Abstract
Autoimmune diseases are commonly associated with a polygenic inheritance pattern. In rare instances, causal monogenic variants have been identified. The study by Liu et al. in this issue of the JCI provides an example of monogenic variants occurring in patients with IgG4-related disease (IgG4-RD). The authors investigated a familial cluster of IgG4-RD that consisted of an affected father and two daughters; the mother was unaffected. Genome sequencing of this quad identified a variant in IKZF1 (encoding IKAROS) and another variant in UBR4 (encoding E3 ubiquitin ligase). Both variants were present in the father and both daughters but absent in the unaffected mother. Using multidimensional profiling of immune cells and functional experiments in primary cells, the authors determined a molecular pathway contributing to T cell activation in IgG4-RD. Importantly, the characterization of these variants provides insights into pathogenic mechanisms in IgG4-RD and, potentially, other autoimmune diseases.
IgG4-RD and potential mechanism of pathogenesis
IgG4-related disease (IgG4-RD) is a rare immune-mediated fibroinflammatory condition associated with high serum IgG4 levels and infiltration of IgG4+ plasma cells in affected organs (1). Common organs affected include the pancreas, the lacrimal and salivary glands, the retroperitoneum, and the lymph nodes. A correct diagnosis to distinguish IgG4-RD from malignancy requires results from clinical, radiological, and histological tests. Diagnostic histological characteristics of lesions include storiform (swirling) fibrosis and lymphoplasmacytic infiltration causing vascular inflammation with obstructive clotting (2). These destructive lesions can lead to organ failure if left untreated. Initial treatment to induce remission typically involves glucocorticoids, followed by steroid-sparing immunosuppressive drugs such as azathioprine or cyclophosphamide. Biologics, such as rituximab, have been used to maintain remission (3). Although these therapeutic strategies are successful for treating IgG4-RD, the fact that these therapies also benefit other inflammatory and autoinflammatory conditions highlight the incomplete understanding of the pathogenic mechanisms specific to IgG4-RD.
Although the underlying pathogenesis in IgG4-RD is unclear, molecular features of the disease support the current Th2-centric hypothesis (4), in which increased IgG4 reflects class switching rather than the production of disease-initiating autoantibodies. This idea suggests that Th2 cytokines such as IL-4 and IL-13 promote B cell production and class switching of IgE and IgG4. Indeed, increased production of these Th2 cytokines, as well as of IL-5 and IL-10, have been reported in IgG4-RD (5). In addition, the common atopic manifestations characteristic of many patients diagnosed with IgG4-RD implicates a Th2 response (6). In this issue of the JCI, Liu et al. (7) describe a genetic basis for a proallergic immune imbalance in patients with IgG4-RD.
Genetic basis for Th2 polarization
Genome sequencing of a family consisting of a father and two daughters diagnosed with IgG4-RD and an unaffected mother identified potential candidate variants (7). The father and both daughters, but not the mother, carried two rare variants not annotated in the gnomAD database. One was a missense variant in IKZF1, which encodes the transcription factor IKAROS, at position 548G>A. The resulting amino acid substitution (Arg183His) in the DNA binding domain was predicted to be a gain-of-function (GOF) variant. IKZF1 variants in the DNA binding domain have been identified previously through exome sequencing of four unrelated families with autoimmune, allergic, and lymphoproliferative conditions (8). The other variant was a nonsense variant in UBR4, which encodes an E3 ubiquitin ligase at position 12537T>A and was predicted to result in a truncated protein (Cys4179Ter [UBR4-C4179Ter]) (7).
To assess the affect of these variants on immune cell populations from the three patients and three healthy individuals, PBMCs were immunophenotyped by mass cytometry (CyTOF). While there were some differences in the frequencies of immune cell subsets, the most striking finding was a reproducible increase in the levels of CD45 (a membrane tyrosine phosphatase, encoded by PTPRC) on all immune cells, particularly T cells (7). CD45 regulates T cell receptor (TCR) signaling by dephosphorylating intracellular signaling molecules, and increased phosphatase activity from elevated CD45 is predicted to downregulate TCR signaling via reduced phosphorylation of the Src family protein tyrosine kinase LCK (9–11). Although the authors detected reduced LCK phosphorylation in T cells from the patients, phosphorylation of an LCK downstream target, ZAP70, was increased. To test whether another Src family member was responsible for enhanced TCR signaling in the patients, the authors measured FYN, a tyrosine kinase involved in TCR signaling, and detected elevated protein levels in T cells from the patients. FYN expression was also elevated in EBV-transformed B cells from the patients. Results from overexpression of FYN in T cells from healthy individuals and siRNA-mediated knockdown of FYN in T cells from the three patients demonstrated a requirement of FYN for T cell signaling and activation (7). Interestingly, CD45 acts only on FYN’s inhibitory phosphorylation site (Y527) (12), and testing T cells from the patients revealed reduced phosphorylation only at Y527. These results focused attention on FYN as a primary regulator of T cell activation in IgG4-RD.
The next question Liu and colleagues addressed explored the cause of dysregulated protein levels: Were FYN and CD45 dysregulated transcriptionally or posttranscriptionally in the patients? By measuring gene expression of FYN and PTPRC (encoding CD45), the authors discovered an increased FYN, but not PTPRC, message, indicating transcriptional control of FYN and posttranscriptional control of CD45. The authors tested the association of increased FYN and CD45 with variants in IKZF1 and UBR4. Knockdown of IZKF1 resulted in reduced FYN with no change in CD45, whereas knockdown of UBR4 increased CD45 with no change in FYN. A direct mechanism through transcriptional control of FYN expression by the IKZF1 variant was demonstrated with ChIP experiments and luciferase reporter assays (7). IKAROS-R183H bound the FYN promoter in primary T cells and increased luciferase expression driven by the FYN promoter, confirming this variant’s GOF activity (8). Investigating a potential role of UBR4 truncation in posttranscriptional regulation of CD45, the authors blocked lysosomal protein degradation, which, similar to UBR4 knockdown, stabilized CD45. These data support the authors’ conclusion that increased FYN activity results from IKAROS-R183H driving increased expression of FYN; and UBR4 truncation stabilizes CD45, which removes phosphorylation at the inhibitory Y527 site of FYN (7) (Figure 1).
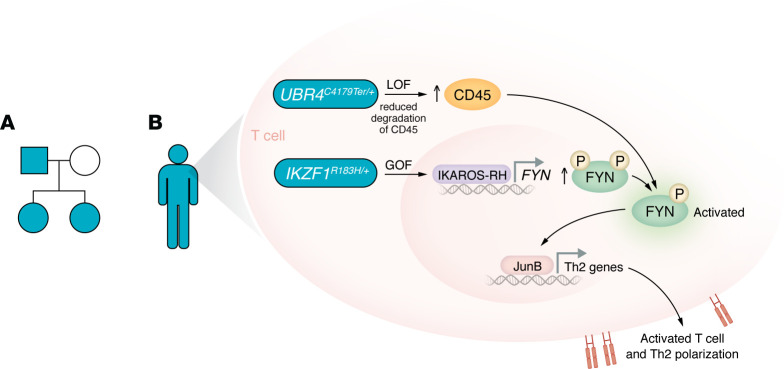
Figure 1. Patients with IgG4-RD possess genetic variants responsible for Th2 polarization and T cell activation.
(A) Three members of a family were affected by IgG4-RD: the father (teal square) and two daughters (teal circles). The mother was unaffected (white circle). (B) Rare variants were detected in the genome sequence of the affected individuals. The GOF IKAROS-R183H variant increases FYN expression, and the LOF UBR4-C4179Ter variant inhibits clearance of the tyrosine phosphatase CD45, which removes inhibitory phosphate, sparing phosphorylation at the activating tyrosine residue and leading to activation of FYN. The consequence of these variants for FYN stabilizes JunB to drive Th2 response genes and T cell activation.
The authors investigated the effect of this synergistic effect on FYN activity as it relates to T cell activation. Using transgenic expression of a low-avidity, self-reactive TCR that recognizes an islet-specific autoantigen (glucose-6 phosphate catalytic subunit–related protein), they showed that T cells from the patients with the IKZF1 GOF and UBR4 truncation variants had a lower threshold for T cell activation compared with T cells from healthy individuals. These data support the existence of a mechanism for self-reactive T cells to break tolerance, whereby increased FYN activity enhances TCR signaling (7).
A hallmark of IgG4-RD is the differentiation of immune cells into Th2 cells. CyTOF data indicated that the patients had increased Th2 and decreased Th1 cell frequencies. In addition, transcripts for the Th2 cytokines IL-4, IL-5, and IL-13 were upregulated in the three patients, while the Th1 and Th17 cytokines IFN-γ (INFG) and IL-17 (IL17) were decreased. Overexpression of FYN in T cells from healthy individuals was sufficient to promote IL-4 production — importantly, without affecting INFG or IL17 mRNA. Evidence that FYN was capable of inducing Th2 polarization in vivo came from mouse experiments, in which CD4+ T cells were transduced to overexpress FYN and transferred into mice followed by ovalbumin immunization. This immunization induced Th2 differentiation in mice that had overexpression of FYN (7).
After establishing the importance of FYN in skewing toward Th2 differentiation, Liu et al. (7) investigated a mechanism through which FYN may function. The E3 ubiquitin ligase ITCH is regulated by FYN. FYN phosphorylation of ITCH reduces the ubiquitination of JunB by ITCH, thus stabilizing JunB (13). Indeed, JunB was elevated in the patients’ T cells, and overexpression of FYN in T cells from healthy individuals resulted in higher JunB levels. As a final support for their proposed mechanism for a genetic basis of Th2 polarization, the authors showed that T cells expressing the IKAROS-R183H variant compared with WT IKAROS had elevated FYN and JunB in conjunction with increased expression of the Th2 lineage transcription factor GATA3 (7).
Future directions
The genetic findings and functional studies of Liu et al. (7) are an important advance in understanding the mechanism for a Th2 bias in patients with IgG4-RD and allergy and/or atopy. The GOF IKAROS-R183H variant increased the expression of FYN, and simultaneously, the loss-of-function (LOF) variant UBR4-C4179Ter disrupted lysosome-mediated degradation of CD45, which activated FYN. Activated FYN stabilized JunB, thereby inducing the expression of Th2 response genes (Figure 1). While previous studies implicated IKAROS deficiency in contributing to autoimmunity (14), the results presented here (7), as well as the identification of GOF IKAROS variants in individuals with inflammatory conditions (8), indicate a more complicated picture of IKAROS function. Profiling the regulatory landscape of IKAROS GOF variants could identify additional pathways contributing to T cell activation and potential biomarkers to monitor the response to therapy. More broadly, these findings open the possibility that IKAROS plays a role in other atopic diseases (7). The discovery of defective protein degradation mediated by the UBR4 variant suggests that regulation of protein turnover also contributes to autoimmunity. As Liu and authors point out, profiling the proteome in patients who are deficient for protein degradation could identify biomarkers and pathways involved in the inflammatory response (7). The findings of Liu et al. suggest that defining the IKAROS GOF genomic-binding landscape or profiling the proteome will uncover synergistic mechanisms that modulate immune function.
Version 1. 08/15/2024
Electronic publication
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Copyright: © 2024, Ciavatta et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2024;134(16):e183396. https://doi.org/10.1172/JCI183396.
See the related article at IKZF1 and UBR4 gene variants drive autoimmunity and Th2 polarization in IgG4-related disease.
References
- 1.Kamisawa T, et al. IgG4-related disease. Lancet. 2015;385(9976):1460–1471. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Okazaki K. Diagnosis and treatment of IgG4-related disease. Curr Top Microbiol Immunol. 2017;401:19–33. doi: 10.1007/82_2016_36. [DOI] [PubMed] [Google Scholar]
- 3.Karadeniz H, Vaglio A. IgG4-related disease: a contemporary review. Turk J Med Sci. 2020;50(si-2):1616–1631. doi: 10.3906/sag-2006-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floreani A, et al. IgG4-related disease: changing epidemiology and new thoughts on a multisystem disease. J Transl Autoimmun. 2021;4:100074. doi: 10.1016/j.jtauto.2020.100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zen Y, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45(6):1538–1546. doi: 10.1002/hep.21697. [DOI] [PubMed] [Google Scholar]
- 6.Walker JA, McKenzie ANJ. TH2 cell development and function. Nat Rev Immunol. 2018;18(2):121–133. doi: 10.1038/nri.2017.118. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, et al. IKZF1 and UBR4 gene variants drive autoimmunity and Th2 polarization in IgG4-related disease. J Clin Invest. 2024;134(16):e178692. doi: 10.1172/JCI178692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshino A, et al. Gain-of-function IKZF1 variants in humans cause immune dysregulation associated with abnormal T/B cell late differentiation. Sci Immunol. 2022;7(69):eabi7160. doi: 10.1126/sciimmunol.abi7160. [DOI] [PubMed] [Google Scholar]
- 9.McNeill L, et al. The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity. 2007;27(3):425–437. doi: 10.1016/j.immuni.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Zikherman J, et al. Quantitative differences in CD45 expression unmask functions for CD45 in B-cell development, tolerance, and survival. Proc Natl Acad Sci U S A. 2012;109(1):E3–E12. doi: 10.1073/pnas.1117374108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courtney AH, et al. CD45 functions as a signaling gatekeeper in T cells. Sci Signal. 2019;12(604):eaaw8151. doi: 10.1126/scisignal.aaw8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada M. Regulation of the SRC family kinases by Csk. Int J Biol Sci. 2012;8(10):1385–1397. doi: 10.7150/ijbs.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang C, et al. Negative regulation of the E3 ubiquitin ligase itch via Fyn-mediated tyrosine phosphorylation. Mol Cell. 2006;21(1):135–141. doi: 10.1016/j.molcel.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Sharabi A, et al. The serine/threonine protein phosphatase 2A controls autoimmunity. Clin Immunol. 2018;186:38–42. doi: 10.1016/j.clim.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]