Abstract
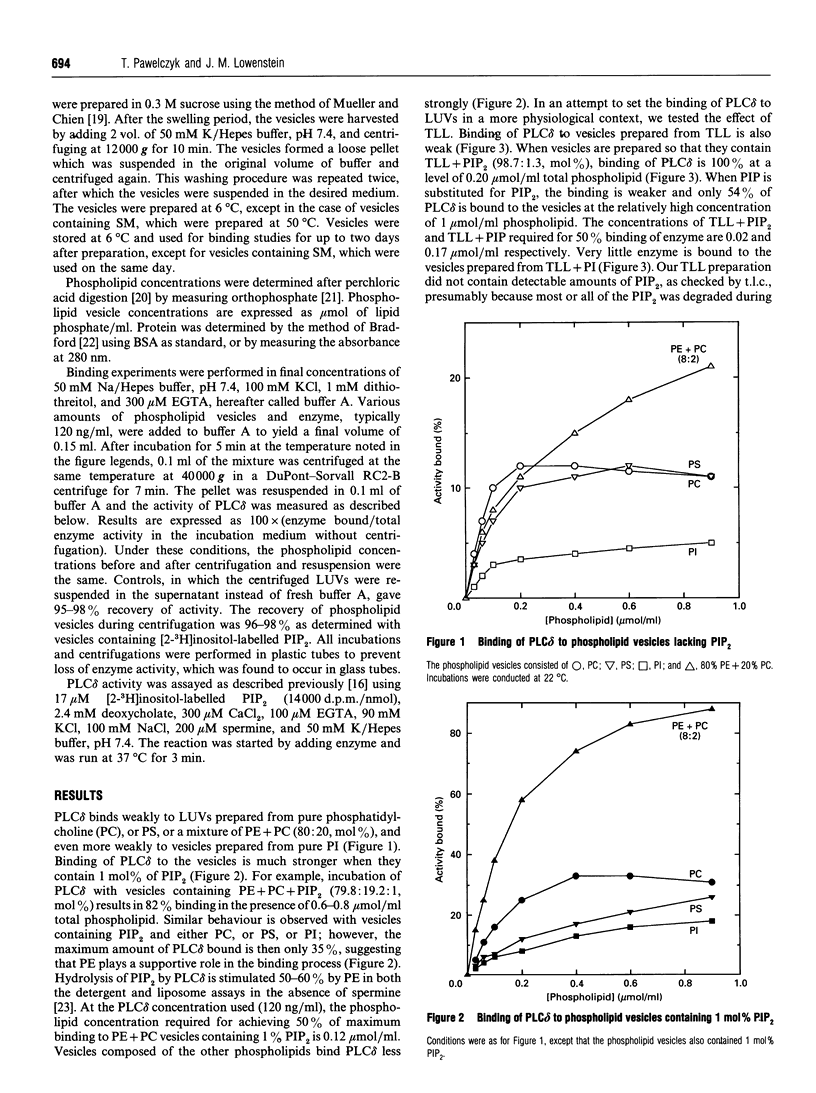
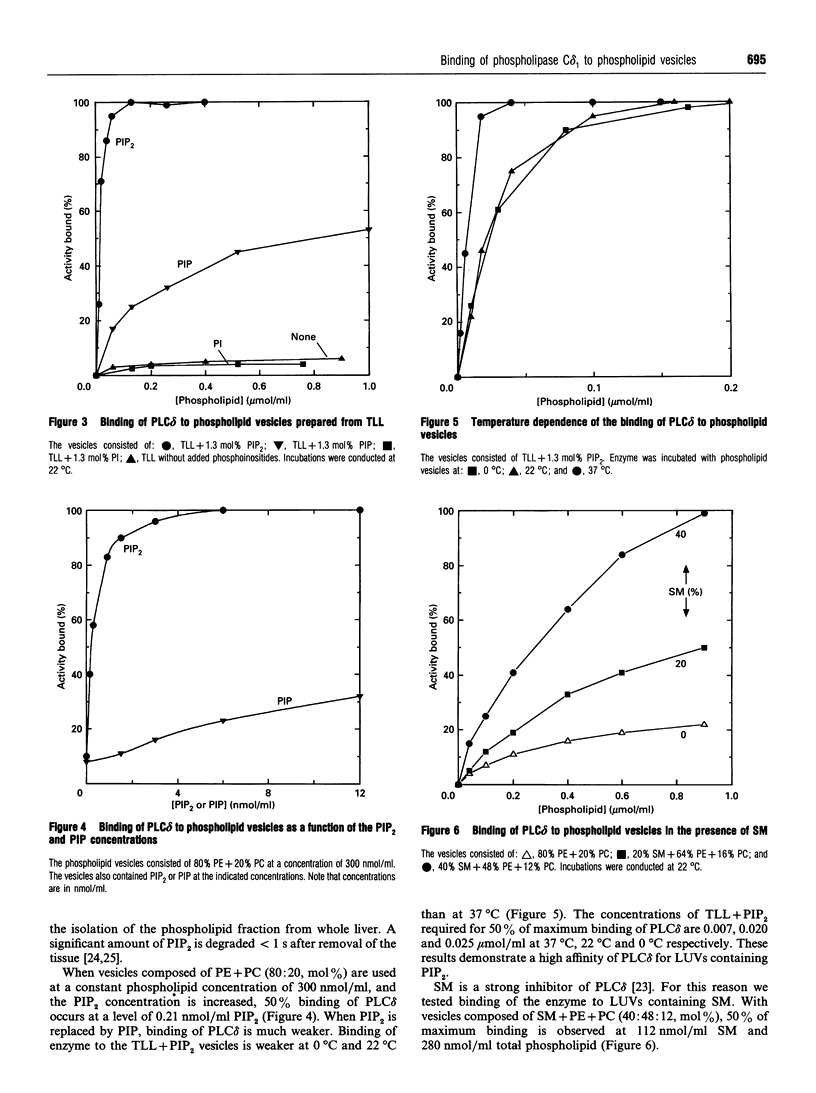
Binding of phospholipase C delta 1 (PLC delta) to phospholipid vesicles was studied using large, unilamellar phospholipid vesicles (LUVs). PLC delta bound weakly to vesicles composed of phosphatidylserine (PS) or phosphatidylcholine (PC) or phosphatidylethanolamine (PE) + PC, and even more weakly to vesicles composed of phosphatidylinositol. The enzyme bound strongly to LUVs composed of PE + PC and phosphatidylinositol 4,5-bisphosphate (PIP2) or sphingomyelin (SM). Binding of 50% of PLC delta occurred at 0.25 nmol/ml PIP2 when LUVs composed of PE + PC (molar ratio of 80:20), plus various amounts of PIP2, were used at a constant phospholipid concentration of 300 nmol/ml. When LUVs composed of PE + PC + PIP2 (molar ratio of 79:20:1) were tested as a function of increasing phospholipid concentration, 50% binding of PLC delta occurred at 1.2 nmol/ml PIP2 and 120 nmol/ml total phospholipid. Similar measurements were conducted with other phospholipids and PIP2 at a molar ratio of 99:1. These showed that 50% binding of PLC delta occurred at a level of 0.9 nmol/ml PIP2 with 80 nmol/ml PC; at 2.2 nmol/ml PIP2 with 170 nmol/ml PS; at 4.2 nmol/ml PIP2 with 320 nmol/ml PI; and at 0.26 nmol/ml PIP2 with 20 nmol/ml total liver phospholipids. Binding to phosphatidylinositol 4-phosphate was much weaker. When LUVs composed of PE + PC + SM (molar ratio 48:12:40) were tested as a function of increasing phospholipid concentration, 50% binding of PLC delta occurred at a level of 96 nmol/ml SM. This is well below the concentration of SM that can be calculated to face the cytosol. Binding of PLC delta to LUVs decreased as the temperature was lowered from 37 degrees C to 0 degree C. Thus PLC delta shows a high degree of specificity for binding to PIP2 and SM. Under physiological conditions a considerable fraction of PLC delta may be bound to cellular membranes, either in an inactive form if bound to PIP2 at low resting Ca2+ concentrations, or in the inhibited form if bound to SM.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banno Y., Yada Y., Nozawa Y. Purification and characterization of membrane-bound phospholipase C specific for phosphoinositides from human platelets. J Biol Chem. 1988 Aug 15;263(23):11459–11465. [PubMed] [Google Scholar]
- Barenholz Y., Thompson T. E. Sphingomyelins in bilayers and biological membranes. Biochim Biophys Acta. 1980 Sep 30;604(2):129–158. doi: 10.1016/0005-2736(80)90572-6. [DOI] [PubMed] [Google Scholar]
- Bennett C. F., Crooke S. T. Purification and characterization of a phosphoinositide-specific phospholipase C from guinea pig uterus. Phosphorylation by protein kinase C in vivo. J Biol Chem. 1987 Oct 5;262(28):13789–13797. [PubMed] [Google Scholar]
- Boegheim J. P., Jr, Van Linde M., Op den Kamp J. A., Roelofsen B. The sphingomyelin pools in the outer and inner layer of the human erythrocyte membrane are composed of different molecular species. Biochim Biophys Acta. 1983 Nov 23;735(3):438–442. doi: 10.1016/0005-2736(83)90160-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dennis E. A., Rhee S. G., Billah M. M., Hannun Y. A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991 Apr;5(7):2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Emori Y., Homma Y., Sorimachi H., Kawasaki H., Nakanishi O., Suzuki K., Takenawa T. A second type of rat phosphoinositide-specific phospholipase C containing a src-related sequence not essential for phosphoinositide-hydrolyzing activity. J Biol Chem. 1989 Dec 25;264(36):21885–21890. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fukui T., Lutz R. J., Lowenstein J. M. Purification of a phospholipase C from rat liver cytosol that acts on phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 4-phosphate. J Biol Chem. 1988 Nov 25;263(33):17730–17737. [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Baldassare J. J., Pollard T. D. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science. 1990 Mar 30;247(4950):1575–1578. doi: 10.1126/science.2157283. [DOI] [PubMed] [Google Scholar]
- Jolles J., Zwiers H., Dekker A., Wirtz K. W., Gispen W. H. Corticotropin-(1--24)-tetracosapeptide affects protein phosphorylation and polyphosphoinositide metabolism in rat brain. Biochem J. 1981 Jan 15;194(1):283–291. doi: 10.1042/bj1940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M., Parker P. J. Purification of phosphoinositide-specific phospholipase C from a particulate fraction of bovine brain. Eur J Biochem. 1987 Oct 15;168(2):413–418. doi: 10.1111/j.1432-1033.1987.tb13435.x. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Sim S. S., Kim U. H., Nishibe S., Wahl M. I., Carpenter G., Rhee S. G. Tyrosine residues in bovine phospholipase C-gamma phosphorylated by the epidermal growth factor receptor in vitro. J Biol Chem. 1990 Mar 5;265(7):3940–3943. [PubMed] [Google Scholar]
- Kim M. Y., Linardic C., Obeid L., Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991 Jan 5;266(1):484–489. [PubMed] [Google Scholar]
- Lange Y., Swaisgood M. H., Ramos B. V., Steck T. L. Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J Biol Chem. 1989 Mar 5;264(7):3786–3793. [PubMed] [Google Scholar]
- Lee K. Y., Ryu S. H., Suh P. G., Choi W. C., Rhee S. G. Phospholipase C associated with particulate fractions of bovine brain. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5540–5544. doi: 10.1073/pnas.84.16.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Mueller P., Chien T. F., Rudy B. Formation and properties of cell-size lipid bilayer vesicles. Biophys J. 1983 Dec;44(3):375–381. doi: 10.1016/S0006-3495(83)84311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Rhee S. G., Carpenter G. Tyrosine phosphorylation of phospholipase C-II in vitro by the epidermal growth factor receptor. J Biol Chem. 1989 Jun 25;264(18):10335–10338. [PubMed] [Google Scholar]
- Nishihara M., Keenan R. W. Inositol phospholipid levels of rat forebrain obtained by freeze-blowing method. Biochim Biophys Acta. 1985 Jul 9;835(2):415–418. doi: 10.1016/0005-2760(85)90300-5. [DOI] [PubMed] [Google Scholar]
- Pawelczyk T., Lowenstein J. M. Regulation of phospholipase C delta activity by sphingomyelin and sphingosine. Arch Biochem Biophys. 1992 Sep;297(2):328–333. doi: 10.1016/0003-9861(92)90680-u. [DOI] [PubMed] [Google Scholar]
- Rath H. M., Fee J. A., Rhee S. G., Silbert D. F. Characterization of phosphatidylinositol-specific phospholipase C defects associated with thrombin-induced mitogenesis. J Biol Chem. 1990 Feb 25;265(6):3080–3087. [PubMed] [Google Scholar]
- Rawyler A. J., Roelofsen B., Op den Kamp J. A., Van Deenen L. L. Isolation and characterization of plasma membranes from Friend erythroleukaemic cells. A study with sphingomyelinase C. Biochim Biophys Acta. 1983 Apr 21;730(1):130–138. doi: 10.1016/0005-2736(83)90325-5. [DOI] [PubMed] [Google Scholar]
- Rhee S. G., Suh P. G., Ryu S. H., Lee S. Y. Studies of inositol phospholipid-specific phospholipase C. Science. 1989 May 5;244(4904):546–550. doi: 10.1126/science.2541501. [DOI] [PubMed] [Google Scholar]
- Sanui H. Measurement of inorganic orthophosphate in biological materials: extraction properties of butyl acetate. Anal Biochem. 1974 Aug;60(2):489–504. doi: 10.1016/0003-2697(74)90259-0. [DOI] [PubMed] [Google Scholar]
- Soukup J. F., Friedel R. O., Shanberg S. M. Microwave irradiation fixation for studies of polyphosphoinositide metabolism in brain. J Neurochem. 1978 Mar;30(3):635–637. doi: 10.1111/j.1471-4159.1978.tb07819.x. [DOI] [PubMed] [Google Scholar]
- Stahl M. L., Ferenz C. R., Kelleher K. L., Kriz R. W., Knopf J. L. Sequence similarity of phospholipase C with the non-catalytic region of src. Nature. 1988 Mar 17;332(6161):269–272. doi: 10.1038/332269a0. [DOI] [PubMed] [Google Scholar]
- Todderud G., Wahl M. I., Rhee S. G., Carpenter G. Stimulation of phospholipase C-gamma 1 membrane association by epidermal growth factor. Science. 1990 Jul 20;249(4966):296–298. doi: 10.1126/science.2374928. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]


