Abstract
Breast cancer is the most prevalent cancer among African women, with high mortality rates in Ghana. Nuclear factor kappa B (NF-kB) has been associated with tumor progression in breast cancer. However, its clinical validation is controversial and understudied with no known published data on NF-kB (p65) among breast cancer patients in Ghana and other African countries. This study assessed the prognostic significance of NF-kB (p65) expression and its association with various clinicopathological features in breast cancer patients. Ninety formalin-fixed breast cancer tissues and 15 normal breast tissues were used to determine the expression of NF-kB (p65) using immunohistochemistry. We explored the correlation between expression of NF-kB (p65) and clinicopathological features. NF-kB (p65) was expressed in 86.7% of breast cancer tissues. There was a significant relationship between NF-kB (p65) expression and tumor grade, proliferation index (Ki67), and molecular subtype. High NF-kB (p65) expression in tumor grade 3 was about 10 times that of grade 1 (54.2% vs. 5.1%), and Ki67 > 20 was 79.7% compared to 20.3% for Ki67 ≤ 20. Patients with triple-negative breast cancer (TNBC) had 49.1% overexpression of NF-kB (p65) compared to 17%, 25.4%, and 8.5% for luminal A, luminal B, and HER2 cases, respectively. This study demonstrates that NF-kB (p65) was highly expressed among breast cancer patients at Cape Coast Teaching Hospital, Ghana, especially in TNBC. NF-kB (p65) could serve as a biomarker for cancer stage, progression, prognosis and as a therapeutic target.
Keywords: Breast cancer, NF-kB (p65), Tumor grade, Ki67
Highlights of the Study
The expression of NF-kB (p65) and its correlation with some clinicopathological features were evaluated among breast cancer patients.
High expression of NF-kB (p65) in breast cancer tissues might explain their aggressive action in breast cancer, especially in patients with triple-negative breast cancer patients.
The association of NF-kB (p65) with tumor grade, proliferation index (Ki67), and molecular subtype indicates its involvement in breast cancer progression.
Introduction
Breast cancer (BC) is the most prevalent cancer among women aged 20–50 years worldwide and is one of the primary causes of death in women [1]. BC surpassed lung cancer as the most common tumor worldwide in the Global Cancer statistics for 2020 with annual cases continuing to rise [2, 3]. There is evidence for an increase in cases of BC in Africa, although the generalization of these estimates is questionable due to a lack of proper data in several regions [4]. It is the most common cancer among women and cancer-related deaths among women in Ghana. Despite advances in treatment, some patients still experience recurrence and eventually develop metastatic disease that is generally incurable. Our understanding of the cellular and molecular processes underlying breast cancer development, recurrence, and metastasis is not entirely clear; therefore, it is essential to further explore these processes to identify molecular targets that can predict prognosis and/or guide targeted therapy.
Transcription factors have been recognized for their roles in cancer progression. Nuclear factor kappa B (NF-kB), a transcription factor with five family members, exists as a dimer that binds to inhibitors of nuclear factor kappa B (IKBs) degradation of IKBs under external stimuli via ubiquitin-proteasome pathway releases NF-kB (p65) dimers that translocate to the nucleus and stimulate various target genes [5, 6]. NF-kB has been linked to chronic inflammation and malignant tumors including BC, through increased expression of target genes [7]. Contrary to expectations, clinical studies have shown that chemotherapy drugs and radiotherapy not only induce cell death in BC cells but also stimulate the NF-kB pathway, which stifles caspase cascade signaling by increasing the expression levels of anti-apoptotic proteins [8]. Inhibiting the NF-kB pathway has been shown in some studies to induce apoptosis, repress proliferation and invasion, and boost chemosensitivity in different kinds of tumor cells [9, 10]. Thus, the inhibition of NF-kB might provide an effective strategy for cancer treatment. However, there is a general paucity of data on the prognostic worth of NF-kB among BC patients with no known published data in the African context in which genetic makeup and other contributory factors may affect its expression level. This study, therefore, seeks to evaluate the expression level of NF-kB (p65) among patients with breast cancer at Cape Coast Teaching Hospital (CCTH)-Ghana and its association with clinicopathological features.
Materials and Methods
Study Design, Patients, and Tissue Samples
This cross-sectional study was approved by the Institutional Review Board of CCTH (CCTHERC/EC/2023/060); laboratory work was carried out at Kumasi Center for Collaborative Research (KCCR). Archival formalin-fixed paraffin embedded tissues (FFPE) were retrospectively selected by purposive sampling from breast cancer patient with complete medical history, no history of preoperative chemotherapy treatment and no history of other cancers from January 2019 to March 2023 at CCTH. Clinicopathological information such as age, pathological lymph node status, tumor location, tumor size, histologic type, tumor grade, ki67, perineural invasion, lymphovascular invasion, HER2, hormonal status of ER and PR, were obtained from the patients’ laboratory request form and matched with the corresponding FFPE tissue block number stored at CCTH. All tissues were confirmed with hematoxylin-eosin stain for pathological classification of breast cancer before selection. In all, 90 breast cancer tissues and 15 normal breast tissues obtained from non-breast cancer subjects were selected.
Determination of Expression of NF-kB (p65)
FFPE sectioned into 4 μm and fixed on positively charged slides were deparaffinized and rehydrated with xylene (2 changes for 10 min each) and various grades of ethanol (100%, 90%, and 70% for 3 min each), respectively. Antigens were retrieved by using Novocastra epitome retrieval solution (Leica, Newcastle, UK) diluted buffer at a pH 9.0 for 30 min in a medical decloaking chamber at a temperature of 95°. The tissues were further allowed to cool for 35 min in the buffer before washing with Ventana reaction buffer (Ventana Medical systems, Tucson, AZ, USA) solution for 2 min each. Tissues were then blocked with 3% hydrogen peroxide for 10 min and protein blocker (Biocare Medical, Makati city, Philippines), respectively. Subsequently, they were then incubated for 2 h in NF-kB (p65) rabbit polyclonal antibody IgG (Proteintech, Wuhan, China) diluted in Da Vinci green diluent (Biocare Medical, Makati city, Philippines) at 1:400 dilutions, respectively. Following incubation, tissues were rinsed twice for 3 min each before incubating with MACH 1 Universal horse radish peroxidase (HRP) polymer (Biocare Medical, Makati city, Philippines) for 40 min. Two washes were done for 3 min each and 3,3 diaminobenzidine (DAB) (Biocare Medical, Makati city, Philippines) was added afterward. Mayer’s hematoxylin was then used for counter staining (1–2 min) followed by dehydration with ethanol (70%, 90%, and 100% for 3 min each) and clearing in xylene (2 changes for 5 min each), respectively.
Slides were visualized by two pathologists using a digital microscope (Opto-Edu, Beijing, China), and BC cells showing NF-kB (p65) positive or negative reactions were recorded. The presence of brownish yellow color was considered positive either in the cytoplasm or nucleus or both. The expression of NF-kB (p65) was recorded by using the semi-quantitative method consisting of a combination of the intensity of stain (0 = no staining, 1 = mild stain, 2 = moderate stain, 3 = strong stain) and proportion of the brownish yellow stained cells (0 = negative, 1 < 25%, 2 = 25–50%, 3 = 51–75%, 4 = 76–100%). Normal breast tissues were used as negative controls.
Statistical Analysis
Descriptive and Pearson χ2 analysis was done for all clinicopathological data using SPSS version 25 and presented in tables and figures with p values <0.05 considered significant. Logistic regression analysis was also performed to check the association within the significant groups. Xlstat was used to draw receiver operating characteristic curve.
Results
Patient Characteristics and Immunohistochemical Expression of NF-kB (p65) in Breast Cancer Tissues
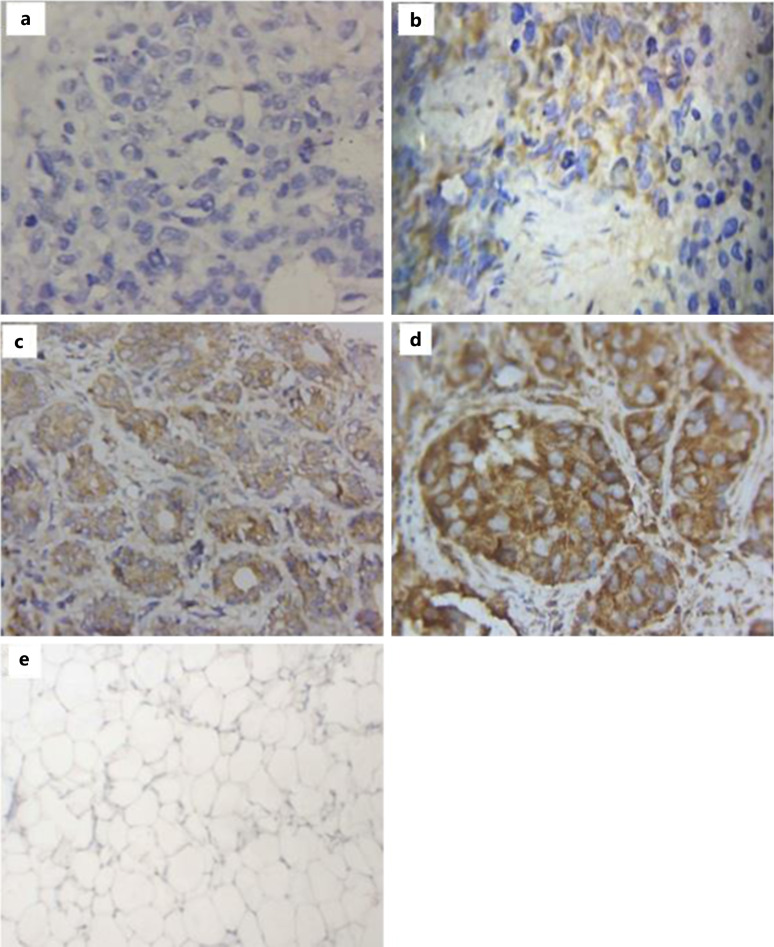
The majority of the 90 patients with breast cancer studied were above 50 years (63.3%). Tumor grades 3, 2 and 1 were 43 (47.8%), 37 (41.1%), and 10 (11.1%), respectively, with invasive carcinoma (no specific type) representing 69 (76.7%) of the study subjects. Majority of the hormonal receptors (estrogen and progesterone) were negative. Sixty-four (71.1%) had high Ki67 (>20) compared to 26 (28.9%) which were low (≤20). Triple-negative 38 (42.2%) were the majority with equal cases of luminal A and luminal B 19 (21.1%), while HER2-enriched was 10 (11.1%) and 4 (4.4%) classified as others (Table 1). Out of the 90 cases, 59 (65.6%) breast cancer tissues expressed high NF-kB (p65) as compared to 31 (34.4%) low expression. The 59 tissues that showed high NF-kB (p65) protein expression consisted of 25 (27.8%) and 34 (37.8%) moderately and strongly stained, respectively, whereas the 31 low expression was made up of 12 (13.3%) negative and 19 (21.1%) mild, respectively, as depicted in Figure 1. NF-kB (p65) immunostaining was seen in either cytoplasm only or both cytoplasm and nucleus of study subjects. Seventy-eight (86.7%) of the 90 breast cancer tissues were positive for NF-kB (p65). Cytoplasm stain alone was 68 (87.2%), both cytoplasm and nuclear stain were 10 (12.8%) and no nuclear stain alone was recorded.
Table 1.
General clinicopathological features of breast cancer cases from CCTH
| Variables | Frequency, N (%) |
|---|---|
| Age group | |
| 0–50 years | 33 (36.70) |
| >50 years | 57 (63.3) |
| Tumor grade | |
| Grade 1 | 10 (11.1) |
| Grade 2 | 37 (41.1) |
| Grade 3 | 43 (47.8) |
| Tumor size | |
| 0–2 cm | 15 (16.7) |
| 2.1–5.0 cm | 46 (51.1) |
| >5 cm | 29 (32.2) |
| Laterality | |
| Left | 59 (65.6) |
| Right | 31 (34.4) |
| Estrogen receptor | |
| Negative | 53 (58.9) |
| Positive | 37 (41.1) |
| Progesterone receptor | |
| Negative | 73 (81.1) |
| Positive | 17 (18.9) |
| HER2 | |
| Negative | 70 (77.8) |
| Positive | 13 (14.4) |
| Equivocal | 7 (7.8) |
| Ki67 | |
| High | 64 (71.1) |
| Low | 26 (28.9) |
| Molecular subtype | |
| Triple-negative | 38 (42.2) |
| Luminal A | 19 (21.1) |
| Luminal B | 19 (21.1) |
| Her2-enriched | 10 (11.1) |
| Others | 4 (4.4) |
| Pathological tumor stage | |
| pT0 | 6 (6.7) |
| pT1 | 13 (14.4) |
| pT2 | 41 (45.6) |
| pT3 | 19 (21.1) |
| pT4 | 11 (12.2) |
| Pathological lymph node stage | |
| pNX | 21 (23.3) |
| pN0 | 21 (23.3) |
| pN1 | 23 (25.6) |
| pN2 | 16 (17.8) |
| pN3 | 9 (10.0) |
| Lymphovascular invasion | |
| Absent | 43 (47.8) |
| Present | 47 (52.2) |
| Perineural invasion | |
| Absent | 78 (86.7) |
| Present | 12 (13.3) |
| Type of breast cancer | |
| Invasive carcinoma (NST) | 69 (76.7) |
| Others | 21 (23.3) |
Fig. 1.
Images of NF-kB (p65) expression level in various invasive carcinoma (no specific type) grade 3 from triple-negative breast cancer tissues. a Tissue showing negative expression. b Tissue showing mild expression. c Tissue showing moderate expression. d Tissue with strong expression. e Normal breast tissue with negative expression. ×400 images.
Association between NF-kB (p65) Status and Histopathological Features
NF-kB (p65) was significantly associated with tumor grade (p = 0.029) with grade 3 having 54.2% high expression followed by grade 2 (40.7%) and grade 1 (5.1%), respectively, (Table 2). There was a moderate odds ratio (Table 4) of expressing high NF-kB (p65) in grade 3 tumors (OR = 6.79, 95% CI [1.49–30.92], p = 0:013). There was no association between NF-kB (p65) expression and age, tumor size, laterality, pathological lymph node stage, pathological tumor stage, lymphovascular invasion, and type of breast carcinoma. NF-kB (p65) was highly expressed among patients with absence of perineural invasion than those with perineural invasion (84.7% vs. 15.3%), but this association was not statistically significant (p = 0.534) (Table 2).
Table 2.
Association between NF-kB (p65) status and histopathological features
| Variables | NF-kB (p65) status | χ2 | p value | ||
|---|---|---|---|---|---|
| total (90), n (%) | high (59), n (%) | low (31), n (%) | |||
| Age group | |||||
| 0–50 years | 33 (36.7) | 22 (37.3) | 11 (35.5) | 0.028 | 1.000 |
| >50 years | 57 (63.3) | 37 (62.7) | 20 (64.5) | ||
| Tumor grade | |||||
| Grade 1 | 10 (11.1) | 3 (5.1) | 7 (22.6) | 7.102 | 0.029 |
| Grade 2 | 37 (41.1) | 24 (40.7) | 13 (41.9) | ||
| Grade 3 | 43 (47.8) | 32 (54.2) | 11 (35.5) | ||
| Tumor size | |||||
| 0–2 cm | 15 (16.7) | 7 (11.9) | 8 (25.8) | 3.043 | 0.218 |
| 2.1–5.0 cm | 46 (51.1) | 31 (52.5) | 15 (48.4) | ||
| >5.0 cm | 29 (32.2) | 21 (35.6) | 8 (25.8) | ||
| Laterality | |||||
| Left | 59 (65.6) | 36 (61.0) | 23 (74.2) | 1.563 | 0.249 |
| Right | 31 (34.4) | 23 (39.0) | 8 (25.8) | ||
| Pathological tumor stage | |||||
| pT0 | 6 (6.7) | 5 (8.5) | 1 (3.2) | 7.302 | 0.121 |
| pT1 | 13 (14.4) | 5 (8.5) | 8 (25.8) | ||
| pT2 | 41 (45.6) | 28 (47.5) | 13 (42.0) | ||
| pT3 | 19 (21.1) | 15 (25.4) | 4 (12.9) | ||
| pT4 | 11 (12.2) | 6 (10.1) | 5 (16.1) | ||
| Pathological lymph node stage | |||||
| pNX | 21 (23.3) | 12 (20.3) | 9 (29.0) | 7.604 | 0.107 |
| pN0 | 21 (23.3) | 15 (25.4) | 6 (19.4) | ||
| pN1 | 23 (25.6) | 12 (20.3) | 11 (35.5) | ||
| pN2 | 16 (17.8) | 11 (18.7) | 5 (16.1) | ||
| pN3 | 9 (10.0) | 9 (15.3) | 0 (0) | ||
| Lymphovascular invasion | |||||
| Absent | 43 (47.8) | 25 (42.4) | 18 (58.1) | 2.006 | 0.186 |
| Present | 47 (52.2) | 34 (57.6) | 13 (41.9) | ||
| Perineural invasion | |||||
| Absent | 78 (86.7) | 50 (84.7) | 28 (90.3) | 0.547 | 0.534 |
| Present | 12 (13.3) | 9 (15.3) | 3 (9.7) | ||
| Type of breast cancer | |||||
| Invasive carcinoma (NST) | 69 (76.7) | 48 (81.4) | 21 (67.8) | 2.106 | 0.191 |
| Others | 21 (23.3) | 11 (18.6) | 10 (32.2) | ||
NST, no specific type; pNX, pathological lymph node stage not assessed; NF-kB, nuclear factor kappa B.
Table 4.
Logistic regression analysis for NF-kB (p65) versus tumor grade, molecular subtypes, and Ki67
| Variables | Total (90), n (%) | High (59), n (%) | Low (31), n (%) | cOR (95% CI) | p value |
|---|---|---|---|---|---|
| Tumor grade | |||||
| Grade 1 | 10 (11.1) | 3 (5.1) | 7 (22.6) | 1 | |
| Grade 2 | 37 (41.1) | 24 (40.7) | 13 (41.9) | 4.31 (0.95–19.53) | 0.058 |
| Grade 3 | 43 (47.8) | 32 (54.2) | 11 (35.5) | 6.79 (1.49–30.92) | 0.013 |
| Molecular subtypes | |||||
| Luminal A | 19 (21.1) | 10 (17.0) | 9 (29.0) | 1 | |
| Luminal B | 19 (21.1) | 15 (25.4) | 4 (13.0) | 3.38 (0.81–14.02) | 0.94 |
| Triple-negative | 38 (42.2) | 29 (49.1) | 9 (29.0) | 2.90 (0.90–9.35) | 0.75 |
| Her2-enriched | 10 (11.1) | 5 (8.5) | 5 (16.0) | 0.90 (0.20–4.17) | 0.893 |
| Others | 4 (4.5) | 0 (0) | 4 (13.0) | 0.00 (0.0) | 0.999 |
| Ki67 | |||||
| Low | 26 (28.9) | 12 (20.3) | 14 (45.2) | 1 | |
| High | 64 (71.1) | 47 (79.7) | 17 (54.8) | 3.23 (1.24–8.34) | 0.016 |
OR, odds ratio; CI, confidence interval.
Association between NF-kB (p65) and Prognostic Molecular Markers
NF-kB (p65) expression was statistically significantly associated with proliferation index Ki67 (p = 0.026) and molecular subtypes (p = 0.009) (Table 3). Patients with high Ki67 > 20 had a significant odds ratio (OR = 3.27, 95% CI [1.28–8.34], p = 0:016) of expressing high NF-kB (p65) (Table 4). In contrast, there was no association between the expression of NF-kB (p65) and estrogen receptors, progesterone receptor, and HER2.
Table 3.
Association between NF-kB (p65) and prognostic molecular markers
| Variables | NF-kB (p65) status | χ2 | p value | ||
|---|---|---|---|---|---|
| total (90), n (%) | high (59), n (%) | low (31), n (%) | |||
| Estrogen receptor | |||||
| Negative | 53 (58.9) | 35 (59.3) | 18 (58.1) | 0.013 | 1.000 |
| Positive | 37 (41.1) | 24 (40.7) | 13 (41.9) | ||
| Progesterone receptor | |||||
| Negative | 73 (81.1) | 47 (79.7) | 26 (83.9) | 0.235 | 0.779 |
| Positive | 17 (18.9) | 12 (20.3) | 5 (16.1) | ||
| HER2 | |||||
| Negative | 70 (77.8) | 48 (81.4) | 22 (71.0) | 1.972 | 0.373 |
| Positive | 13 (14.4) | 8 (13.6) | 5 (16.1) | ||
| Equivocal | 7 (7.8) | 3 (5.0) | 4 (12.9) | ||
| Ki67 | |||||
| High | 64 (71.1) | 47 (79.7) | 17 (54.8) | 6.095 | 0.026 |
| Low | 26 (28.9) | 12 (20.3) | 14 (45.2) | ||
| Molecular subtype | |||||
| Luminal A | 19 (21.1) | 10 (17.0) | 9 (29.0) | 13.548 | 0.009 |
| Luminal B | 19 (21.1) | 15 (25.4) | 4 (13.0) | ||
| Triple-negative | 38 (42.2) | 29 (49.1) | 9 (29.0) | ||
| Her2-enriched | 10 (11.1) | 5 (8.5) | 5 (16.0) | ||
| Others | 4 (4.5) | 0 (0) | 4 (13.0) | ||
Diagnostic Performance of NF-kB (p65) among the Molecular Subtypes Breast Cancer Patients
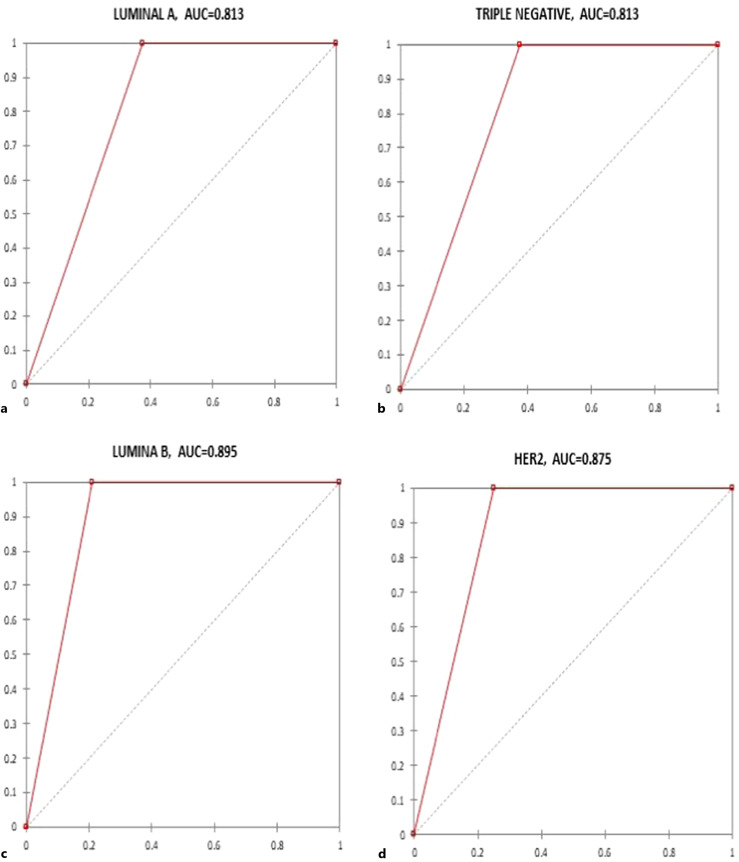
To determine the diagnostic performance of NF-kB(p65) among the molecular subtypes of breast cancer patients in Cape Coast Teaching Hospital, receiver operating characteristic curves were drawn with Xlstat, and the corresponding area under the curve, sensitivity, specificity, positive predictive value, and negative predictive value were determined as shown in Figure 2 and Table 5, respectively. Overall luminal B had a better area under the curve of 0.89, sensitivity 1.00, specificity 0.79, positive predictive value 0.79, and negative predictive value 1.00 (Table 5).
Fig. 2.
Graphical representation of the receiver operating characteristic (ROC) curve of NF-kB (p65) marker among the molecular subtypes of breast cancer patients in Cape Coast Teaching Hospital. a Luminal A. b Triple-negative. c Luminal B. d Her2.
Table 5.
The diagnostic performance of NF-kB (p65) marker among the molecular subtypes of breast cancer patients in CCTH
| Molecular subtype | AUC | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Luminal A | 0.81 (0.71–0.91) | 1.00 (0.67–1.00) | 0.63 (0.43–0.79) | 0.54 (0.30–0.75) | 1.000 |
| Triple-negative | 0.81 (0.71–0.91) | 1.00 (0.86–1.00) | 0.63 (0.43–0.79) | 0.76 (0.63–0.90) | 1.000 |
| Luminal B | 0.89 (0.801–0.99) | 1.00 (0.76–1.00) | 0.79 (0.56–0.92) | 0.79 (0.60–0.97) | 1.000 |
| Her2 | 0.87 (0.78–0.97) | 1.00 (0.51–1.00) | 0.75 (0.53–0.89) | 0.50 (0.19–0.81) | 1.000 |
AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value.
Discussion
This study aimed to assess the prognostic significance of NF-kB (p65) expression and its association with various clinicopathological features in breast cancer patients at Cape Coast Teaching Hospital. The study found that NF-kB (p65) was expressed in 86.7% of breast cancer tissues. There was a significant relationship between NF-kB (p65) expression and tumor grade, proliferation index (Ki67), and molecular subtype. High expression of NF-kB (p65) was more common in tumor grade 3 compared to grade 1, and Ki67 > 20 had higher expression of NF-kB (p65) compared to Ki67 ≤ 20. Triple-negative breast cancer patients had the highest overexpression of NF-kB (p65) compared to other molecular subtypes. Based on histopathological features of this study, the average age of 54.3 ± 13.9 years follows similar trends in other studies in Ghana [11, 12]. In contrast, investigations carried out in Nigeria [13] and Uganda [14] had lower than 50 years mean ages, respectively. Invasive carcinoma (no specific type) representing 76.7% of this current research work was the commonest just like other literature reports [12, 13, 15]. Pathological lymph node stage 3 was the least observed which is similar to a previous [7]. Research on breast cancer has demonstrated that vascular (lymphovascular or perineural) invasion form a significant part of the overall outcome of the disease. In this study, 65.5% of BC patients had vascular invasion which is comparable to another study in Ghana with 70.6% [15] in contrast to a 2013 report of 5.1% [16]. These data emphasize the dismal prognosis for BC among women in Ghana.
NF-kB (p65) was initially recognized as an important factor in the initiation of immune responses, but it is now being investigated as a potential prognostic marker and a target for cancer treatment. There is paucity of data on the prognostic relevance of NF-kB (p65). Currently, there are no known published data on the level of Nuclear Factor kappa B expression in breast cancer patients from the African population, of which Ghana is a part. NF-kB (p65) functions as a transcription mediator for target genes associated with carcinogenesis. It also causes resistance to growth inhibitor signals, unregulated proliferation, angiogenesis, metastasis, and a decrease in apoptosis, these being typical cancer hallmarks [5, 6, 17]. Nevertheless, multiple factors influence NF-kB (p65) activation, including the expression of various proteins which regulate its functionality [5, 6]. This current study in CCTH, Ghana showed that NF-kB (p65) was overexpressed in breast cancer tissues as determined by immunostaining. A majority of the patients showed only cytoplasmic staining (87.2%), while 12.8% of the patients had both cytoplasmic and nuclear staining for NF-kB (p65).
Literature on the relationship between NF-kB (p65) expression and clinicopathological aspects of breast cancer is contradictory [17]; moreover, there are no data on this from Africa. An earlier study by Montagut et al. [18], linked nuclear NF-kB (p65) staining to chemoresistance, Baba et al. [19], found no established association between nuclear expression of NF-kB (p65) and response to chemotherapy or prognosis. Baba et al. [19], 2016 again suggested that almost all the 33 triple-negative tumors studied had cytoplasmic expression of NF-kB (p65) before treatment and that tumors with strong cytoplasmic expression of NF-kB (p65) responded successfully and became free of the disease after initiation of chemotherapy. However, a recent study suggested nuclear NF-kB (p65) status as a prognostic marker for different chemotherapy regimens [20]. Another recent study found that breast cancer cases with low nuclear NF-kB (p65) level typically respond more effectively to chemotherapy compared to individuals with high expression level of NF-kB (p65) [21]. The significance of cytoplasmic NF-kB (p65) is unknown, although it is thought that there is a link between increased NF-kB (p65) expression and the molecular modifications that give rise to its activation; deactivation of NF-kB (p65) is believed to retain it in the cytoplasm, which contributes to an improved outcome in especially triple-negative breast cancer patients [17].
Tumor grading has been shown to have predictive and prognostic significance in breast cancer patients in the initial 5 years after diagnosis. Additionally, a significant link was observed between histological tumor grading, pathological response and survival rate in breast cancer patients with tumor grade 3 and 2 having a poorer outcome compared to tumor grade 1 [22, 23]. NF-kB (p65) expression in this current study was reported to be significantly associated with tumor grade. This finding substantiates earlier reports on the association between NF-kB (p65) expression level and tumor grade [17, 24]. On the contrary, some researchers have reported that neither cytoplasmic nor nuclear NF-kB (p65) expression was found to relate to tumor grade [7, 25]. The current study provides evidence of the aggressive role of NF-kB (p65) in breast cancer patients in Ghana and its potential impact on disease progression.
There was no statistically significant relationship between NF-kB (p65) expression and histological type, age group, tumor size, lymphovascular invasion, perineural invasion, pathological lymph node stage, pathological tumor stage, or laterality in this study. A previous study also discovered no link between cytoplasmic or nuclear NF-kB (p65) expression and histological type, tumor size, age group, pathological lymph node stage, pathological tumor stage, or laterality [7, 26]. However, others have reported a significant link between cytoplasmic expression of NF-kB (p65) and tumor size [17, 24].
In support of various studies conducted, our study also did found no association between hormonal estrogen or progesterone receptors and NF-kB (p65) status [7, 17]. In contrast, other studies reported a significant relationship between hormonal receptors and NF-kB (p65) expression [20, 24, 25].
HER2 belongs to the receptor tyrosine kinases (RTK) family and regulates cell growth, differentiation and survival. Approximately 15–20% of breast cancer patients express high level of HER2 and are classified as HER2 positive. These tumors typically grow rapidly and have a poor prognosis [27]. A similar trend was seen in our study; 14.4% of breast cancer patients were HER2 positive. NF-kB (p65) was highly expressed in HER2 negative tissues (81.4%) compared to HER2 positive (13.6%) in this study. Although not statistically significant, this is consistent with other reports which did not find any link between NF-kB (p65) status and HER2 expression [7, 17]. Gershtein et al. [26], (2010) observed that HER2 has a significant link with NF-kB (p65). This inconsistency requires further probe among breast cancer researchers.
Detection of Ki67 is performed to assess the proliferation status of the tumor although its clinical utility is limited. In this study, Ki67 was significantly associated with NF-kB (p65) which is similar to studies conducted in India [24] and the UK [25]. Moreover, studies in Poland [7] and Kuwait [17], however, did not observe any strong link between NF-kB (p65) and Ki67.
In this study, NF-kB (p65) was significantly associated with molecular subtypes of our patients with patients with triple-negative breast cancer patients having the highest NF-kB (p65) expression level (49.1%). Triple-negative cases are known to be common among West African women with a poorer prognosis and high mortality [28]. Based on our study we suggest that NF-kB (p65) regulation could be a potential contributory factor for its aggressiveness. The activated NF-kB pathway in cancer cells has been shown to promote resistance to chemotherapeutics and ionizing radiation, while its inhibition strongly upregulates the responsiveness of cancer cells to these agents [29]. This is a preliminary study hence the small sample size used resulted in smaller patient samples with grade one breast cancer, further investigation will be conducted in a longitudinal manner with a larger sample size.
Conclusions
This study on the prognostic significance of NF-kB (p65) among breast cancer patients in Ghana showed that NF-kB (p65) is highly expressed, especially in TNBC patients. This high expression is also linked to tumor grade, proliferation index (Ki67) and molecular subtypes and, therefore, suggest its involvement in breast cancer progression. NF-kB (p65) could be a biomarker for breast cancer stage, progression, and prognosis. However, the number of patient samples classified as tumor grade 1 in this current study was few. Further studies with a larger sample size combined with in vitro experiments are needed to fully clarify the role and relationship of NF-kB (p65) expression in breast cancer progression to evaluate it as a possible prognostic marker and a potential therapeutic target.
Statement of Ethics
This study was approved by the Cape Coast Teaching Hospital Institutional Review Board, Cape Coast, Ghana (clearance number CCTHERC/EC/2023/060).
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Funding Sources
This study received funding from Directorate of Research, Innovation, and Consultancy (RSG/GRP/CoHAS/2022/104), University of Cape Coast and Samuel and Emelia Brew-Butler/School of Graduate Studies research grant, University of Cape Coast.
Author Contributions
Conceptualization: Roland Osei Saahene. Study design: Precious Barnes, Leonard Derkyi-Kwarteng, and Roland Osei Saahene. Funding acquisition: Precious Barnes, Leonard Derkyi-Kwarteng, Benjamin Amoani, George Adjei, and Roland Osei Saahene. Manuscript writing and editing: Precious Barnes, Abraham Mensah, Leonard Derkyi-Kwarteng, Ernest Adankwa, Elvis Agbo, Benjamin Amoani, Patrick Kafui Akakpo, Faustina Halm-Lai, Kwabena Dankwa, Daniel Amoako-Sakyi, Samuel Victor Nuvor, and Dorcas Obiri-Yeboah. Literature review: Abraham Mensah, Elvis Agbo, Benjamin Amoani, Kwabena Dankwa, Daniel Amoako-Sakyi, Samuel Victor Nuvor, and Dorcas Obiri-Yeboah. Laboratory investigations: Abraham Mensah, Ernest Adankwa, and Samuel Mingyigilougu Apewe Ka-Chungu. Data collection: Ewura Seidu Yahaya, George Adjei, and Roland Osei Saahene. Data Analysis: Precious Barnes, Abraham Mensah, Ernest Adankwa, Elvis Agbo, Ewura Seidu Yahaya, George Adjei, Samuel Mingyigilougu Apewe Ka-Chungu, and Roland Osei Saahene. Data interpretation: Patrick Kafui Akakpo, Faustina Halm-Lai, Kwabena Dankwa, Daniel Amoako-Sakyi, Samuel Victor Nuvor, and Dorcas Obiri-Yeboah. Manuscript preparation and review: Ewura Seidu Yahaya, George Adjei, Samuel Mingyigilougu Apewe Ka-Chungu, and Roland Osei Saahene.
Funding Statement
This study received funding from Directorate of Research, Innovation, and Consultancy (RSG/GRP/CoHAS/2022/104), University of Cape Coast and Samuel and Emelia Brew-Butler/School of Graduate Studies research grant, University of Cape Coast.
Data Availability Statement
All relevant data have been included in this report.
References
- 1. Iacoviello L, Bonaccio M, de Gaetano G, Donati MB. Epidemiology of breast cancer, a paradigm of the “common soil” hypothesis. Semin Cancer Biol. 2021;72:4–10. [DOI] [PubMed] [Google Scholar]
- 2. Britt KL, Cuzick J, Phillips K-A. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20(8):417–36. [DOI] [PubMed] [Google Scholar]
- 3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 4. Adeloye D, Bowman K, Chan KY, Patel S, Campbell H, Rudan I. Global and regional child deaths due to injuries: an assessment of the evidence. J Glob Health. 2018;8(2):021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Concetti J, Wilson CL. NFKB1 and cancer: friend or foe? Cells. 2018;7(9):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu J, Li Y, Chen C, Ma J, Sun W, Tian Z, et al. NF-κB p65 overexpression promotes bladder cancer cell migration via FBW7-mediated degradation of RhoGDIα protein. Neoplasia. 2017;19(9):672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agrawal AK, Pielka E, Lipinski A, Jelen M, Kielan W, Agrawal S. Clinical validation of nuclear factor kappa B expression in invasive breast cancer. Tumour Biol. 2018;40(1):1010428317750929. [DOI] [PubMed] [Google Scholar]
- 8. Zeng A, Liang X, Zhu S, Liu C, Luo X, Zhang Q, et al. Baicalin, a potent inhibitor of NF-κB signaling pathway, enhances chemosensitivity of breast cancer cells to docetaxel and inhibits tumor growth and metastasis both in vitro and in vivo. Front Pharmacol. 2020;11:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Capece D, Verzella D, Di Francesco B, Alesse E, Franzoso G, Zazzeroni F. NF-κB and mitochondria cross paths in cancer: mitochondrial metabolism and beyond. Semin Cell Dev Biol. 2020;98:118–28. [DOI] [PubMed] [Google Scholar]
- 10. Kani K, Momota Y, Harada M, Yamamura Y, Aota K, Yamanoi T, et al. γ-tocotrienol enhances the chemosensitivity of human oral cancer cells to docetaxel through the downregulation of the expression of NF-κB-regulated anti-apoptotic gene products. Int J Oncol. 2013;42(1):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Der EM, Gyasi RK, Tettey Y, Edusei L, Bayor MT, Jiagge E, et al. Triple-negative breast cancer in Ghanaian women: the Korle Bu teaching hospital experience. Breast J. 2015;21(6):627–33. [DOI] [PubMed] [Google Scholar]
- 12. Ohene-Yeboah M, Adjei E. Breast cancer in kumasi, Ghana. Ghana Med J. 2012;46(1):8–13. [PMC free article] [PubMed] [Google Scholar]
- 13. Ngwogu K, Offiah S, Ngwogu A, Ndubuka G, Ekperi O. Prevalence and histopathological pattern of breast cancer among patients at Abia state university teaching hospital, aba, south eastern Nigeria. Int J Basic Appl Innovat Res. 2017;6(4):100–6. [Google Scholar]
- 14. Galukande M, Wabinga H, Mirembe F, Karamagi C, Asea A. Molecular breast cancer subtypes prevalence in an indigenous Sub Saharan African population. Pan Afr Med J. 2014;17:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Derkyi-Kwarteng L, Agyemang-Yeboah F, Ahenkorah Fondjo L, Gustav Imbeah E, Kafui Akakpo P. Clinicohistologic characteristics of breast cancer in Ghanaian patients. Ann Pathol Lab Med. 2020;7(8):A385–393. [Google Scholar]
- 16. Edmund DM, Naaeder SB, Tettey Y, Gyasi RK. Breast cancer in Ghanaian women: what has changed? Am J Clin Pathol. 2013;140(1):97–102. [DOI] [PubMed] [Google Scholar]
- 17. Al-Mutairi MS, Habashy HO. Nuclear factor-κb clinical significance in breast cancer: an immunohistochemical study. Med Princ Pract. 2023;32(1):33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montagut C, Tusquets I, Ferrer B, Corominas J, Bellosillo B, Campas C, et al. Activation of nuclear factor-kappa B is linked to resistance to neoadjuvant chemotherapy in breast cancer patients. Endocr Relat Cancer. 2006;13(2):607–16. [DOI] [PubMed] [Google Scholar]
- 19. Baba M, Takahashi M, Yamashiro K, Yokoo H, Fukai M, Sato M, et al. Strong cytoplasmic expression of NF-κB/p65 correlates with a good prognosis in patients with triple-negative breast cancer. Surg Today. 2016;46(7):843–51. [DOI] [PubMed] [Google Scholar]
- 20. Manginstar C, Manginstar C, Islam AA, Sampepajung D, Hamdani W, Bukhari A, et al. The relationship between NFKB, HER2, ER expression and anthracycline-based neoadjuvan chemotherapy response in local advanced stadium breast cancer: a cohort study in Eastern Indonesia. Ann Med Surg. 2021;63:102164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sampepajung E, Hamdani W, Sampepajung D, Prihantono P. Overexpression of NF-kB as a predictor of neoadjuvant chemotherapy response in breast cancer. Breast Dis. 2021;40(s1):S45–S53. [DOI] [PubMed] [Google Scholar]
- 22. Engstrøm MJ, Opdahl S, Hagen AI, Romundstad PR, Akslen LA, Haugen OA, et al. Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res Treat. 2013;140(3):463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12(5):320–7. [DOI] [PubMed] [Google Scholar]
- 24. Jana D, Das S, Sarkar DK, Mandal S, Maji A, Mukhopadhyay M. Role of nuclear factor-κB in female breast cancer: a study in Indian patients. Asian Pac J Cancer Prev. 2012;13(11):5511–5. [DOI] [PubMed] [Google Scholar]
- 25. Tannahill C, Obondo C, Al-Murri A, Doughty J, Lannigan A, Wilson C, et al. The relationship between tumour NF-kB expression, hormone status, and clinicopathological factors in primary invasive breast cancer. Cancer Res. 2009;69(2_Suppl):4038. [Google Scholar]
- 26. Gershtein E, Scherbakov A, Platova A, Tchemeris GY, Letyagin V, Kushlinskii N. The expression and DNA-binding activity of NF-κB nuclear transcription factor in the tumors of patients with breast cancer. Bull Exp Biol Med. 2010;150(1):71–4. [DOI] [PubMed] [Google Scholar]
- 27. Saini KS, Azim HA Jr, Metzger-Filho O, Loi S, Sotiriou C, de Azambuja E, et al. Beyond trastuzumab: new treatment options for HER2-positive breast cancer. Breast. 2011;20(Suppl 3):S20–7. [DOI] [PubMed] [Google Scholar]
- 28. Hercules SM, Alnajar M, Chen C, Mladjenovic SM, Shipeolu BA, Perkovic O, et al. Triple-negative breast cancer prevalence in Africa: a systematic review and meta-analysis. BMJ open. 2022;12(5):e055735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luo C, Zhang H. The role of proinflammatory pathways in the pathogenesis of colitis-associated colorectal cancer. Mediators Inflamm. 2017;2017:5126048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data have been included in this report.