Abstract
Objective
Biotin has widespread popularity as a hair supplement. We sought to review the literature regarding biotin’s efficacy as a hair supplement.
Methods
We conducted a literature search of PubMed for articles specifically studying the use of oral biotin for hair growth or quality. Case reports and case series were excluded.
Results
Three studies met our inclusion criteria. The first study was the highest quality, with a double-blind and placebo-controlled study design, but their results found no difference between the biotin and placebo groups for hair growth. The other two studies investigated specific patient populations (patients on isotretinoin and female patients post-sleeve gastrectomy). Both studies were susceptible to multiple potential biases and neither had striking results in favor of biotin.
Limitations
Our review is limited by lack of available studies.
Conclusion
Given the widespread popularity of biotin as a hair supplement, one would presume that this claim must be grounded in strong evidence; however, there is a large discrepancy between the public’s perception of its efficacy and the scientific literature. The utility of biotin as a hair supplement is not supported by high-quality studies.
Keywords: Biotin, vitamin B-7, vitamin H, supplement, hair loss, alopecia
Biotin is vitamin B7, also known as vitamin H from the German words “Haar und Haut,” which mean “hair and skin.” Biotin has long been glamorized as a hair supplement, but the scientific evidence supporting this claim has been surprisingly lacking.1 Biotin is an essential cofactor for five mammalian carboxylase enzymes involved in gluconeogenesis, fatty acid synthesis, and amino acid catabolism.2,3 Mammals cannot synthesize biotin, however deficiency is thought to be rare in industrialized countries since it is available in a wide range of foods (namely meat, fish, eggs, nuts, dairy, and some vegetables) and is produced by intestinal flora.4,5 A balanced Western diet provides 35–70mcg of biotin daily, which exceeds the daily adequate intake of 30mcg.4,6–8 Biotin deficiency can be either acquired or inherited. Acquired forms can be seen with pregnant and breastfeeding women, alcoholism, smoking, malabsorption, malnutrition, total parenteral nutrition, excessive consumption of raw egg whites, prolonged antibiotic use altering the gut microbiota, and associated with various medications including valproic acid and isotretinoin.2,5,9,10 Inherited deficiencies are due to a genetic mutation in either biotinidase or holocarboxylase, both involved in the biotin cycle.
METHODS
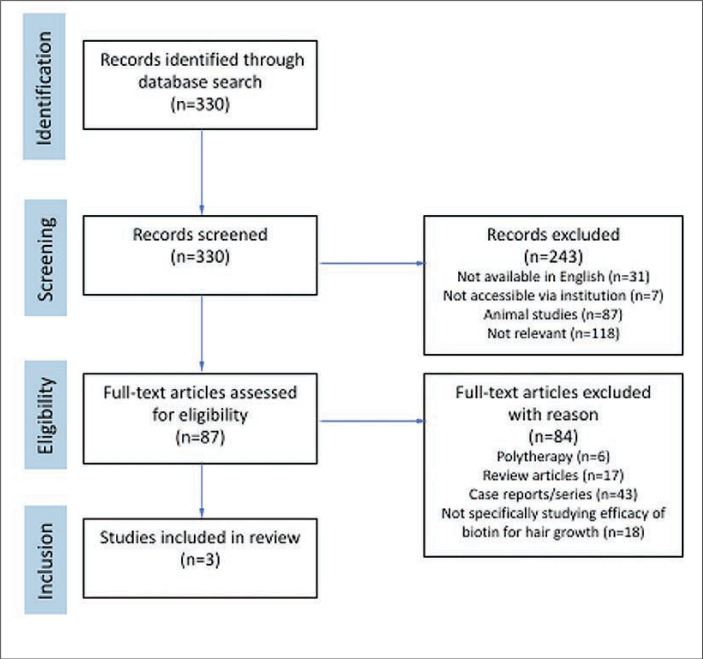
In this review, we sought to delve deeper into the existing high-quality scientific evidence regarding biotin for hair loss by conducting a literature review using PubMed on June 6th, 2023, for the following search terms: biotin AND (hair OR alopecia). There were 330 results. Results were narrowed by relevance, those available in English, and those accessible through the authors’ institutional library (Figure 1). Relevance was defined as human studies evaluating the use of biotin monotherapy for improving hair growth or quality. With the purpose of isolating high-quality evidence, case reports and case series were excluded.
FIGURE 1.
Summary of review conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines
RESULTS
Only three studies met our criteria (Table 1). One double-blind, placebo-controlled study was identified dating back to 1966.11 In this study on female participants with diffuse alopecia, 28 patients took 10mg of biotin daily compared to 18 patients given placebo. After four weeks, both groups improved from baseline and there was no significant difference in hair growth or sebum excretion between the groups. In the second study, Aksac et al12 had 60 patients taking isotretinoin and 30 of them were also given biotin 10 mg daily.12 Using trichoscopy, both groups displayed a decrease in the terminal hair density while on isotretinoin therapy; however, the biotin group showed a significant shift toward anagen hairs compared to baseline. In the final study, Sen et al13 investigated the efficacy of biotin for hair loss after sleeve gastrectomy. Of 112 female patients with self-reported hair loss post-sleeve gastrectomy, 22 were biotin-deficient. Of these biotin-deficient patients, 23 percent reported subjective improvement in hair loss with 1mg of biotin daily. Interestingly, 38 percent of biotin-sufficient patients reported improvement in hair loss as well. No other case-control studies, cohort studies, nor controlled clinical trials specifically investigating the efficacy of biotin for improving hair growth or quality were identified.
TABLE 1.
Summary of the three studies that met our inclusion criteria
| STUDY | POPULATION | DOSE | DESIGN | RESULTS |
|---|---|---|---|---|
| Pawlowski et al 196611 | Females with diffuse pattern hair loss | 10mg daily x 4 weeks | Double-blinded, placebo controlled; Group 1: 28 patients took biotin; Group 2: 18 patients took placebo | Both groups improved from baseline. No significant difference in hair growth or sebum production between groups. |
| Aksac et al 202112 | Patients taking isotretinoin | 10mg daily x 4 months | Group 1: 30 patients took isotretinoin and biotin; Group 2: 30 patients took isotretinoin only | Using trichoscopy, both groups had a decrease in terminal hair density. Group 1 had a significant shift toward anagen hairs compared to baseline. |
| Sen et al 202113 | Females with hair loss after sleeve gastrectomy | 1mg daily | Group 1: 22 biotin-deficient patients took biotin x 3 months; Group 2: 29 biotin-sufficient patients took biotin x 2.5 months on average | 23 percent of Group 1 reported remarkable decline in hair loss. 38 percent of Group 2 reported remarkable decline in hair loss. There was no significant difference between groups. |
DISCUSSION
The study by Pawlowski et al11 is almost 60 years old and did not support the use of biotin for diffuse female alopecia. The study on biotin’s utility in isotretinoin-associated alopecia as well as the study on sleeve gastrectomy patients focused on niche patient populations and, therefore, are not generalizable.12,13 These studies were not blinded, and therefore the results may have been affected by observer bias; regardless, the results were not remarkably supportive of biotin. The level of evidence for the sleeve gastrectomy study in particular is especially low because it relied on subjective perception of hair loss.
Given the dearth of publications that met our complete search criteria, we will touch on noteworthy articles that resulted from our initial search terms, including numerous case reports and small case series, as well as studies investigating serum biotin levels in various causes of alopecia. Of the reported cases, the majority were pediatric cases, and all patients had an underlying pathology to explain their poor hair growth.
Biotin supplementation has been utilized in many pediatric hair conditions. Doses ranging from 300mcg three times daily to 5,000mcg daily have shown improvement of hair thickness and combability at 3 to 4 months in cases of uncombable hair syndrome, even with normal baseline biotin levels.14,15 A retrospective chart analysis of 25 patients with short anagen syndrome demonstrated that biotin alone or with topical minoxidil produced clinical improvement in the length and diameter of hair shafts.16
Additionally, there are ample case reports of patients with inherited biotin-deficiency due to defective biotinidase or holocarboxylase enzymes that display subjective improvement of alopecia after biotin supplementation.1,17 Likewise, many cases of alopecia from acquired causes of biotin deficiency have shown improvement with biotin supplementation, including malnutrition from formula, parenteral nutrition, or surgical resection of bowel.18–21
Biotin-responsive alopecia has been seen with a few medications, as seen with the isotretinoin trial mentioned above. Accordingly, Schulpis et al22 discovered that isotretinoin-associated telogen effluvium has significantly diminished biotinidase activity compared to controls. Alopecia in the setting of valproic acid has also shown subjective improvement with 10mg of daily biotin administration.23,24 The exact mechanism is unclear since one study of 20 children on valproic acid showed no significant difference in serum biotin level or biotinidase activity compared to controls, yet another study did show a dose-related reduction of biotinidase activity with valproic acid; nonetheless, the biotin-responsiveness of alopecia in both studies suggests some interference of the biotin cycle.23,24
Biotin has been studied in polytherapy regimens for hair loss. In a compounded topical formulation with minoxidil, finasteride, and caffeine citrate, biotin has promoted normal hair growth in androgenetic alopecia.25 Similarly, in an intradermal mesotherapy preparation with minoxidil, finasteride, and D-panthenol, biotin produced significant improvement in hair density and thickness.26 In children with alopecia areata, oral biotin with concurrent oral zinc and topical clobetasol administration produced complete hair regrowth in 3 of 9 pediatric patients compared to none of the nine children that received systemic steroids.27 Intramuscular biotin and dexpanthenol injections improved total hair density and decreased hair fall count compared to baseline in a study of 50 patients with diffuse pattern hair loss.28 Of course, studies on polytherapy formulations do not give any true insight on the impact of biotin in producing the result.
A few studies have evaluated serum biotin concentrations in patients with hair loss and yielded conflicting results. On one side, Trueb et al29 reported a remarkable 38 percent of 541 female patients (9–92 years old) complaining of hair loss were biotin-deficient (<100 ng/L), and 89 percent of these biotin-deficient individuals had no identifiable risk factors for biotin deficiency. On the other side, Rahman et al30 found no difference in biotin levels between 60 patients with telogen effluvium and 20 controls. In androgenetic alopecia, 60 male patients had biotin levels significantly lower than 60 controls, though neither group had any individuals that were truly biotin-deficient.9 The gender differences and type of alopecia may help explain the discrepant outcomes. Plus, serum biotin levels are thought to be unreliable; instead, measuring urine metabolites is considered a more precise measure of biotin deficiency.1,5,9,29,31 More studies are needed to corroborate these findings.
Some authors have investigated public perception of biotin efficacy for hair loss. John et al32 analyzed Amazon.com reviews for biotin products revealing 27.2 percent of reviewers stated it was helpful for their hair. That same group performed an anonymous survey of dermatology patients where 27.4 percent of 152 biotin users reported subjective improvement in hair and nails.33
Overall, existing low-grade evidence only supports that biotin may improve hair growth or quality in the following settings: uncombable hair syndrome, short anagen syndrome, biotinidase and holocarboxylase deficiency, insufficient biotin intake (formula, parenteral nutrition, surgical bowel resection), and medications (isotretinoin and valproic acid). There have been no studies demonstrating biotin supplementation to be beneficial for hair growth in healthy individuals.
It is well-known that patients who are biotin-deficient can display alopecia.5 From this, one can surmise that biotin must play a significant role in hair growth. Residing on a fallacious rationale, the media extrapolated that excess supplementation of this vitamin would be useful for hair growth and started marketing biotin as an essential hair supplement. Herein lies a large discrepancy between the public’s perception of biotin’s efficacy for hair loss and the current scientific literature. As we can see above, the current literature does not support biotin’s use in adults with sufficient levels of biotin. Nonetheless, use of this vitamin by the public is pervasive. In 2016, 29 percent of the population was found to take a biotin-containing supplement.34 In another study of 1,944 emergency room visits at Mayo Clinic, 7.7 percent of patients knowingly reported taking biotin.35 The survey by John et al33 exhibited that 33.7 percent of dermatology patients reported current or past biotin use, and 28.8 percent of them were recommended to start biotin by a physician. Despite insufficient evidence, physicians continue to recommend biotin for hair concerns. A national survey of 147 physicians found that 43.9 percent recommended biotin to patients, and of these, 59 percent recommended it for hair disorders.36 Though, Trueb et al29 did raise the possibility that a substantial proportion of the hair loss community may be biotin-deficient, and thus, warrants further investigation into possible biotin deficiency in alopecia patients.
FDA warning. Being a water-soluble vitamin, biotin toxicity is essentially unheard of; however, biotin supplementation does not come without risk. Elevated serum biotin levels can interact with various laboratory immunoassays that could lead to a missed diagnosis, needless workups, undue distress, or fatal consequences. For instance, a death has been reported where biotin interference of a troponin test led to the missed diagnosis of a heart attack.37
Many immunoassays rely on a streptavidin-biotin interaction. Interference from excess serum biotin may induce both false-positive and false-negative laboratory results based on the assay design. Competitive assays result in positive interference, meaning lab results are falsely elevated, and sandwich (non-competitive) assays can have negative interference, meaning lab results are falsely diminished.38 Table 2 displays a list of laboratory tests that may have false-positive or false-negative results due to biotin interference. There are many reports of biochemical Graves’ disease in euthyroid individuals due to biotin interference, one almost leading to an unnecessary thyroidectomy.43–50Another notable interference is with qualitative urine hCG tests, which may pose a problem since biotin is often a component of prenatal vitamins.42
TABLE 2.
| POSITIVE INTERFERENCE (FALSELY ELEVATED) | NEGATIVE INTERFERENCE (FALSELY DIMINISHED) |
|---|---|
| Aldosterone | ACTH, FSH, HGH, LH, PTH, SHBG, TSH |
| Androstenedione | AFP |
| Anti-HAV Total | Anti-CCP |
| Anti-TSH receptor | Anti-HAV IgM |
| Anti-TPO | Anti-HBc IgM & HBsAg |
| Anti-Thyroglobulin (both) | Anti-HCV |
| Cortisol | Calcitonin & Procalcitonin |
| Cyclosporine | Cancer Ags (125, 15-3, 19-9, CEA, HE4, PSA) |
| DHEAS | Cardiac biomarkers (Troponin, CK-MB, Myoglobin) |
| Digoxin | |
| Estradiol | EPO |
| Folate | Ferritin |
| FT3, FT4, T3, T4 | Gastrin |
| Progesterone | HIV 1/2 Ag/Ab [41] |
| Testosterone [40] | Insulin, IGF-1, C-peptide |
| Vitamin B12 | IgE |
| Vitamin D Total | NT-proBNP |
| 17-OH-progesterone | Prolactin |
| 25-Hydroxyvitamin D | Qualitative hCG [42] |
In 2017, the Food and Drug Administration (FDA) released a safety communication to spread awareness that biotin can seriously interfere with certain lab tests.37 In 2019, they issued another statement reminding the public, healthcare providers, lab personnel, and lab test developers about such lab interference with particular concern about interference with troponin laboratory tests.51 They also published a list of troponin lab tests that are subject to biotin interference that was last updated June 2022.52
Fortunately, high doses of biotin are required in order to pass most thresholds for interference and biotin is eliminated quickly.38,50 Each individual immunoassay has its own threshold, some being more sensitive to interference than others. It is concerning that troponin is one of the affected tests that has a low threshold for interference.50 Biotin’s serum concentration depends on the dose, duration of treatment, time of last dose, and the patient’s renal function. Microgram (mcg) doses have a half-life of around 1.8 h, while 100–300 mg doses have a half-life ranging from 7.8-18.8 hours.50 Standard microgram doses (30–60 mcg daily) often found in multivitamins are believed not to interfere with streptavidin-biotin assays.50 Milligram doses (5–10 mg daily) can be found in over the counter supplements marketed for hair, skin, and nail growth, and can cause biotin interference, particularly in more sensitive assays like troponin.50 As a reminder, the recommended daily intake for biotin is 30mcg daily, meaning that these products contain >10,000 percent adequate intake. High-dose biotin therapy (100–300 mg daily), used for treatment of multiple sclerosis, produced significant interference in all tested assays.50 Cessation of biotin therapy is recommended at least two days prior to blood tests, and up to a week may be needed for high-dose therapy, especially for sensitive tests.50,53 In reality, at least an 8-hour washout is likely sufficient for patients who have consumed 5–10 mg of biotin or less.38
Despite these FDA warnings, many consumers and even physicians are unaware of biotin’s potential laboratory interference. The John et al. survey showed that only 6.6 percent of biotin users were aware of the FDA warning and only 4 percent were informed of potential laboratory interference by their physician.33 Waqas et al36 published a national survey of physicians revealing that 19.5 percent were unaware of any laboratory interference.
There are many safeguards that can be instituted to prevent the unwanted implications of biotin laboratory interference. Increased public and physician awareness will help mitigate potential errors. It is important for physicians to inquire about biotin supplementation in all patients since 54.6 percent of users have been shown to self-prescribe.33 Physicians should educate patients about such laboratory interference and recommend for patients to discontinue biotin two days prior to scheduled laboratory work. If the patient does not have sufficient notice for a blood test as in the case of an emergency, then the laboratory should be notified that the patient is taking biotin.54 Surprisingly, biotin product labels are not mandated to mention the FDA warning, but biotin manufacturers should also be responsible for educating the public about this.32,55 The onus is also on laboratories and assay manufacturers to update immunoassays recognizing the fact that much of the public is taking biotin. For laboratory tests subject to biotin interference, serum biotin concentration could be reported with the test results with an explanation as to whether it’s above the interference threshold.56 Alternatively, biotin could be removed from the serum prior to the assay to prevent interference.57,58 Lastly, it is vital for providers to have an open discussion with patients about the risks and scarcity of evidence supporting biotin’s use for hair loss in the first place.
CONCLUSION
Biotin is not an enemy here; misinformation is. The glorification of its uncertain efficacy for hair loss combined with the lack of awareness about laboratory interference poses a potential hazard. For those concerned about inadequate biotin consumption, it can be innocuously administered in microgram doses to ensure sufficient intake while being subthreshold for laboratory interference; however, excessive daily doses are inadvisable. Our review displays that the widespread marketing of biotin for hair loss in healthy individuals is unsubstantiated. The current literature comprised of low-quality studies merely shows that biotin may be useful in patients who are biotin-deficient or in pediatric patients with an underlying hair pathology. It does not support superfluous administration in individuals with sufficient biotin. In order to appropriately justify or dispel biotin’s popularity as a hair supplement, randomized-controlled studies on biotin monotherapy for improving hair growth or quality in the target population (i.e., healthy individuals with self-perceived hair loss) are especially needed.
REFERENCES
- Patel DP, Swink SM, Castelo-Soccio L. A review of the use of biotin for hair loss. Skin Appendage Disord. 2017;3(3):166–169. doi: 10.1159/000462981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempleni J, Hassan YI, Wijeratne SS. Biotin and biotinidase deficiency. Expert Rev Endocrinol Metab. 2008;3(6):715–724. doi: 10.1586/17446651.3.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistas KG, Tadi P. Biotin. [Updated 23 May 2023]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2023. https://www.ncbi.nlm.nih.gov/books/NBK554493/ Jan. Available from:
- https://ods.od.nih.gov/factsheets/Biotin-HealthProfessional/ National Institutes of Health Office of Dietary Supplements. Biotin – fact sheet for health professionals. (2022). Accessed: 15 Aug 2023.
- Nosewicz J, Spaccarelli N, Roberts KM et al. The epidemiology, impact, and diagnosis of micronutrient nutritional dermatoses. Part 2: B-complex vitamins. J Am Acad Dermatol. 2022;86(2):281–292. doi: 10.1016/j.jaad.2021.06.900. [DOI] [PubMed] [Google Scholar]
- Almohanna HM, Ahmed AA, Tsatalis JP et al. The role of vitamins and minerals in hair loss: a review. Dermatol Ther (Heidelb). 2019;9(1):51–70. doi: 10.1007/s13555-018-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem F, Soos MP. Treasure Island (FL): StatPearls Publishing; 2023. Biotin Deficiency. 2023 Feb 20. In: StatPearls [Internet]. Jan. PMID: 31613531. [PubMed] [Google Scholar]
- https://www.ncbi.nlm.nih.gov/books/NBK114297/ Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US); 1998. 11, Biotin. Available from. [PubMed]
- El-Esawy FM, Hussein MS, Ibrahim Mansour A. Serum biotin and zinc in male androgenetic alopecia. J Cosmet Dermatol. 2019;18(5):1546–1549. doi: 10.1111/jocd.12865. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Kim N. Dietary supplements in dermatology: a review of the evidence for zinc, biotin, vitamin D, nicotinamide, and Polypodium. J Am Acad Dermatol. 2021;84(4):1042–1050. doi: 10.1016/j.jaad.2020.04.123. [DOI] [PubMed] [Google Scholar]
- Pawlowski A, Kostanecki W. Effect of biotin on hair roots and sebum excretion in women with diffuse alopecia. Pol Med J. 1966;5(2):447–452. [PubMed] [Google Scholar]
- Aksac SE, Bilgili SG, Yavuz GO et al. Evaluation of biophysical skin parameters and hair changes in patients with acne vulgaris treated with isotretinoin, and the effect of biotin use on these parameters. Int J Dermatol. 2021;60(8):980–985. doi: 10.1111/ijd.15485. [DOI] [PubMed] [Google Scholar]
- Şen O, Türkçapar AG. Hair loss after sleeve gastrectomy and effect of biotin supplements. J Laparoendosc Adv Surg Tech A. 2021;31(3):296–300. doi: 10.1089/lap.2020.0468. [DOI] [PubMed] [Google Scholar]
- Boccaletti V, Zendri E, Giordano G et al. Familial uncombable hair syndrome: ultrastructural hair study and response to biotin. Pediatr Dermatol. 2007;24(3):E14–E16. doi: 10.1111/j.1525-1470.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Shelley WB, Shelley ED. Uncombable hair syndrome: observations on response to biotin and occurrence in siblings with ectodermal dysplasia. J Am Acad Dermatol. 1985;13(1):97–102. doi: 10.1016/s0190-9622(85)70150-8. [DOI] [PubMed] [Google Scholar]
- Starace M, Gurioli C, Carpanese MA et al. Short anagen syndrome: a case series and algorithm for diagnosis. Pediatr Dermatol. 2021;38(5):1157–1161. doi: 10.1111/pde.14750. [DOI] [PubMed] [Google Scholar]
- Walth CB, Wessman LL, Wipf A et al. Response to: "Rethinking biotin therapy for hair, nail, and skin disorders". J Am Acad Dermatol. 2018;79(6):e121–e124. doi: 10.1016/j.jaad.2018.07.055. [DOI] [PubMed] [Google Scholar]
- Sato Y, Wakabayashi K, Ogawa E et al. Low serum biotin in Japanese children fed with hydrolysate formula. Pediatr Int. 2016;58(9):867–871. doi: 10.1111/ped.12937. [DOI] [PubMed] [Google Scholar]
- Fujimoto W, Inaoki M, Fukui T et al. Biotin deficiency in an infant fed with amino acid formula. J Dermatol. 2005;32(4):256–261. doi: 10.1111/j.1346-8138.2005.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Daniells S, Hardy G. Hair loss in long-term or home parenteral nutrition: are micronutrient deficiencies to blame? Curr Opin Clin Nutr Metab Care. 2010;13(6):690–697. doi: 10.1097/MCO.0b013e32833ece02. [DOI] [PubMed] [Google Scholar]
- Yazbeck N, Muwakkit S, Abboud M et al. Zinc and biotin deficiencies after pancreaticoduodenectomy. Acta Gastroenterol Belg. 2010;73(2):283–286. [PubMed] [Google Scholar]
- Schulpis KH, Georgala S, Papakonstantinou ED et al. The effect of isotretinoin on biotinidase activity. Skin Pharmacol Appl Skin Physiol. 1999;12(1–2):28–33. doi: 10.1159/000029843. [DOI] [PubMed] [Google Scholar]
- Schulpis KH, Karikas GA, Tjamouranis J et al. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001;42(10):1359–1362. doi: 10.1046/j.1528-1157.2001.47000.x. [DOI] [PubMed] [Google Scholar]
- Castro-Gago M, Gómez-Lado C, Eirís-Puñal J et al. Serum biotinidase activity in children treated with valproic acid and carbamazepine. J Child Neurol. 2010;25(1):32–35. doi: 10.1177/0883073809336118. [DOI] [PubMed] [Google Scholar]
- Marotta JC, Patel G, Carvalho M et al. Clinical efficacy of a topical compounded formulation in male androgenetic alopecia: minoxidil 10 percent, finasteride 0.1 percent, biotin 0.2 percent, and caffeine citrate 0.05 percent hydroalcoholic solution. Int J Pharm Compd. 2020;24(1):69–76. [PubMed] [Google Scholar]
- Melo DF, de Mattos Barreto T, Plata GT et al. Excellent response to mesotherapy as adjunctive treatment in male androgenetic alopecia. J Cosmet Dermatol. 2020;19(1):75–77. doi: 10.1111/jocd.12983. [DOI] [PubMed] [Google Scholar]
- Camacho FM, García-Hernández MJ. Zinc aspartate, biotin, and clobetasol propionate in the treatment of alopecia areata in childhood. Pediatr Dermatol. 1999;16(4):336–338. doi: 10.1111/j.1525-1470.1999.pdele65.x. [DOI] [PubMed] [Google Scholar]
- Samadi A, Ketabi Y, Firooz R et al. Efficacy of intramuscular injections of biotin and dexpanthenol in the treatment of diffuse hair loss: A randomized, double-blind controlled study comparing two brands. Dermatol Ther. 2022;35(9):e15695. doi: 10.1111/dth.15695. [DOI] [PubMed] [Google Scholar]
- Trüeb RM. Serum biotin levels in women complaining of hair loss. Int J Trichology. 2016;8(2):73–77. doi: 10.4103/0974-7753.188040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel Rahman SH, Mohammed Salem R, Hassan Sabry J. Biotin deficiency in telogen effluvium: fact or fiction? J Clin Aesthet Dermatol. 2020;13(3):37–40. [PMC free article] [PubMed] [Google Scholar]
- Soleymani T, Lo Sicco K, Shapiro J. The infatuation with biotin supplementation: is there truth behind its rising popularity? A comparative analysis of clinical efficacy versus social popularity. J Drugs Dermatol. 2017;16(5):496–500. [PubMed] [Google Scholar]
- John JJ, Lipner SR. Consumer perception of biotin supplementation. J Cutan Med Surg. 2019;23(6):613–616. doi: 10.1177/1203475419871046. [DOI] [PubMed] [Google Scholar]
- John JJ, Cooley V, Lipner SR. Assessment of biotin supplementation among patients in an outpatient dermatology clinic. J Am Acad Dermatol. 2019;81(2):620–621. doi: 10.1016/j.jaad.2018.12.045. [DOI] [PubMed] [Google Scholar]
- Kantor ED, Rehm CD, Du M et al. Trends in dietary supplement use among US adults from 1999–2012. JAMA. 2016;316(14):1464–1474. doi: 10.1001/jama.2016.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman BM, Lueke AJ, Donato LJ et al. Prevalence of biotin supplement usage in outpatients and plasma biotin concentrations in patients presenting to the emergency department. Clin Biochem. 2018;60:11–16. doi: 10.1016/j.clinbiochem.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Waqas B, Wu A, Yim E et al. A survey-based study of physician practices regarding biotin supplementation. J Dermatolog Treat. 2022;33(1):573–574. doi: 10.1080/09546634.2020.1770178. [DOI] [PubMed] [Google Scholar]
- https://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm586505.htm U.S. Food and Drug Administration (FDA). The FDA warns that biotin may interfere with lab tests: FDA safety communication. Washington, DC: FDA, U.S. Department of Health and Human Services; 2017. Updated 28 Nov 2017.
- Li D, Ferguson A, Cervinski MA et al. AACC guidance document on biotin interference in laboratory tests. J Appl Lab Med. 2020;5(3):575–587. doi: 10.1093/jalm/jfz010. [DOI] [PubMed] [Google Scholar]
- Ostrowska M, Bartoszewicz Z, Bednarczuk T et al. The effect of biotin interference on the results of blood hormone assays. Endokrynol Pol. 2019;70(1):102–121. doi: 10.5603/EP.a2018.0084. [DOI] [PubMed] [Google Scholar]
- Stieglitz HM, Korpi-Steiner N, Katzman B et al. Suspected testosterone-producing tumor in a patient taking biotin supplements. J Endocr Soc. 2018;2(6):563–569. doi: 10.1210/js.2018-00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haleyur Giri Setty MK, Lee S, Lathrop J et al. Biotin interference in point of care hiv immunoassay. Biores Open Access. 2020;9(1):243–246. doi: 10.1089/biores.2020.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR, Cervinski MA, Nerenz RD. Assessment of biotin interference with qualitative point-of-care hCG test devices. Clin Biochem. 2018;53:168–170. doi: 10.1016/j.clinbiochem.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Piketty ML, Polak M, Flechtner I et al. False biochemical diagnosis of hyperthyroidism in streptavidin-biotin-based immunoassays: the problem of biotin intake and related interferences. Clin Chem Lab Med. 2017;55(6):780–788. doi: 10.1515/cclm-2016-0606. [DOI] [PubMed] [Google Scholar]
- Frame IJ, Joshi PH, Mwangi C et al. Susceptibility of cardiac troponin assays to biotin interference. Am J Clin Pathol. 2019;151(5):486–493. doi: 10.1093/ajcp/aqy172. [DOI] [PubMed] [Google Scholar]
- Sharma A, Baumann NA, Shah P. Biotin-induced biochemical Graves disease: a teachable moment. JAMA Intern Med. 2017;177(4):571–572. doi: 10.1001/jamainternmed.2016.9295. [DOI] [PubMed] [Google Scholar]
- Kummer S, Hermsen D, Distelmaier F. Biotin treatment mimicking Graves’ disease. N Engl J Med. 2016;375(7):704–706. doi: 10.1056/NEJMc1602096. [DOI] [PubMed] [Google Scholar]
- Elston MS, Sehgal S, Du Toit S et al. Factitious Graves’ disease due to biotin immunoassay interference-a case and review of the literature. J Clin Endocrinol Metab. 2016;101(9):3251–3255. doi: 10.1210/jc.2016-1971. [DOI] [PubMed] [Google Scholar]
- Batista MC, Ferreira CES, Faulhaber ACL et al. Biotin interference in immunoassays mimicking subclinical Graves’ disease and hyperestrogenism: a case series. Clin Chem Lab Med. 2017;55(6):e99–e103. doi: 10.1515/cclm-2016-0628. [DOI] [PubMed] [Google Scholar]
- Odhaib SA, Mansour AA, Haddad NS. How biotin induces misleading results in thyroid bioassays: case series. Cureus. 2019;11(5):e4727. doi: 10.7759/cureus.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trambas C, Lu Z, Yen T et al. Characterization of the scope and magnitude of biotin interference in susceptible Roche Elecsys competitive and sandwich immunoassays. Ann Clin Biochem. 2018;55(2):205–215. doi: 10.1177/0004563217701777. [DOI] [PubMed] [Google Scholar]
- https://public4.pagefreezer.com/content/FDA/16-06-2022T13:39/https:/www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication U.S. Food and Drug Administration (FDA). UPDATE: The FDA Warns that Biotin May Interfere with Lab Tests: FDA Safety Communication. Washington, DC: FDA, U.S. Department of Health and Human Services; 2019. Updated 5 Nov 2019.
- https://www.fda.gov/medical-devices/in-vitro-diagnostics/biotin-interference-troponin-lab-tests-assays-subject-biotin-interference U.S. Food and Drug Administration (FDA). Biotin Interference with Troponin Lab Tests - Assays Subject to Biotin Interference. Washington, DC: FDA, U.S. Department of Health and Human Services; 2022. Updated 21 Jun 2022.
- Gifford JL, de Koning L, Sadrzadeh SMH. Strategies for mitigating risk posed by biotin interference on clinical immunoassays. Clin Biochem. 2019;65:61–63. doi: 10.1016/j.clinbiochem.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Lipner SR. Rethinking biotin therapy for hair, nail, and skin disorders. J Am Acad Dermatol. 2018;78(6):1236–1238. doi: 10.1016/j.jaad.2018.02.018. [DOI] [PubMed] [Google Scholar]
- Perez-Sanchez AC, Burns EK, Perez VM et al. Safety concerns of skin, hair and nail supplements in retail stores. Cureus. 2020;12(7):e9477. doi: 10.7759/cureus.9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann A, Plomgaard P, Hilsted LM et al. Quantification of biotin in plasma samples by column switching liquid chromatography - tandem mass spectrometry. Scand J Clin Lab Invest. 2021;81(2):127–136. doi: 10.1080/00365513.2020.1871504. [DOI] [PubMed] [Google Scholar]
- Trambas C, Lu Z, Yen T et al. Depletion of biotin using streptavidin-coated microparticles: a validated solution to the problem of biotin interference in streptavidin-biotin immunoassays. Ann Clin Biochem. 2018;55(2):216–226. doi: 10.1177/0004563217707783. [DOI] [PubMed] [Google Scholar]
- Piketty ML, Prie D, Sedel F et al. High-dose biotin therapy leading to false biochemical endocrine profiles: validation of a simple method to overcome biotin interference. Clin Chem Lab Med. 2017;55(6):817–825. doi: 10.1515/cclm-2016-1183. [DOI] [PubMed] [Google Scholar]