed using BBDuk.22 Quality-controlled transcripts were mapped against UniRef90, which consists of clusters of nonredundant protein sequences in UniProt with 90% identity and 80% coverage of the longest sequence in the cluster.23 These mappings were weighted by alignment quality, coverage, and gene sequence length to estimate community-wide weighted gene family abundances.24 MetaCyc25 was used to annotate gene families to enzyme reactions to reconstruct and quantify MetaCyc metabolic pathways in the community.24
Due to the interest in B. oleronius and rosacea development,26 a separate analysis was performed because B. oleronius is not currently included in the database. By counting the number of raw reads mapped (subsampled to 3 million reads each with reformat of the BBMap suite) against the reference genome using Bowtie 2,27 the relative abundance of B. oleronius in the metagenomics samples was estimated. The National Center for Biotechnology Information currently identifies the reference genome as Heyndrickxia oleronia.
Statistical analysis. The statistical analysis plan was finalized before unblinding of study treatments. Data manipulation, descriptive statistics, and inferential statistics were performed using SAS version 9.4 (SAS Institute). All tests were two-tailed; P≤0.05 was considered statistically significant.
The sample size for this study is based on clinical considerations only and no formal sample size calculations were performed a priori because the influence of topical E-BPO on the microbiome in rosacea is a novel area with no previous preexisting data. An enrollment of 30 participants aged 18 years or older, allowing for a 10-percent dropout rate, with at least 24 participants completing the study (12 evaluable participants in each arm), was considered adequate statistical power.
RESULTS
Participants. Thirty-one participants were enrolled and randomly assigned 1:1 to E-BPO or vehicle cream. The Treatment 1-2 group (n=15) was randomized to E-BPO (8 weeks) and then switched to vehicle cream (4 weeks). The Treatment 2-1 group (n=16) was randomized to vehicle cream (8 weeks) and then switched to E-BPO (4 weeks) (Figure 2). Demographic characteristics were comparable between groups (Table 1).
FIGURE 2.
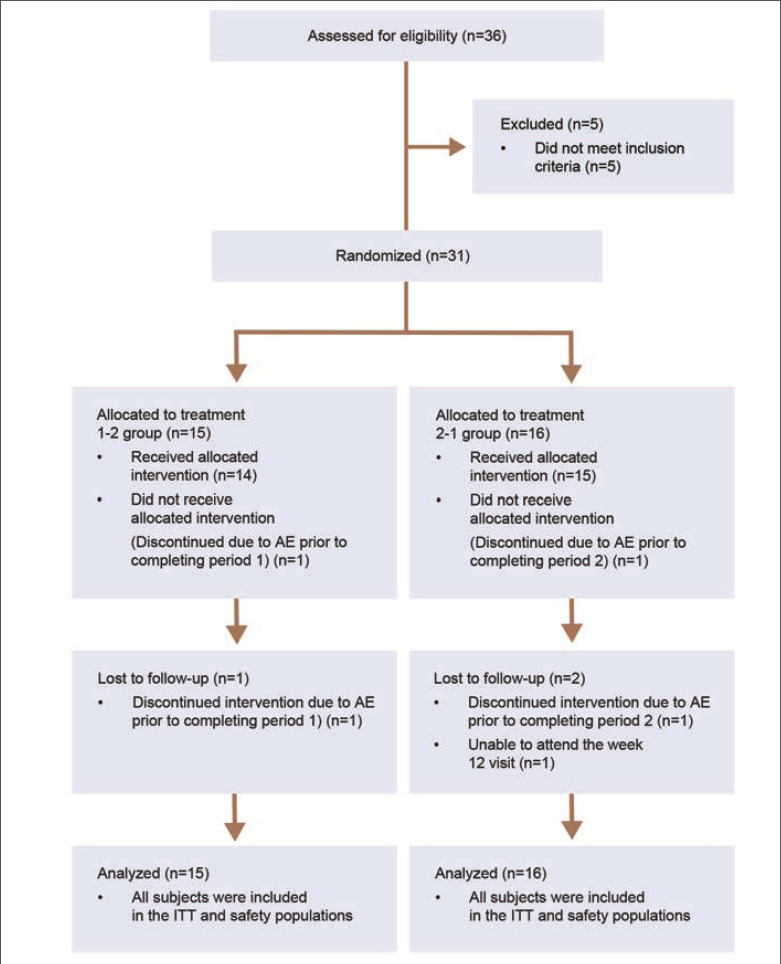
CONSORT Flow Diagram AE, adverse event; ITT, intent-to-treat
TABLE 1.
Participant demographics (intent-to-treat population)
| DEMOGRAPHIC ITEM | TREATMENT 1-2 (n=15) | TREATMENT 2-1 (n=16) | ALL PARTICIPANTS (n=31) |
|---|---|---|---|
| Age | |||
| Mean (SD) | 48.80 (13.06) | 50.88 (15.36) | 49.87 (14.09) |
| Median | 52.00 | 55.00 | 52.00 |
| Min | 28.00 | 26.00 | 26.00 |
| Max | 67.00 | 73.00 | 73.00 |
| Sex, n (%) | |||
| Female | 13 (86.67) | 9 (56.25) | 22 (70.97) |
| Male | 2 (13.33) | 7 (43.75) | 9 (29.03) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 3 (20.00) | 3 (18.75) | 6 (19.35) |
| Not Hispanic or Latino | 12 (80.00) | 10 (62.50) | 22 (70.97) |
| Not reported | 0 | 1 (6.25) | 1 (3.23) |
| Unknown | 0 | 2 (12.50) | 2 (6.45) |
| Race, n (%) | |||
| Not reported | 0 | 2 (12.50) | 2 (6.45) |
| Unknown | 0 | 1 (6.25) | 1 (3.23) |
| White | 13 (86.67) | 12 (75.00) | 25 (80.65) |
| Asian | 2 (13.33) | 0 | 2 (6.45) |
| American Indian or Alaska Native | 0 | 1 (6.25) | 1 (3.23) |
Min: minimum; Max: maximum
Microbiome analysis. Within the Treatment 1-2 group (E-BPO), a marked reduction in the relative abundance of Staphylococcus (P=0.186) was accompanied by an increase in Cutibacterium (P=0.391) from baseline to Week 8 (Figure 3). A noticeable increase in the treated group from baseline to Week 8 was revealed at the species level with a relative abundance of C. acnes (P=0.338), accompanied by a decrease in abundance of S. epidermidis (P=0.652; Figure 4). No noticeable difference in the vehicle group was detected at the genus or species level. At the bacterial species level, a slight increase in microbial richness and evenness of bacterial species was observed in E-BPO–treated samples versus baseline samples within the Treatment 1-2 group (not statistically significant) (Figure 3). Microbiome alterations in other genera and species were also detected, but their role is unknown.
FIGURE 3.
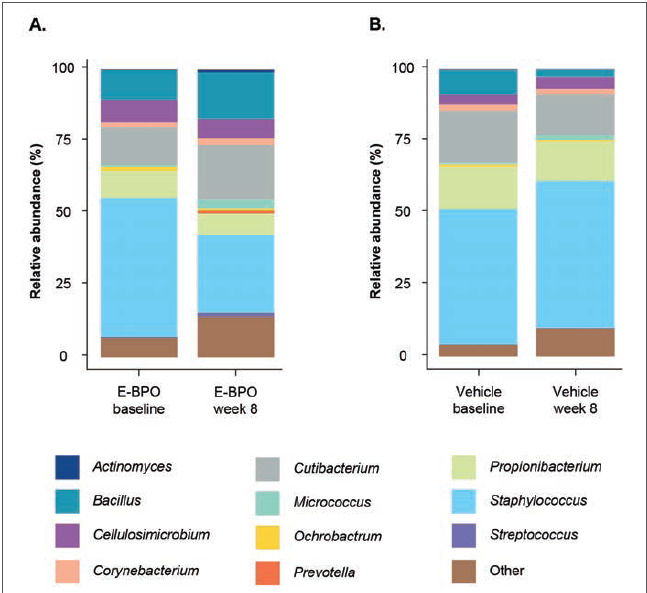
Relative abundance of major bacterial genera in skin microbiomes of participants with rosacea at baseline (untreated) compared with Week 8 in subjects treated with (A) E-BPO or (B) vehicle in the intent-to-treat population. Each bar represents the average relative abundance of the top 25 features in each cohort at the genus level. The features were selected based on the most abundant organisms relative to each cohort. E-BPO: microencapsulated benzoyl peroxide cream, 5%
FIGURE 4.
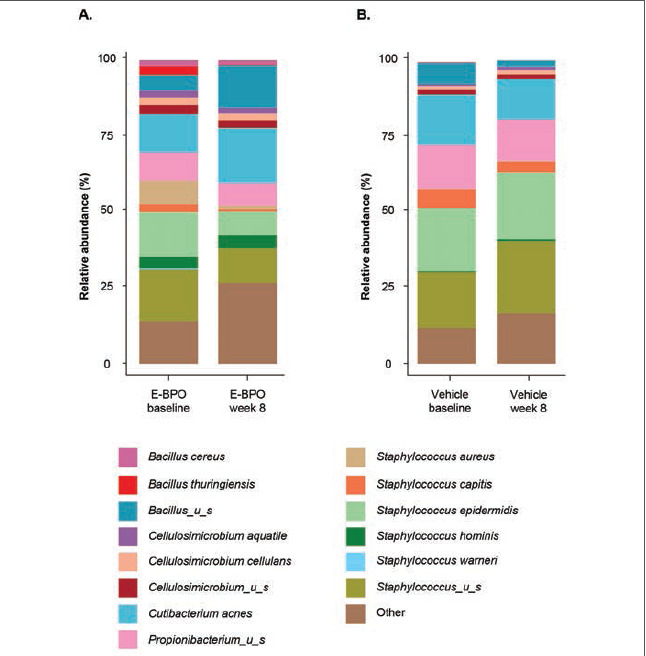
Relative abundance of major bacterial species in skin microbiomes of participants with rosacea at baseline compared with Week 8 in subjects treated with (A) E-BPO or (B) vehicle in the intent-to-treat population. Each bar represents the average relative abundance of the top 25 features in each cohort at the species level. The features were selected based on the most abundant organisms relative to each cohort. E-BPO: microencapsulated benzoyl peroxide cream, 5%
Comparison of microbiome samples at Week 8 did not reveal significant differences between groups based on the Shannon diversity index (Figure 5), a metric used to account for the number of species in a habitat (species richness). This finding suggests similar bacterial species diversity profiles for all participants at Week 8.
FIGURE 5.
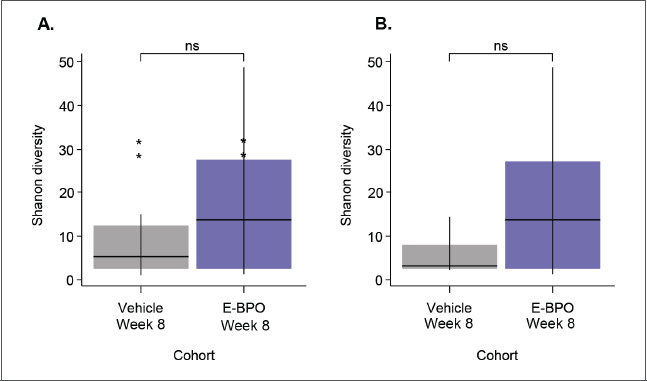
Comparison of bacterial Shannon diversity in skin microbiomes of participants with rosacea (A) before treatment (baseline) and after treatment with E-BPO or (B) E-BPO and vehicle cream at Week 8/Visit 6 (intent-to-treat population). Box plot illustrating the comparison of bacterial Shannon alpha diversity in skin microbiomes of E-BPO cream, 5%, and vehicle cream–treated participants at Week 8. The line in the box is a median of index scores, the boxes represent the interquartile range, the whiskers represent the lowest and highest values, and the dots represent outliers. Wilcoxon rank sum test was performed.
E-BPO: microencapsulated benzoyl peroxide cream, 5%; ns: not significant
After crossover of the Treatment 2-1 vehicle group at eight weeks to E-BPO, the relative abundance of S. epidermidis was markedly lowered, and the abundance of C. acnes was slightly increased. In the Treatment 1-2 group, after crossing over from eight weeks of treatment with E-BPO to treatment with vehicle cream, the relative abundance of S. epidermidis and C. acnes at Weeks 12 and 16 remained at the levels observed at Week 8, with S. epidermidis reduced and C. acnes increased from baseline.
Identification of enriched metabolic pathways. Linear discriminant analysis effect size (LEfse) was performed to identify metabolic pathways that were significantly enriched or depleted in response to E-BPO. Based on the LEfse results, several significantly enriched pathways were identified (Figure 6). The histidine degradation III pathway was the most significantly enriched pathway in the Treatment 1-2 group at Week 8.
FIGURE 6.
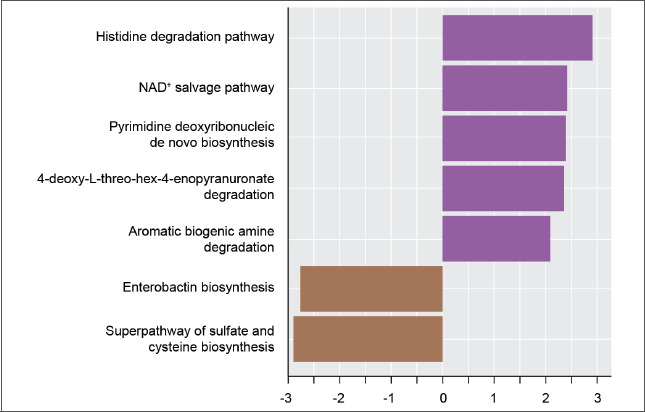
Metabolic pathways with statistically different abundance in skin microbiomes of subjects with rosacea before treatment (baseline) and after treatment with E-BPO (Week 8/Visit 6) (Intent-to-Treat Population). LDA effect size (LEfse) rank plot of differentially abundant pathways in untreated (baseline) versus E-BPO cream, 5%, treated samples (Week 8). Computed LDA scores (log10) of the relative abundance difference between untreated and treated groups are presented. Negative LDA scores (brown) are enriched in the untreated (baseline) group, while positive LDA scores (purple) are enriched in the E-BPO cream, 5%, group, and the length is the LDA effect size. LDA score ≥2.0 or ≤–2.0 with a P-value ≤0.05 was considered significantly enriched using Kruskal-Wallis (to detect features with significant differential abundance) and Wilcoxon tests (for pairwise comparisons). E-BPO: microencapsulated benzoyl peroxide cream; LDA: linear discriminant analysis; NAD: nicotinamide adenine dinucleotide
Skin sebum properties. Within the Treatment 1-2 group treated with E-BPO, the sebum level significantly increased from baseline to Week 8 (31.68 µg/cm2; P=0.0110). However, within the Treatment 2-1 group treated with vehicle, the sebum level slightly decreased (−2.15; P=0.7340; Table 2). The difference between the groups at Week 8 was statistically significant (P=0.0087). After crossing from vehicle cream to E-BPO, a statistically significant increase was observed in the Treatment 2-1 group from baseline to Week 12 (13.42 µg/cm2; P=0.0131), which decreased during follow-up (P=0.5893).
TABLE 2.
Mean absolute changes from baseline in sebum excretion (µg/cm2) of left and right cheek by randomization sequence (intent-to-treat population)
| VISITa | TREATMENT 1-2 | TREATMENT 2-1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NO. OF PATIENTS | MEAN | SD | P-VALUEb | NO. OF PATIENTS | MEAN | SD | P-VALUEb | Pr > |t|c | |
| Visit 3: period 1, Week 1 | 14 | 9.91 | 15.81 | 0.0355 | 16 | 1.49 | 25.25 | 0.8166 | 0.2913 |
| Visit 4: period 1, Week 2 | 14 | 3.64 | 11.85 | 0.2708 | 16 | 9.19 | 26.79 | 0.1901 | 0.4805 |
| Visit 5: period 1, Week 4 | 14 | 16.97 | 31.28 | 0.0634 | 16 | 2.08 | 32.82 | 0.8029 | 0.2158 |
| Visit 6: period 1, EOT, Week 8 | 14 | 31.68 | 39.98 | 0.0110 | 16 | −2.15 | 24.84 | 0.7340 | 0.0087 |
| Visit 7: period 2 (crossover), EOT, Week 12 | 14 | 30.23 | 47.80 | 0.0342 | 15 | 13.42 | 18.31 | 0.0131 | 0.2159 |
| Visit 8: FU, Week 16, EOS/early termination | 14 | 28.41 | 47.20 | 0.0422 | 14 | 5.76 | 38.91 | 0.5893 | 0.1776 |
aBaseline used was Day 1
bP-value is from a paired t-test
cThe 2-sample t-test was used EOS: end of study; EOT: end of treatment; FU: follow-up; SD: standard deviation
Investigator Global Assessments. Greater improvement in IGA was observed from baseline with E-BPO versus vehicle cream based on the percentage of participants achieving a score of “clear”/“almost clear” (Table 3). In the Treatment 1-2 group, the percentage of participants with IGA success remained the same (78.57%) four weeks after crossing from E-BPO to vehicle cream. In the Treatment 2-1 group, a drastic improvement was seen with IGA success (80%) four weeks after crossing from vehicle to E-BPO. During follow-up, the percentages generally remained the same in both groups (78.57% [treatment group] vs. 71.43% [vehicle group]).
TABLE 3.
IGA from baseline to Week 16 with E-BPO versus vehicle cream: Percent of patients achieving a score of “clear” or “almost clear”
| TIME POINT | TREATMENT 1-2 (n=15) | TREATMENT 2-1 (n=16) |
|---|---|---|
| Baseline | 0 | 0 |
| Week 2 | 64.29 | 12.50 |
| Week 4 | 85.71 | 31.25 |
| Week 8 | 78.57 | 37.50 |
| Week 12 | 78.57 | 80.00 |
| Week 16 | 78.57 | 71.43 |
| Range | 0–85.71 | 0–80.00 |
E-BPO: microencapsulated benzoyl peroxide cream, 5%; IGA: Investigator Global Assessment
Facial erythema. More participants achieved improvement from baseline in facial erythema with E-BPO than with vehicle cream based on the percentages of participants who had no/mild erythema at Week 8 (Table 4). No changes occurred four weeks after switching to vehicle cream in the Treatment 1-2 group (85.71%). Shifting to E-BPO in the Treatment 2-1 group showed improvement because many participants then presented with no/mild erythema (80.00%). During follow-up, the percentages remained comparable for both groups (78.57%).
TABLE 4.
Facial erythema from baseline to Week 16 with E-BPO versus vehicle cream: Percent of patients achieving no or mild erythema
| TIME POINT | TREATMENT 1-2 (n=15) | TREATMENT 2-1 (n=16) |
|---|---|---|
| Baseline | 0 | 0 |
| Week 2 | 71.43 | 25.00 |
| Week 4 | 71.43 | 25.00 |
| Week 8 | 85.71 | 43.75 |
| Week 12 | 85.71 | 80.00 |
| Week 16 | 78.57 | 78.57 |
| Range | 21.43–85.71 | 25.00–80.00 |
E-BPO: microencapsulated benzoyl peroxide cream, 5%; IGA: Investigator Global Assessment
Inflammatory grade. Greater improvement in rosacea inflammatory grade was observed from baseline with E-BPO than with vehicle cream based on the percentages of participants with no/mild inflammation (Table 5). In the Treatment 1-2 group, the percentages remained high four weeks after crossing from E-BPO to vehicle treatment (92.86%) and throughout the four-week follow-up (85.72%). In the Treatment 2-1 group, a noticeable increase in the percentages of participants with no/mild inflammation was observed four weeks after crossing from vehicle to E-BPO (80.00%). It remained high throughout the follow-up (78.58%).
TABLE 5.
Inflammatory grades from baseline to Week 16 with E-BPO versus vehicle cream: Percent of patients achieving no or mild inflammation
| TIME POINT | TREATMENT 1-2 (n=15) | TREATMENT 2-1 (n=16) |
|---|---|---|
| Baseline | 0 | 0 |
| Week 2 | 71.43 | 18.75 |
| Week 4 | 92.85 | 50 |
| Week 8 | 92.85 | 37.50 |
| Week 12 | 92.86 | 80.00 |
| Week 16 | 85.72 | 78.58 |
| Range | 0–92.86 | 0–80.00 |
E-BPO: microencapsulated benzoyl peroxide cream, 5%; IGA: Investigator Global Assessment
Safety and tolerability. Three participants experienced four AEs when treated with E-BPO (viral gastroenteritis [n=1], neuralgia [n=1], contact dermatitis [n=2]). Four participants experienced five AEs when treated with vehicle cream (medical device site inflammation, streptococcal pharyngitis, upper respiratory tract infection, ankle fracture, and panic attack). The AEs were mild or moderate in severity. Except for one participant, who was treated with E-BPO in the Treatment 1-2 group and developed contact dermatitis, AEs were not considered treatment related. No serious AEs were reported.
DISCUSSION
In this study, microbiome shifts associated with E-BPO correlated with rosacea improvement. Specifically, the relative abundance of S. epidermidis was reduced and correlated with an improvement in rosacea. Our findings agree with a previous study showing that S. epidermidis was overexpressed in rosacea,7,8 suggesting that S. epidermidis might contribute to rosacea pathogenesis, but this remains unclear. Our findings that S. epidermidis was reduced in correlation with clinical improvement further support a possible pathogenic role of S. epidermidis. However, further mechanistic analyses are needed to assess the role of S. epidermidis in rosacea, including follicular-based collections, because S. epidermidis likely has a different response in anaerobic conditions.28 Additionally, the relative abundance of C. acnes and Cutibacterium increased with an increase in sebum excretion rate. While C. acnes has been implicated in the pathogenesis of acne, there is a sebum production deficiency with rosacea.12 Accordingly, higher levels of C. acnes, as noted in this study with E-BPO treatment, were not always inflammatory and might reflect the increased sebum load of the skin. This finding, if repeatable in future studies, could lead to a paradigm shift in our understanding of the role of C. acnes in inflammatory follicular disorders. No significant shift in the relative abundance of B. oleronius was observed in this study, indicating that the Demodex-related microbiome was not significantly affected by E-BPO treatment. Demodex was not measured directly as its genome was not available in the genome libraries used. Although microbiome alterations in other genera and species were also detected, their roles are currently unknown. Overall, the bacterial diversity did not significantly change, suggesting that more than diversity alone may be needed to assess the microbiome. Individual changes in species quantity may be more important than overall diversity shifts in rosacea. More data are needed to define a “balanced” skin microbiome.
The current study revealed several significantly enriched pathways in response to E-BPO, including the histidine degradation III pathway, the most significantly enriched pathway in the treatment group at Week 8, followed by the NAD salvage pathway. Enrichment of the histidine degradation pathway suggests that the relative proportion of microbes engaging in histidine degradation was increased, perhaps due to increased histidine at the skin surface. Skin histidine content has been linked to improved skin barrier and skin health. In some patients with atopic dermatitis, there are deficiencies in the histidine-rich skin barrier protein, filaggrin.29 An increase in L-histidine increased filaggrin in human keratinocytes, improving stratum corneum acidity and skin hydration. These factors correlated with an improved skin barrier in patients with atopic dermatitis.30 An intermediate histidine metabolite, urocanic acid (UCA), has been shown to accumulate in the stratum corneum. There is robust evidence in an animal model of a photoprotective role for endogenous UCA against the damaging effects of ultraviolet-B radiation.31 While L-histidine was not directly measured in this study, the functional analysis suggested skin histidine content might be important in rosacea. Further studies that specifically correlate histidine levels with clinical response are warranted. An integrative analysis of skin surface samples collected from healthy infants identified histidine as a core metabolite in the microbiome and metabolome of healthy skin.32 This analysis also found that nicotinamide, a precursor in the NAD salvage pathway, was highly correlated with bacterial genera present in the core skin microbiome.32,33 Nicotinamide also supports skin barrier function, and its application improved skin hydration in patients with rosacea.34 The relative importance of the identified metabolic pathways needs to be clarified and requires additional investigation.
The response to E-BPO showed selective shifts in specific microorganisms rather than overall extermination of all species, contrasting with the results following treatment with unencapsulated BPO in patients with acne. In a small study (N=52), the effect of BPO, 5%, gel on acne and the microbiome was investigated. Despite improvements in acne, unencapsulated BPO reduced the skin microbiome diversity and damaged the skin barrier.35 In another study, a similar reduction in microbiome diversity was reported in preadolescent patients with acne treated with unencapsulated BPO..36 In contrast with unencapsulated BPO, E-BPO is released slowly onto the skin over time. Safety, efficacy, and stability may be improved when using microencapsulated treatments.37 Further, the results reported here indicate that E-BPO had a distinct effect on the skin microbiome and skin barrier that contrasts with the effects previously reported for unencapsulated BPO.
Limitations. This study had several limitations. First, this was a single-site study in a relatively small population of subjects. Shifts were seen in relative abundance, but a larger population may be necessary to confirm the statistical significance of these results. Despite fewer participants, the whole-genome sequencing method offered comprehensive insight into taxonomic and functional characteristics of the microbiome in rosacea. Shotgun whole-genome sequencing provides more information than 16S or internal transcribed spacer approaches. Shotgun whole-genome sequencing can simultaneously assess for fungi, archaea, bacteria, specific strains, and the presence of genes, giving more taxonomic and functional insight beyond the typical 16S approach. The functional gene results are also important in illuminating the critical roles of the microbiome. This study also evaluated change in specific Cutibacterium phylotypes, and future work is needed to understand the impact of E-BPO to each phylotype. Finally, this study was limited by using surface swab collections to assess the skin microbiome and did not include absolute assessments of microbial burden on the skin. While this is similar to other published studies on rosacea, the knowledge gained from surface swab collections cannot be extended to the hair follicle environment. Future researchers should consider evaluating the follicular microbiome in rosacea in addition to measuring the absolute abundance of microbial species.
E-BPO was well tolerated, and the results reported here suggest that it may improve the overall microbial environment in rosacea, shifting toward a more balanced cutaneous microbiome in correlation with clinical improvement. Importantly, E-BPO should be differentiated from conventionally available, unencapsulated BPO, which may have adverse effects on the skin microbiome and is more irritating than E-BPO.
Meeting presentations. Part of the data included in this article were presented at Fall Clinical 2022, October 20-23, in Las Vegas, Nevada; Maui Derm 2023, January 23-27, in Maui, Hawaii; and the World Congress of Dermatology 2023, July 3-8. Part of the data included in this article will be presented at DERM 2023, August 3-6, in Las Vegas, Nevada.
Availability of data and material. The data that support the findings of this study are not openly available due to capacity and the company proprietary information and are available from the corresponding author upon reasonable request.
ACKNOWLEDGMENTS
The authors would like to thank Kim Tran-Kerr, MD, and Alyssa Theodore, PhD, from Simpson Healthcare for their writing support and editorial assistance. Their support was provided by Galderma.
REFERENCES
- Tan J, Berg M. Rosacea: Current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27–S35. doi: 10.1016/j.jaad.2013.04.043. [DOI] [PubMed] [Google Scholar]
- Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6(6):348–354. [PMC free article] [PubMed] [Google Scholar]
- Wollina U. Recent advances in the understanding and management of rosacea. F1000Prime Rep. 2014;6:50. doi: 10.12703/P6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daou H, Paradiso M, Hennessy K et al. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11(1):1–12. doi: 10.1007/s13555-020-00460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55(2):77–81. doi: 10.1016/j.jdermsci.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AD. Potential role of microorganisms in the pathogenesis of rosacea. J Am Acad Dermatol. 2013;69(6):1025–1032. doi: 10.1016/j.jaad.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Whitfeld M, Gunasingam N, Leow LJ et al. Staphylococcus epidermidis: a possible role in the pustules of rosacea. J Am Acad Dermatol. 2011;64(1):49–52. doi: 10.1016/j.jaad.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Woo YR, Lee SH, Cho SH et al. Characterization and analysis of the skin microbiota in rosacea: impact of systemic antibiotics. J Clin Med. 2020;9(1):185. doi: 10.3390/jcm9010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer BM, Thompson KG, Antonescu C et al. Characterization and analysis of the skin microbiota in rosacea: a case-control study. Am J Clin Dermatol. 2020;21(1):139–147. doi: 10.1007/s40257-019-00471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Farhat M, Na J et al. Bacterial and fungal microbiome characterization in patients with rosacea and healthy controls. Br J Dermatol. 2020;183(6):1112–1114. doi: 10.1111/bjd.19315. [DOI] [PubMed] [Google Scholar]
- Marples RR, Downing DT, Kligman AM. Control of free fatty acids in human surface lipids by Corynebacterium acnes. J Invest Dermatol. 1971;56(2):127–131. doi: 10.1111/1523-1747.ep12260695. [DOI] [PubMed] [Google Scholar]
- Ni Raghallaigh S, Bender K, Lacey N et al. The fatty acid profile of the skin surface lipid layer in papulopustular rosacea. Br J Dermatol. 2012;166(2):279–287. doi: 10.1111/j.1365-2133.2011.10662.x. [DOI] [PubMed] [Google Scholar]
- Medgyesi B, Dajnoki Z, Beke G et al. Rosacea is characterized by a profoundly diminished skin barrier. J Invest Dermatol. 2020;140(10):1938–1950.e5. doi: 10.1016/j.jid.2020.02.025. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Di Nardo A, Bardan A et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13(8):975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- Lacey N, Delaney S, Kavanagh K et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157(3):474–481. doi: 10.1111/j.1365-2133.2007.08028.x. [DOI] [PubMed] [Google Scholar]
- Montes LF, Cordero AA, Kriner J et al. Topical treatment of acne rosacea with benzoyl peroxide acetone gel. Cutis. 1983;32(2):185–190. [PubMed] [Google Scholar]
- Green LJ, Lain E, Prunty T et al. Enhancing topical pharmacotherapy for acne and rosacea: vehicle choices and outcomes. J Clin Aesthet Dermatol. 2022;15(5):36–40. [PMC free article] [PubMed] [Google Scholar]
- Bhatia N, Lain E, Baldwin H et al. Encapsulated benzoyl peroxide (E-BPO): a novel formulation of BPO for long-term management of rosacea. SKIN. 2022;6(2):s14. [Google Scholar]
- Wilkin J, Dahl M, Detmar M et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50(6):907–912. doi: 10.1016/j.jaad.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Wilkin J, Dahl M, Detmar M et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46(4):584–587. doi: 10.1067/mjd.2002.120625. [DOI] [PubMed] [Google Scholar]
- Gallo RL, Granstein RD, Kang S et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78(1):148–155. doi: 10.1016/j.jaad.2017.08.037. [DOI] [PubMed] [Google Scholar]
- https://jgi.doe.gov/data-and-tools/software-tools/bbtools/bb-tools-user-guide/bbduk-guide BBDuk Guide. Joint Genome Institute. Accessed August 1, 2022.
- https://docs.cosmosid.com/docs/functional-analysis Functional classification. CosmosID-HUB Microbiome and GenBook. CosmosID. Accessed 17 Feb 2023.
- Franzosa EA, McIver LJ, Rahnavard G et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat Methods. 2018;15(11):962–968. doi: 10.1038/s41592-018-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi R, Foerster H, Fulcher CA et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2008;36:D623–D631. doi: 10.1093/nar/gkm900. database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly N, Bergin D, Reeves EP et al. Demodex-associated bacterial proteins induce neutrophil activation. Br J Dermatol. 2012;166(4):753–760. doi: 10.1111/j.1365-2133.2011.10746.x. [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe-Alvarez C, Chiquete-Felix N, Contreras-Zentella M et al. Staphylococcus epidermidis: metabolic adaptation and biofilm formation in response to different oxygen concentrations. Pathog Dis. 2016;74(1):ftv111. doi: 10.1093/femspd/ftv111. [DOI] [PubMed] [Google Scholar]
- Palmer CN, Irvine AD, Terron-Kwiatkowski A et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- Tan SP, Brown SB, Griffiths CE et al. Feeding filaggrin: effects of l-histidine supplementation in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:403–411. doi: 10.2147/CCID.S146760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi C, Stremnitzer C, Mlitz V et al. Increased sensitivity of histidinemic mice to UVB radiation suggests a crucial role of endogenous urocanic acid in photoprotection. J Invest Dermatol. 2011;131(1):188–194. doi: 10.1038/jid.2010.231. [DOI] [PubMed] [Google Scholar]
- Roux PF, Oddos T, Stamatas G. Deciphering the role of skin surface microbiome in skin health: an integrative multiomics approach reveals three distinct metabolite-microbe clusters. J Invest Dermatol. 2022;142(2):469–479.e5. doi: 10.1016/j.jid.2021.07.159. [DOI] [PubMed] [Google Scholar]
- Chen AC, Damian DL. Nicotinamide and the skin. Australas J Dermatol. 2014;55(3):169–175. doi: 10.1111/ajd.12163. [DOI] [PubMed] [Google Scholar]
- Mohammed D, Crowther JM, Matts PJ et al. Influence of niacinamide containing formulations on the molecular and biophysical properties of the stratum corneum. Int J Pharm. 2013;441(1𠄔2):192–201. doi: 10.1016/j.ijpharm.2012.11.043. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chen L, Liu X et al. The influence of benzoyl peroxide on skin microbiota and the epidermal barrier for acne vulgaris. Dermatol Ther. 2022;35(3):e15288. doi: 10.1111/dth.15288. [DOI] [PubMed] [Google Scholar]
- Coughlin CC, Swink SM, Horwinski J et al. The preadolescent acne microbiome: a prospective, randomized, pilot study investigating characterization and effects of acne therapy. Pediatr Dermatol. 2017;34(6):661–664. doi: 10.1111/pde.13261. [DOI] [PubMed] [Google Scholar]
- Casanova F, Santos L. Encapsulation of cosmetic active ingredients for topical application—a review. J Microencapsul. 2016;33(1):1–17. doi: 10.3109/02652048.2015.1115900. [DOI] [PubMed] [Google Scholar]


