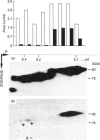
Abstract
Vitamin K-dependent coagulation factors undergo several post-translational modifications before the proteins are secreted into the blood as functional zymogens of the coagulation system. The modifications include Asn-linked glycosylation, Asn/Asp hydroxylation, removal of a signal peptide for translocation of the polypeptide into the endoplasmic reticulum and removal of a propeptide which, when attached to the intracellular coagulation factor precursor, directs the protein for vitamin K-dependent gamma-carboxylation. gamma-Carboxylation of targeted Glu residues results in formation of Ca(2+)-binding gamma-carboxyglutamic acid (Gla) residues. Ca2+ binding by these residues induces a conformational change in the protein which is a necessary event for optimal activation or activity of the clotting factor in blood. In the present study we have monitored the intracellular prothrombin precursor in the secretory pathway of liver cells to determine the effect that the propeptide has on Ca(2+)-dependent folding of the protein. The data provide evidence that the Ca(2+)-induced conformational change required for activation of prothrombin coincides with release of the propeptide in the trans-Golgi apparatus of the liver cell and elucidates an important function for the endoproteinase furin in biosynthesis of vitamin K-dependent clotting factors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borowski M., Furie B. C., Bauminger S., Furie B. Prothrombin requires two sequential metal-dependent conformational transitions to bind phospholipid. Conformation-specific antibodies directed against the phospholipid-binding site on prothrombin. J Biol Chem. 1986 Nov 15;261(32):14969–14975. [PubMed] [Google Scholar]
- Diuguid D. L., Rabiet M. J., Furie B. C., Liebman H. A., Furie B. Molecular basis of hemophilia B: a defective enzyme due to an unprocessed propeptide is caused by a point mutation in the factor IX precursor. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5803–5807. doi: 10.1073/pnas.83.16.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernlund P., Stenflo J., Roepstorff P., Thomsen J. Vitamin K and the biosynthesis of prothrombin. V. Gamma-carboxyglutamic acids, the vitamin K-dependent structures in prothrombin. J Biol Chem. 1975 Aug 10;250(15):6125–6133. [PubMed] [Google Scholar]
- Furie B., Furie B. C. Molecular basis of vitamin K-dependent gamma-carboxylation. Blood. 1990 May 1;75(9):1753–1762. [PubMed] [Google Scholar]
- Furie B., Furie B. C. The molecular basis of blood coagulation. Cell. 1988 May 20;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Krause K. H. Ca(2+)-storage organelles. FEBS Lett. 1991 Jul 22;285(2):225–229. doi: 10.1016/0014-5793(91)80806-e. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg I. The new eukaryotic precursor processing proteinases. Mol Endocrinol. 1991 Oct;5(10):1361–1365. doi: 10.1210/mend-5-10-1361. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Oda K., Fujiwara T., Takami N., Tashiro K., Ikehara Y. Functional expression of furin demonstrating its intracellular localization and endoprotease activity for processing of proalbumin and complement pro-C3. J Biol Chem. 1991 Sep 5;266(25):16954–16959. [PubMed] [Google Scholar]
- Nelsestuen G. L. Role of gamma-carboxyglutamic acid. An unusual protein transition required for the calcium-dependent binding of prothrombin to phospholipid. J Biol Chem. 1976 Sep 25;251(18):5648–5656. [PubMed] [Google Scholar]
- Nelsestuen G. L., Suttie J. W. The purification and properties of an abnormal prothrombin protein produced by dicumarol-treated cows. A comparison to normal prothrombin. J Biol Chem. 1972 Dec 25;247(24):8176–8182. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rehemtulla A., Kaufman R. J. Preferred sequence requirements for cleavage of pro-von Willebrand factor by propeptide-processing enzymes. Blood. 1992 May 1;79(9):2349–2355. [PubMed] [Google Scholar]
- Stanton C., Taylor R., Wallin R. Processing of prothrombin in the secretory pathway. Biochem J. 1991 Jul 1;277(Pt 1):59–65. doi: 10.1042/bj2770059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton C., Wallin R. Processing and trafficking of clotting factor X in the secretory pathway. Effects of warfarin. Biochem J. 1992 May 15;284(Pt 1):25–31. doi: 10.1042/bj2840025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. C., Suttie J. W. Vitamin K dependent in vitro production of prothrombin. Biochemistry. 1982 Nov 9;21(23):6011–6018. doi: 10.1021/bi00266a044. [DOI] [PubMed] [Google Scholar]
- Wallin R., Culp E. N., Coleman D. B., Goodman S. R. A structural model of human erythrocyte band 2.1: alignment of chemical and functional domains. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4095–4099. doi: 10.1073/pnas.81.13.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin R., Turner R. Propeptide recognition by the vitamin K-dependent carboxylase in early processing of prothrombin and factor X. Biochem J. 1990 Dec 1;272(2):473–478. doi: 10.1042/bj2720473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J., Diuguid D. L., Liebman H. A., Rabiet M. J., Kasper C. K., Furie B. C., Furie B., Stafford D. W. Factor IX San Dimas. Substitution of glutamine for Arg-4 in the propeptide leads to incomplete gamma-carboxylation and altered phospholipid binding properties. J Biol Chem. 1989 Jul 5;264(19):11401–11406. [PubMed] [Google Scholar]
- Welsch D. J., Nelsestuen G. L. Amino-terminal alanine functions in a calcium-specific process essential for membrane binding by prothrombin fragment 1. Biochemistry. 1988 Jun 28;27(13):4939–4945. doi: 10.1021/bi00413a052. [DOI] [PubMed] [Google Scholar]