Abstract
Herbal extracts have been successfully used as feed additives in fish culture with attractive growth-promoting, immunostimulant, antimicrobial, and antioxidant properties for several fish and shellfish species. Therefore, we have designed a feeding trial to assess the impacts of dietary incorporation of Perovskia abrotanoides extract (PAE) on common carp (Cyprinus carpio). For this purpose, five isonitrogenous (35% protein) and isocaloric (~4,000 kcal/kg) diets have been supplied by supplementing PAE at the varying inclusion levels as 0.0%, 0.25%, 0.5%, 1.0%, and 2.0% diets, and growth performance and feed utilization, digestive enzyme activities, serum biochemical variables, antioxidant responses, and immunological factors were studied. The experiment continued for 60 days. At the termination of the experiment, the mean final weight, weight gain percentage (WG%), feed conversion rate (FCR), and specific growth rate (SGR) have been improved significantly in all fish groups fed PAE-based diets with regard to those fed the reference diets. A second-order polynomial regression equations indicate that the optimum dietary supplementation level of PAE in fish diets was ~1%. Serum cortisol, glucose, triglyceride, cholesterol, and malondialdehyde levels as well as catalase, alanine aminotransferase, and aspartate aminotransferase activities were significantly decreased generally in all PAE-supplemented groups compared to the control groups before and/or after high-temperature stress (32°C). Moreover, serum total protein, albumin, and total immunoglobulin levels as well as ACH50, lysozyme, superoxide dismutase, and glutathione peroxidase activities were increased before and/or after high-temperature stress (32°C). In conclusion, the results showed, for the first time, that dietary supplementation with ~1% PAE can improve growth performance, stimulated the digestive enzymes, and enchanced antioxidant status as well as immune parameters and prevented high-temperature stress of common carp.
1. Introduction
With the global population steadily expanding, the imperative to enhance food production becomes increasingly urgent. The alteration in human consumption trends, encompassing a movement toward aquatic sources of protein, especially derived from aquaculture, emerges as a wholesome and ecologically viable substitute for diets centered around meat [1, 2]. Aquaculture is a significant economic sector that offers humans a useful and necessary protein supply [3, 4]. Nowadays, aquaculture provides over 50% of the global fish supply intended for human consumption [5]. The cyprinid fish family has gained high economic importance as it contributes more than 20 million metric tons of fish to world fish production, constituting approximately 40% of the total output from aquaculture worldwide and encompassing 70% of freshwater aquaculture yield [5]. Carps are an environmentally friendly alternative to other major aquaculture species such as salmon and shrimp. They are omnivorous/filter fish and require less fish meal and fish oil when farmed. Common carp (Cyprinus carpio) is the main member of the cyprinid fish family, which is farmed in more than 100 countries. It accounts for up to 10% (over 3 million tons) of the global annual freshwater aquaculture production [6]. The common carp ranks fourth among the most common cultivated fish species in the world and is highly valued in many countries of the Middle East, including Iran [7]. In its natural habitat, this species is found in the Caspian Sea, where it is actively farmed in most freshwater basins, including aquaculture farms located in the southern part of the Caspian Sea [8].
Fish have evolved to survive in a specific range of environmental variations, and anything outside of that ranges threatens their physiology and health [9, 10]. Also, intensive aquaculture systems may cause some environmental factor changes that facilitate the occurrence of disease outbreaks due to imposed stress conditions and subsequent fish immunosuppression [11]. Hence, the management of environmental factors holds paramount importance, with temperature emerging as the most crucial factor among them. It influences all aspects of aquatic animal life such as their survival, metabolism, feeding habits, growth performance, and disease resistance. Severe heat stress depresses the growth, physiological function, feed efficiency, oxidative status, and immune response in the organs of aquatic animal [12] and, subsequently, reduced the fish ability to defend against infectious diseases [13]. In this regard, improving the immune system of fish during heat stress is of great importance for fish farmers.
Nowadays, the use of medicinal plants is recommended for stress relief in aquaculture. The Perovskia genus, Labiatae family, ranks among the frequently utilized medicinal plants and comprises seven distinct species. From this family, three species including P. abrotanoides, P. atriplicifolia, and P. artemisoides are observed in the nature of Iran [14]. P. abrotanoides represents a perennial herbaceous plant that thrives in various geographical areas such as Iran, Afghanistan, Turkmenistan, and Pakistan, known by various names including Brazambal, Domou, and Gevereh. Some pharmacological actions of the plant have been verified including leishmanicidal, antiplasmodial, and antinociceptive and antiinflammatory activities [15, 16, 17]. Employing P. abrotanoides oil in the treatment of secondary infections associated with leishmaniasis, particularly in relation to S. aureus, holds potential advantages [18]. Tanshinone, a diterpene molecule found in P. abrotanoides roots, has been demonstrated to have a variety of biological actions, including antibacterial, antioxidant, and anticancer characteristics [14, 16]. Local communities use the herb to treat typhoid fever, migraine, arteriosclerosis, vomiting, gonorrhea, cough, toothache, and cardiovascular and liver disease [19, 20, 21]. It has antiseptic, sedative, analgesic, and cooling effect [15, 22]. GC and GC-MS were used to examine the essential oil extracted from the flowering aerial portions of P. abrotanoides. There were 21 components were found. The composition of the essential oil includes mainly camphor (34.1%), 1.8-cineole (18.0%), b-caryophyllene (8.2%), and a-humulene (8.2%) [23].
Herbal feed supplements are renowned for their wide range of beneficial properties, such as antistress and antioxidant effects, immune-boosting capabilities, and hepatoprotective properties. Therefore, nutritional herbal supplements are a promising option for mitigating the heat stress of fish in aquaculture conditions [24]. For example, the previous study has shown that hyssop (Hyssopus officinalis) extract inclusion into the diet improves the physiological and antioxidant responses of young rainbow trout (Oncorhynchus mykiss) exposed to thermal stress [25]. However, as far as literature survey could ascertain, the protective effect of P. abrotanoides extract (PAE) in heat stressed carp juveniles has yet to be determined. Also, the effect of P. abrotanoides extract on growth efficiency in carp juveniles (C. carpio) has not been documented before in prior records. Hence, the main goal of this research was to examine the impact of orally administering P. abrotanoides extract on the productivity, biochemical, and immune characteristics of carp.
2. Materials and Methods
2.1. Plant Collection and Extraction of Phenolic Compounds
Samples of Proviskia abrotanoides were collected in the Vamnan region located in the Azadshahr county, Golestan province, in northern Iran, at the flowering period. The collected plant sample of P. abrotanoides was botanically authenticated by the colored flora of Iran (code no. GKU/803894). The collected samples were divided into different parts of the root, stem, leaf, and flower. The samples were washed with distilled water and then dried in the shade for 3 days and then dried in an oven at 40°C. The samples were powdered with a grinder and passed through an eight-mesh sieve and stored in plastic bags at a temperature of −20°C before beginning the experiment.
Hot extraction method was used to extract the phenolic compounds. The methanolic extract of P. abrotanoides has the maximum ability to extract the phenolic compounds as compared to other solvents [26]. Thus, 0.1 g of the powdered samples of the entire P. abrotanoides organ was weighed with a digital scale, and then 20 mL of 80% methanol was added to it; the resulting mixture was placed in a shaker for 6 hr. Then it was placed in a water bath with a temperature of 50°C for 2 hr. After this period, the obtained extracts were filtered through filter paper and brought to final volume of 25 mL with 80% methanol solvent. The profile of the studied aerial plant part of P. abrotanoides in the studied place was previously reported by Alamdari et al. [27] according to the Table 1 (GC-MS analysis).
Table 1.
GC-MS analysis of aerial parts of P abrotanoides at flowering stage.
| Row | Name of the compound | Composition percentage | Retention time |
|---|---|---|---|
| 1 | IR-alpha-pinene | 0.268 | 3.302 |
| 2 | Eucalyptol | 1.396 | 4.705 |
| 3 | Bicyclo(2,2,1)heptan-2-one, 1,7,7-trimethyl (1S) | 1.024 | 7.040 |
| 4 | Bornyl acetate | 1.654 | 10.026 |
| 5 | Cyclohexene-1-methanol,alpha-4-trimethyl-propanoate | 3.395 | 11.537 |
| 6 | Copaene | 0.779 | 12.208 |
| 7 | 1H-Cycloprop(e)azulene,1a.2.3,4,4a,5,6,7b-octahydro-1,1,4,7-tetramethyl-(1 a R1a alpha .4.alpha, 4 a. beta, 7 balpha) | 0.952 | 12.981 |
| 8 | Caryophyllene | 2.370 | 13.343 |
| 9 | Azulene,1,2,3,4,5,6,7,8-octahydro1,4-dimethyl-7-(1-methyle)-(1S)-(1.alpha,4.alpha,7.alpha) | 2.220 | 14.213 |
| 10 | 1H-Cycloprop(e)azulene,decahdro-1,1,7-trimethyl-4-methylene(1a, alpha,4 a.beta,7 alpha,7 beta, 7 b. alpha) | 0.788 | 14.306 |
| 11 | Naphthalene,1,2, 3, 4,4a,5,6,8a-octahydro-7-methyl-4-methylene-1-1 methylethyl)-(1 alpha,4 a beta, 8 a alpha) | 2.858 | 15.570 |
| 12 | Naphthalene,1,2,3,5,6,8-a-hexahydro 4,7-dimethyl-1,(1-methylethy) (1S-cis) | 3.736 | 15.655 |
| 13 | 2,3,4-Trifluorobenzoic acid,2,4,6 trichlorophenyl ester | 1.908 | 15.773 |
| 14 | Isoaromadendrene epoxide | 1.473 | 17.279 |
| 15 | Aristolene epoxide | 1.720 | 17.936 |
| 16 | Cubenol | 1.597 | 18.013 |
| 17 | Beta-Guaiene | 1.204 | 18.276 |
| 18 | 1-Naphthalenol,decahydro-1,4a-dimethyl-7,(1-methylethylid) 1 R-(1.alpha,4a.beta,8a.alpha) | 3.644 | 18.575 |
| 19 | Naphthalene,1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)(1S-cis) | 14.251 | 18.773 |
| 20 | Patchoulene | 5.516 | 18.847 |
| 21 | Ir,4s,7s.11R-2,2,4,8 -Tetramethyltricyclo (5,3,1,0 (4,11))undec-8ene | 1.805 | 19.042 |
| 22 | Agarospirol | 7.233 | 19.419 |
| 23 | 1H-Cyclopropeazulene,1a,2,3,4,4a,5,6,7b-octahydro-1,1,4,7-tetramethyl-(1a.alpha.4alpha,4a.beta),7b.alpha | 2.865 | 19.765 |
| 24 | Carotol | 31.105 | 20.009 |
| 25 | 7-1-Isopropyl-1,1,4a,trimethyl,1,2,3,4,4a,9,10,10a-octahydrophenanthrene | 0.904 | 26.918 |
| 26 | 1R,4s,7s,11R-2,2,4,8-tetramethyl tricyclo(5, 3, 1,0 (4,1))undec-8-ene | 1.017 | 27.511 |
Bold values indicate more of these compounds in the extract.
2.2. Diet Preparation and Extract Supplementation
The main dietary formula (control) was prepared by combining the appropriate ingredients, including fish meal, meat meal, soy meal, wheat meal, fish oil, soybean oil, corn flour, lysine, methionine, and vitamins and mineral premix, in the required proportions. Then, it moistened by adding sterilized water. The prepared dough was divided into five equal parts. The control and experimental diets were prepared in the same way by adding 0%, 0.25%, 0.5%, 1%, and 2% of P. abrotanoides extract of dough that thoroughly mixed (Table 2). The dough that was prepared underwent extrusion using a meat grinder die. Subsequently, the resulting strands were crushed to achieve the desired size and then air-dried at room temperature. The juveniles were fed with the control and experimental diets at 2.5% of biomass for 60 days [28].
Table 2.
Dietary formulation and proximate composition analysis of experimental diets (% on dry matter basis) containing different levels of phenolic compounds of P. abrotanoides extract.
| Ingredients (%) | Experimental diets | ||||
|---|---|---|---|---|---|
| Fishmeal1 | 10 | 10 | 10 | 10 | 10 |
| Meat meal2 | 20 | 20 | 20 | 20 | 20 |
| Soybean meal | 23 | 23 | 23 | 23 | 23 |
| Wheat meal | 34.9 | 34.65 | 34.4 | 33.9 | 32.9 |
| Fish oil | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| Soybean oil | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| Corn flour | 9 | 9 | 9 | 9 | 9 |
| L-Lysine3 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| L-Methionine 1003 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Vitamin premixa | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Mineral premixb | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
|
| |||||
| Perovskia extract | 0 | 0.25 | 0.5 | 1 | 2 |
|
| |||||
| Dry matter | 89.18 | 89.15 | 89.13 | 89.09 | 89.00 |
| Crude protein (%) | 35.03 | 35.03 | 35.03 | 35.02 | 35.00 |
| Crude fat (%) | 5.78 | 5.78 | 5.78 | 5.78 | 5.78 |
| Crude ash (%) | 5.82 | 5.82 | 5.82 | 5.82 | 5.82 |
| Energy (kcal/kg) | 4,052.99 | 4,052.01 | 4,051.03 | 4,049.07 | 4,045.15 |
1Pars Kilka Co., Mazandaran, Iran (Kilka powder analysis; protein, 70%–72%; fat, 8%–11%; ash, 11.6%; moisture, 7%–9%). 2Makianmehr Co., Golestan, Iran. 3Morghenojan.Co., Tehran, Iran. aVitamin premix (per kg of diet): vitamin A, 2,000 IU; vitamin B1 (thiamin), 5 mg; vitamin B2 (riboflavin), 5 mg; vitamin B6, 5 mg; vitamin B12, 0.025 mg; vitamin D3, 1,200 IU; vitamin E, 63 mg; vitamin K3, 2.5 mg; folic acid, 1.3 mg; biotin, 0.05 mg; pantothenic acid calcium, 20 mg; inositol, 60 mg; ascorbic acid (35%), 110 mg; niacinamide, 25 mg. bMineral premix (per kg of diet): MnSO4, 10 mg; MgSO4, 10 mg; KCl, 95 mg; NaCl, 165 mg; ZnSO4, 20 mg; KI, 1 mg; CuSO4, 12.5 mg; FeSO4, 105 mg; Co, 1.5 mg.
2.3. Fish and Experimental Conditions
The research was conducted at the Fisheries Laboratory of Gonbad Kavous University in Golestan, Iran, in full compliance with the standards and guidelines set forth by the Animal Care Committee of our university. Carp juveniles (C. carpio) were acclimated for 14 days in our laboratory conditions. During acclimation period, they fed 2.5% biomass three times a day (at 09:00, 13:00, and 17:00) with a commercial diet without supplementation. Four hundred fifty carp juveniles (initial weight 17.79 ± 0.86 gr) were placed in a randomized manner across 15 tanks (60 L) in five treatments (with three replicates). Water temperature, dissolved oxygen, pH, and total ammonia concentration were 26.1 ± 0.29°C, 6.76 ± 0.39 mg/L, 7.7 ± 0.21, and 0.10 ± 0.022 mg/L, respectively.
2.3.1. High-Temperature Stress
At the experiment's conclusion, a batch of 10 fish per tank (totaling 30 fish per treatment) was subjected to elevated temperatures (32°C) in their own tanks for 48 hr. It should be mentioned that water temperature gradually increased as 1°C/30 min by help of aquarium heaters [29]. During this high-temperature stress period, the fish fasted completely, and the tanks were aerated continuously [30]. After 48 hr, blood samples were drawn, and the biochemical stress-related factors (glucose and cortisol) were measured.
2.3.2. Sample Collection
At the experiment's conclusion, three fish were removed from each tank and then anesthetized in a clove powder bath (200 ppm) [31]. Blood samples were taken from the caudal vessel of each fish using nonheparinized syringes. In each repetition, the blood of three fish was equally mixed with each other (a total of three blood samples from each treatment). Blood was centrifuged at 5,000 rpm for 10 min. The obtained serum was preserved at a temperature of −80°C and then used for subsequent biochemical assays.
2.4. Analysis
2.4.1. Growth Performance and Nutrient Efficiency Indices
At the conclusion of the 60-day experimental period, all fish were weighted (BW) by digital scale, and their lengths (TL) were measured by ruler. The fish growth and nutrient efficiency indices were calculated as follows [32]:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
2.4.2. Digestive Enzyme Activity Assays
At the conclusion of the 60-day experimental period, three fish from each tank were captured. Then, the fish belly was disinfected with alcohol. The intestine completely removed from the body, washed with physiological serum, and homogenized. After that, the homogenate was centrifuged (2,500xg, 4°C, 20 min), and the supernatant was snap frozen in liquid nitrogen [31]. The samples were weighed with a digital scale with an accuracy of 0.001 g and then weighed according to the weight-to-volume ratio (1–9) with a buffer solution (100 mM Tris-Hcl, 0.1 mM EDTA, 0.1% Triton at pH 7.8) was homogenized [33, 34].
Amylase activity was evaluated following the procedure outlined by [35], utilizing a substrate of 0.3% soluble starch that was dissolved in NaH2PO4 buffer (pH 7.4). Lipase activity was assessed over a 15-min period at 30°C using p-nitrophenyl myristate as the substrate, which was dissolved in 0.25 M Tris-HCl (pH 9.0) as per the method described by Iijima et al. [36]. Protease activity was determined at 25°C, employing a substrate of 1% (w/v) casein sourced from Sigma, USA, in 0.2 M phosphate buffer with a pH of 7.0, following the methodology of Walter [37].
2.4.3. Biochemical and Immune Parameters
Using commercial kits (Pars Azmun, Karaj, Iran) following the manufacturer's instructions, calorimetric measurements were performed to determine serum total protein and albumin concentrations. Globulin concentrations were determined by subtracting the albumin content from the overall serum protein. Serum cholesterol and triglycerides were analyzed using commercial kits (ZiestChem Diagnostics, Tehran, Iran) using the method proposed by the manufacturer.
The evaluation of serum lysozyme activity followed the approach outlined by Demers and Bayne [38], which involves the lysis of the lysozyme-sensitive bacterium Micrococcus luteus (Sigma). A standard was established using hen egg white lysozyme (Sigma), with dilutions ranging from 0 to 20 μL mL−1, prepared in a 0.1 M phosphate citrate buffer at pH 5.8. The standard, along with an undiluted serum sample (25 μL), was then placed in triplicate within the wells of a 96-well plate. Subsequently, 175 μL of M. luteus suspension (75 mg mL−1), prepared in the same buffer, was introduced into each well. Following rapid mixing, alterations in turbidity were gauged every 30 s over a 5-min span at 450 nm and approximately 20°C, utilizing a microplate reader. The measurement of alternative complement (ACH50) activity was conducted employing the methodology introduced by Sunyer and Tort [39], with adjustments as previously detailed by Yeh et al. [40]. The serum complement volume responsible for inducing 50% hemolysis (ACH50) was established, and the number of ACH50 U mL−1 was computed for the specimen. For serum total immunoglobulin (Ig) levels, precipitation of Ig using polyethylene glycol solution was employed as described by Siwicki and Anderson [41].
2.4.4. Stress Parameters
Serum glucose levels were assessed through a commercially available kit (Pars Azmun, Karaj, Iran) in adherence to the manufacturer's protocol. The quantification of serum cortisol concentrations was performed using the competitive ELISA technique, utilizing a commercial kit (IBL Co., Gesellschaft für Immunchemie und Immunbiologie). For serum enzymatic activities including alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate transaminase (AST), commercial kits (Pars Azmun, Karaj, Iran) [42] were employed in conjunction with an automated biochemical analyzer (Beckman Coulter, USA).
2.4.5. Antioxidant Enzyme Activities
Serum glutathione peroxidase (GPx) and superoxide dismutase (SOD) activities were assessed by evaluating the speed of glutathione oxidation and the pace of cytochrome C reduction, correspondingly. These evaluations were conducted using commercially available kits (ZellBio GmbH, Veltinerweg). The measurement of serum catalase activity was executed by determining the rate of hydrogen peroxide decomposition, following the methodology outlined by Goth [43]. Additionally, serum malondialdehyde (MDA) levels were determined using a commercial kit (ZellBio GmbH, Veltinerweg) based on the thiobarbituric acid technique.
2.5. Statistical Analysis
Initially, the conformity of the data to a normal distribution was assessed via the Shapiro–Wilk test. Subsequently, the homogeneity of variance was examined using Levene's test. Following this, distinctions among the groups were identified through a one-way analysis of variance (ANOVA), accompanied by Duncan's multiple range test, with a significance level set at P < 0.05. Lastly, a two-way ANOVA was conducted to assess the impacts two factors: thermal stress and P. abrotanoides extract levels (PAE). All statistical analyses were carried out using SPSS software (version 22), and a significance level of P < 0.05 was adopted as the threshold for acceptance.
3. Results
3.1. Growth and Feed Assimilation of Carp Juveniles Fed on P. abrotanoides Extract
Upon the conclusion of the 60-day feeding trial, the survival rate (%) of the fish was uniform across all experimental groups, with no instances of recorded mortality. Carp juveniles that were provided diets enriched with extracts (0.25%, 0.5%, 0.1%, and 2%) from P. abrotanoides for 60 days showed higher final weight (FW), weight gain (WG), and SGR than fish fed a control diet (P < 0.001). Moreover, FCR improved significantly (displayed lower values) in fish-fed extract-supplemented diets (P=0.003). The highest FW, WG, and WG% were found in fish-fed diets supplemented with 0.5% and 1% P. abrotanoides extract, whereas the best FCR values were found in fish-fed diets supplemented with 1% and 0.5% extract. Figure 1 illustrates the correlations between WG, SGR, and FCR of common carp concerning varying levels of dietary PAE. The second-order polynomial regression equations indicate an approximate optimal dietary PAE supplementation level of around 1% in fish diets.
Figure 1.
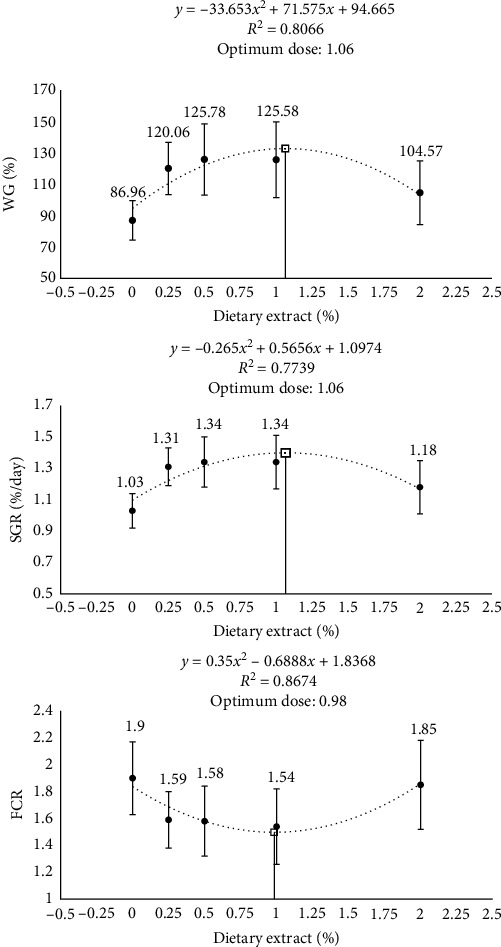
The relationship between weight gain (WG%), specific growth rate (%/day), and feed conversion ratio (FCR) against dietary P. abrotanoides extract levels (% diet) that are supplied in the diets of common carp for 60 days as described by second-order polynomial regression analysis.
Values of growth and feed assimilation of carp juveniles fed on P. abrotanoides extract are presented in Table 3.
Table 3.
Growth performance and feed efficiency of common carp fed with P. abrotanoides extract after 60 days.
| Control | 0.25 | 0.5 | 1 | 2 | P-value | |
|---|---|---|---|---|---|---|
| IW (gr) | 18.17 ± 0.71 | 17.74 ± 0.88 | 17.55 ± 0.96 | 17.50 ± 0.89 | 18.02 ± 0.86 | P > 0.001 |
| FW (gr) | 33.97 ± 2.50c | 38.99 ± 2.79ab | 39.51 ± 3.31a | 39.36 ± 3.33a | 36.73 ± 2.47b | P < 0.001 |
| WG (gr) | 15.79 ± 2.29c | 21.24 ± 2.72a | 21.96 ± 3.45a | 21.85 ± 3.61a | 18.71 ± 3.00b | P < 0.001 |
| WG (%) | 86.96 ± 12.61c | 120.06 ± 16.66ab | 125.78 ± 22.74a | 125.58 ± 24.09a | 104.57 ± 20.28b | P < 0.001 |
| SGR (%/d) | 1.03 ± 0.11c | 1.31 ± 0.12ab | 1.34 ± 0.16a | 1.34 ± 0.17a | 1.18 ± 0.17b | P < 0.001 |
| FCR | 1.90 ± 0.27a | 1.59 ± 0.21b | 1.58 ± 0.26b | 1.54 ± 0.28b | 1.85 ± 0.33a | P=0.003 |
| SR (%) | 100 | 100 | 100 | 100 | 100 | P > 0.001 |
Different letters within a row indicate significant differences among the treatments (n = 3; Duncan test).
3.2. Digestive Enzyme Activities
All fish fed P. abrotanoides extract-enriched diets had significantly increased protease and lipase activities than control fish (Figures 2(a) and 2(b); P < 0.001). The highest protease and lipase activity was found in fish-fed diets supplemented with 1% and 0.5% P. abrotanoides extract, whereas the lowest activity was seen in fish-fed diets supplemented with 2% extract. Amylase activity was much higher in fish-fed enriched diets containing 1% followed by 0.5% P. abrotanoides extract (Figure 2(c); P < 0.001).
Figure 2.
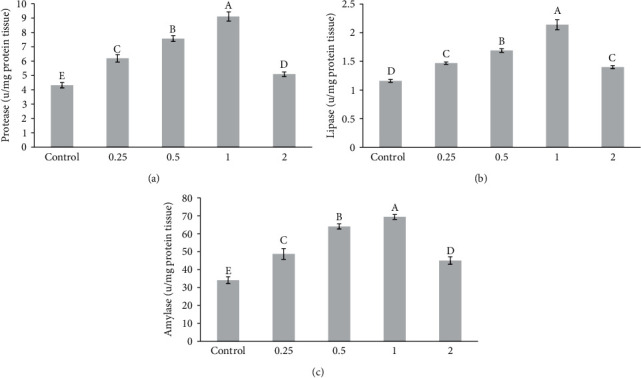
Intestinal digestive enzymes' activities in carp juveniles fed on P. abrotanoides extract after 60 days. Different letters above the bars show significant difference among the treatments (n = 3; Duncan test) (a–c).
3.3. Serum Immune Parameters
The levels of all three immunological indicators examined in this study (ACH50, lysozyme, and total immunoglobulin) improved considerably when carps were exposed to P. abrotanoides extract for 60 days. The highest lysozyme activity was observed in fish that consumed a diet enriched with 1% extract, with the subsequent highest activity seen in those fed a diet containing 0.5% extract (P < 0.001). While fish fed a 0.25% extract-enriched diet exhibited the lowest lysozyme activity. In addition, feed supplementation with 1% and 0.5% P. abrotanoides extract resulted in the highest total immunoglobulin levels (P < 0.001). The highest levels of ACH50 were found in fish fed a diet supplemented with 0.5% followed by 1% extract (P=0.004). The serum immunological parameters of carp juveniles fed P. abrotanoides extract are shown in Table 4.
Table 4.
Serum immunological parameters of carp juveniles fed P. abrotanoides extract after 60 days.
| Experiment groups | Parameters | ||
|---|---|---|---|
| ACH50 (u/mL) | Lysozyme activity (u/mL/min) | Total immunoglobulin (mg/mL) | |
| 0 | 134.66 ± 0.49c | 22.28 ± 0.34c | 17.03 ± 0.60b |
| 0.25 | 136.21 ± 1.09b | 22.60 ± 0.81c | 17.11 ± 0.54b |
| 0.5 | 137.89 ± 1.14a | 26.76 ± 1.87ab | 18.88 ± 0.31a |
| 1.0 | 137.54 ± 0.58ab | 28.50 ± 0.63a | 18.90 ± 0.20a |
| 2.0 | 136.14 ± 0.32b | 25.89 ± 0.53b | 18.16 ± 0.24a |
| P-value | P=0.004 | P < 0.001 | P < 0.001 |
Different letters within a column indicate significant differences among the treatments (n = 3; Duncan test).
3.4. Serum Biochemical Parameters
The blood total protein and albumin levels of fish fed the P. abrotanoides extract for 60 days were considerably greater than those of fish fed the control diet. The highest levels of serum total protein were found in fish fed diets supplemented with 0.25% and 2% P. abrotanoides extract, respectively (P < 0.001), whereas serum albumin levels were the highest in fish-fed diets supplemented with 2% and 1% P. abrotanoides extract, respectively (P < 0.001). The serum total cholesterol levels in fish-fed diets containing 0.25% P. abrotanoides extract were significantly lower. A diet containing 2% extract, however, significantly increased serum total cholesterol levels when compared to a control diet (P < 0.001). Fish that consumed diets containing 1% and 0.25% of P. abrotanoides extract exhibited the least triglyceride levels, respectively (P < 0.001). Results of serum biochemical parameters of carp juveniles fed on P. abrotanoides extract are presented in Table 5.
Table 5.
Serum biochemical parameters of carp juveniles fed on P. abrotanoides extract after 60 days.
| Experiment groups | Parameters | ||||
|---|---|---|---|---|---|
| Total protein (gr/dL) | Albumin (gr/dL) | Globulin (gr/dL) | Cholesterol (mg/dL) | Triglycerides (mg/dL) | |
| 0 | 3.07 ± 0.12d | 1.35 ± 0.036c | 1.72 ± 0.11b | 165.92 ± 2.89b | 150.24 ± 2.75a |
| 0.25 | 3.57 ± 0.08a | 1.37 ± 0.026c | 2.2 ± 0.06a | 141.76 ± 3.06c | 135.04 ± 1.37b |
| 0.5 | 3.21 ± 0.06cd | 1.37 ± 0.026c | 1.84 ± 0.078b | 159.74 ± 1.23b | 146.43 ± 4.39a |
| 1.0 | 3.25 ± 0.05bc | 1.45 ± 0.020b | 1.8 ± 0.036b | 160.66 ± 4.72b | 119.55 ± 0.99c |
| 2.0 | 3.37 ± 0.04b | 1.57 ± 0.010a | 1.8 ± 0.045b | 182.67 ± 3.95a | 150.26 ± 1.31a |
| P-value | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
Different letters within a column indicate significant differences among the treatments (n = 3; Duncan test).
3.5. Antioxidant Enzyme Activities before and after the Stress
The antioxidant status of fish was dramatically increased by supplementing their diet with P. abrotanoides extract. In contrast to the control group, the addition of 1% P. abrotanoides extract significantly elevated SOD levels, while 0.5% extract increased GPx activity (P < 0.001). P. abrotanoides extract at 0.5% considerably reduced MDA levels, while 0.25% extract reduced catalase levels when compared to the control group (P < 0.001). MDA, a lipid peroxidation biomarker, increased considerably in fish that were subjected to heat stress for 48 hr. The highest MDA levels were found in fish fed a control diet, while the lowest MDA levels were found in fish fed an enriched diet (0.5% and 1% P. abrotanoides extract). Catalase levels rose dramatically in fish exposed to heat stress, with fish fed a control diet having the highest levels, and fish provided an enhanced diet containing 1% P. abrotanoides extract having the lowest (P < 0.001). In contrast to the control group, dietary addition of P. abrotanoides extract increased SOD and GPx activity following heat stress (P < 0.001). Fish fed with 0.5% and 1% extract had the highest SOD activity. Furthermore, fish fed 1% extract had the highest GPx activity, followed by fish fed 0.5% extract. Table 6 shows the antioxidant enzyme activities in carp juveniles fed P. abrotanoides extract.
Table 6.
Serum antioxidant enzyme activities (U mg−1 protein) andoxidative status (nmol MDA mg−1 protein) of common carp fed P. abrotanoides extract before and after high-temperature stress (32°C).
| Experiment groups | Parameters | |||
|---|---|---|---|---|
| SOD (U/mL) | GPX (U/mL) | Catalase (U/mL) | MDA (µmol/L) | |
| Before stress | ||||
| 0 | 82.33 ± 1.32dD | 139.09 ± 2.13dG | 122.14 ± 3.34aC | 143.72 ± 5.69aB |
| 0.25 | 87.25 ± 0.90cC | 149.32 ± 1.22cF | 88.32 ± 3.10dF | 88.41 ± 2.62bF |
| 0.5 | 89.81 ± 0.71bB | 190.66 ± 2.71aC | 100.38 ± 3.78cE | 76.54 ± 2.43cG |
| 1.0 | 91.57 ± 0.53aB | 171.43 ± 1.36bD | 90.51 ± 2.06dF | 88.61 ± 5.26bF |
| 2.0 | 87.34 ± 0.49cC | 140.37 ± 2.43dG | 111.58 ± 2.81bD | 90.87 ± 5.96bF |
| After stress | ||||
| 0 | 70.91 ± 1.81dE | 124.88 ± 5.09eH | 163.08 ± 3.10aA | 179.31 ± 3.48aA |
| 0.25 | 85.60 ± 1.04bC | 160.94 ± 5.66dE | 121.21 ± 2.72cC | 120.11 ± 1.89cD |
| 0.5 | 97.26 ± 1.06aA | 201.09 ± 6.95bB | 123.88 ± 7.02cC | 102.47 ± 3.92dE |
| 1.0 | 97.20 ± 0.60aA | 215.40 ± 3.05aA | 108.13 ± 6.82dD | 102.28 ± 2.09dE |
| 2.0 | 81.92 ± 1.23cD | 171.33 ± 6.50cD | 142.33 ± 2.46bB | 129.86 ± 3.10bC |
| Two-way ANOVA | ||||
| Stress | P=0.010 | P < 0.001 | P < 0.001 | P < 0.001 |
| Diet | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| Interaction | P < 0.001 | P < 0.001 | P=0.001 | P < 0.001 |
Different letters within a column indicate significant differences among the treatment (n = 3; Duncan test).
3.6. Levels of Liver Enzymes, Glucose, and Cortisol before and after the Stress
In contrast to the control group, diet supplementation with various levels of P. abrotanoides extract for 60 days significantly reduced liver enzymes, glucose, and cortisol levels. The fish fed diet enriched with 1% P. abrotanoides extract had the lowest AST and ALT levels (P < 0.001). The fish fed diet containing 0.25% extract exhibited the lowest ALP levels, followed by the diet enriched with 0.5% extract (P < 0.001). Furthermore, the diet supplementation with 1% extract revealed the lowest glucose and cortisol levels (P < 0.001). In contrast to the control group, carp juveniles exposed to heat stress and fed a diet enriched with P. abrotanoides extract had lower liver enzymes, glucose, and cortisol levels (P < 0.001). Fish that were fed a diet enriched with 1% P. abrotanoides extract displayed the most minimal AST and ALT levels, while the lowest ALP levels were observed in fish fed with a diet supplemented with 0.25% followed by 0.5% extract, respectively. Diet supplementation with 1% extract showed the lowest glucose and cortisol levels when compared to the control group (P < 0.001). Liver enzymes, glucose, and cortisol levels of carp juveniles fed on P. abrotanoides extract before and after stress are presented in Table 7.
Table 7.
Stress indicators of common carp fed P. abrotanoides extract before and after high-temperature stress(32°C).
| Experiment groups | Parameters | ||||
|---|---|---|---|---|---|
| Cortisol (ng/mL) | Glucose (mg/dL) | AST (u/L) | ALT (u/L) | ALP (u/L) | |
| Before stress | |||||
| 0 | 189.74 ± 3.99aC | 97.45 ± 0.93aBC | 131.62 ± 3.89aCD | 28.39 ± 0.60aD | 140.79 ± 5.03aB |
| 0.25 | 149.41 ± 5.94bE | 84.15 ± 2.77bD | 113.10 ± 1.56bF | 23.60 ± 0.72bE | 111.01 ± 4.22cF |
| 0.5 | 118.51 ± 5.18cG | 83.82 ± 4.43bD | 107.25 ± 2.23cFG | 21.42 ± 0.65cF | 113.22 ± 7.09cEF |
| 1.0 | 111.50 ± 3.41cG | 72.81 ± 4.50cE | 102.36 ± 2.62dG | 20.29 ± 0.66cF | 119.26 ± 2.65bcDEF |
| 2.0 | 183.32 ± 3.62aC | 96.92 ± 1.92aBC | 115.76 ± 1.45bEF | 21.42 ± 0.83cF | 124.56 ± 2.95bCD |
| After stress | |||||
| 0 | 245.42 ± 4.75aA | 138.97 ± 3.59aA | 168.93 ± 3.40aA | 45.89 ± 1.51aA | 150.15 ± 3.87aA |
| 0.25 | 170.09 ± 8.06cD | 101.72 ± 7.18bB | 138.33 ± 7.14bcBC | 35.03 ± 0.76bcBC | 111.48 ± 3.30cF |
| 0.5 | 137.43 ± 4.35dF | 96.24 ± 3.37bBC | 126.89 ± 3.89cdD | 33.31 ± 0.68cC | 115.50 ± 4.77cEF |
| 1.0 | 135.90 ± 5.82dF | 94.17 ± 3.50bC | 122.63 ± 8.54dDE | 29.62 ± 1.55dD | 120.48 ± 4.07cDE |
| 2.0 | 230.37 ± 6.42bB | 132.85 ± 4.04aA | 143.92 ± 8.66bB | 36.48 ± 1.47bB | 129.84 ± 7.27bC |
| Two-way ANOVA | |||||
| Stress | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P=0.045 |
| Diet | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| Interaction | P < 0.001 | P < 0.001 | P=0.043 | P < 0.001 | NS |
Different letters within a column indicate significant differences among the treatment (n = 3; Duncan test).
4. Discussion
This preliminary study investigated the supplementation of P. abrotanoides extract (PAE) at 0.25%–2% and had a positive effect on overall performance, feed utilization, and digestive system of common carp. Effective metabolites, particularly from PAE, could be beneficial to the health status of the fish, which would result in a better growth performance. Moreover, the use of PAE promotes increased secretion of protease, lipase, and amylase enzymes in fish intestines, leading to more efficient nutrient absorption and better fish development. Similar to this study, the addition of herbal extracts to fish feeds increased digestive enzymes activities and growth performance of fish [44, 45, 46]. Moreover, the improvement of performance in common carp fed diets incorporated with PAE may be due to antioxidant exogenous enzymes. These enzymes may reduce to different stressors (physical stressors, environmental pollutants, chemotherapeutic drug applications, etc.) in fish production condition as with other plant extract applications [47, 48, 49]. Alamdari et al. [27] reported that leaf sample of P. abrotanoides contained the highest total phenolic compounds, flavonoids, and antioxidant activity by .17 ± 0.74 mg GAE/100 g dried sample weight, 2.26 ± 0.60 mg QE/g dried sample, and 69.10% ± 0.83%, respectively.
In fish, serum total protein is calculated as the sum of albumin and globulins. Increases in these proteins are considered an indicator of stronger immunity [50]. In this study, it was noticed that the highest serum total protein level was found in fish fed with 0.25% PAE group. Parallel with our study, increased serum protein values have been recorded in C. carpio fed with mix of Malvae sylvestris, Origanum vulgare, and Allium hirtifolium [51], Pandanus tectorius extract [52], and Scrophularia striata extract [53].
In our study, cholesterol and triglyceride levels in the serum decreased when fish was fed PAE supplemented diets, especially at level of 0.25%. Similarly, when herbal extracts like Avena sativa extract [54], dandelion extract [55], Coriandrum sativum, Malva sylvestris and Quercus brantii [56] were added to diets of C. carpio, it was reported that they decreased serum cholesterol or triglyceride levels of fish. These hypolipidemic effects of herbal extracts can be explained by the inhibiting the activity of HMG-CoA reductase in the liver and inhibiting intestinal acyl CoA: cholesterol acyl transferase and/or increases of the serum lipoprotein lipase and hepatic lipase activities as reported previously [57, 58, 59].
ACH50 and lysozyme are important parameters benefitted in the evaluation of innate immune system in fish. In this study, serum ACH50 and lysozyme activities significantly raised in PAE incorporated groups (0.5%–2%) compared to the control group before the stress exposure. Moreover, supplementation with PAE improved the immune system of fish in the present study, demonstrated by the enchanted total immunoglobulin concentration in the serum. Similarly, previous reports suggest that increasing ACH50, lysozyme, and/or total immunoglobulin activities in fish fed diets incorporated with exogenous herbal feed additives [48, 49, 60]. The increase in immune parameters of fish fed with PAE can be attributed to stronger antioxidant defenses, as a positive relationship between antioxidant enzyme activities, and immune responses has been reported in many studies before. In conclusion, the fish seem to be able to develop antioxidant defenses and produce more immune components when PAE is administered. According to the published report, a total of 26 components have been found in P. abrotanoides during the flowering stage. According to the findings, carotol (31.15%) was the most abundant ingredient in the P. abrotanoides extract, followed by naphthalene, 1,2,3,5,6,8a-hexahydro-4, 7-dimethyl-1-(1-methylethyl) (1S-cis) (14.251%), and agarospirol (7.233%). The composition of IR-alpha-pinene (0.268%) was likewise found to have the lowest value [27].
Antioxidant defense systems of aquatic organisms include free radical scavengers, reduced glutathione (GSH), and specific antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx). The increased malondialdehyde (MDA) content is routinely used as a biomarker of lipid peroxidation in the liver [61]. In the present study, increases in SOD and GPx activities by dietary PAE before high-temperature exposure, which corresponds to the improved antioxidant capacity, are supported by lower MDA content. Similar results are in full agreement with findings of earlier studies in common carp, fed different herbal extracts [62, 63, 64]. Moreover, previous studies showed that antioxidant responses exhibit a vital role in antioxidant response in different stress conditions like high temperature [30, 65], ammonia stress [10], high stocking density and/or acute crowding stress [66, 67], hypoxia [65], and cold temperature [68]. As a result, the better antioxidant status following incorporations with PAE observed in this study may be indicative of higher stress prevention capacity in fish. Similarly, oral administration of anthraquinone extract supplement have resulted increased SOD and decreased MDA levels in giant freshwater prawn, Macrobrachium rosenbergii, under high-temperature stress [69].
Cortisol is used as a main stress indicator in fish studies, while serum glucose is a subsequent one [70]. In this study, high-temperature exposure elevated cortisol and glucose levels in fish serum. Increase in serum cortisol level is response to adrenocorticotropic hormone (ACTH) which is the dominant secretagogue of cortisol release from inter-renal tissue under stress conditions [71, 72]. Additionally, high levels of glucose in common carp serum under high-temperature stress may be a result of increased energy requirements during stress [73, 74]. A similar increase in serum glucose and cortisol levels at higher temperature was observed in Wuchang bream, Megalobrama amblycephala [73]. In contrast to our findings, however, reductions in glucose level in rainbow trout serum was observed under higher temperature (21°C) stress conditions [65]. These different results might be associated with differences in fish species, fish size, fish age, rearing conditions, and exposure time.
Previous studies reported that herbal extracts showed a remarkable antistress effect on fish [24]. The role of herbal extracts is probably attributed to the effect of active metabolites in improving the oxidative condition and organ health. Based on the present results, when fish were administered with 0.25%–1% PAE diets, the cortisol and glucose levels in fish serum significantly decreased in the poststress groups. This was supported in a recent study, where anthraquinone extract from R. officinale Bail decrease heat-induced serum glucose and/or cortisol elevation in fish [73].
Serum enzyme variables are accepted important indexes of tissue damages; for example, AST and ALT are indexes of liver damage [75] and ALP for biliary tract damages in fish [76]. Therefore, enhances in AST, ALT, and/or ALP in fish serum could be indicator for liver damage. In this study, serum AST, ALT, and ALP levels were markedly decreased when the fish were fed with different PAE levels before and after high-temperature stress. Previous reports showed that serum AST, ALT, and/or ALP activities provided a decrease when common carps were fed with herbal extracts [51, 77]. To our knowledge, no studies investigated the serum enzyme levels of fish fed with PAE-supplemented feeds were found under the high-temperature stress. Liu et al. [73] reported that AST and ALT activities in Wuchang bream, M. amblycephala, fed a diet containing anthraquinone extract under high-temperature stress were significantly lower than those of the control fish. Moreover, Liu et al. [69] reported that dietary anthraquinone extract removed the adverse effects of high-temperature stress on AST and ALT levels in serum in the freshwater prawn, M. rosenbergii.
5. Conclusions
In summary, the findings of this study indicate that including Perovskia abrotanoides extract (PAE) in the diets significantly enhanced growth performance, bolstered digestive enzymes, and improved immune parameters and antioxidant status in common carp while also providing protection against high-temperature fish stress.
Acknowledgments
This work was supported by Gonbad Kavous University, Gonbad, Golestan, Iran (grant number 6/00/96). This paper has been supported by the RUDN University Strategic Academic Leadership Program dedicated to Morteza Yousefi.
Data Availability
The data are available from the corresponding author upon reasonable request.
Ethical Approval
This project was implemented in meeting number 193287 of the research council of Gonbad Kavos University and approved by the ethics committee (165; 588027) according to the animal sampling protocol.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Hossein Adineh was responsible for the conceptualization, project administration, formal analysis, and writing draft; Saeed Zahedi was responsible for formal analysis and writing draft; Morteza Yousefi was responsible for the conceptualization, methodology, and writing draft; Sevdan Yilmaz was responsible for the investigation, writing draft, and review and editing of the manuscript; Zeynab Sedaghat helped in the implementation of the experiment; Ebrahim Gholamalipour Alamdari was responsible for the field of identification of medicinal plants and preparation and analysis of herbal extracts; Mohammad Farhangi was responsible for methodology and validation.
References
- 1.Thilsted S. H., Thorne-Lyman A., Webb P., et al. Sustaining healthy diets: the role of capture fisheries and aquaculture for improving nutrition in the post-2015 era. Food Policy . 2016;61:126–131. [Google Scholar]
- 2.FAO. The state of world fisheries and aquaculture 2020: sustainability in action. 2020. [DOI]
- 3.Awad E., Awaad A. Role of medicinal plants on growth performance and immune status in fish. Fish & Shellfish Immunology . 2017;67:40–54. doi: 10.1016/j.fsi.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Ahmadifar E., Dawood M. A., Moghadam M. S., Sheikhzadeh N., Hoseinifar S. H., Musthafa M. S. Modulation of immune parameters and antioxidant defense in zebrafish (Danio rerio) using dietary apple cider vinegar. Aquaculture . 2019;513734412 [Google Scholar]
- 5.Stankus A. State of world aquaculture 2020 and regional reviews: FAO webinar series. 2021;63:17–18. FAO Aquaculture Newsletter. [Google Scholar]
- 6.Bostock J., McAndrew B., Richards R., et al. Aquaculture: global status and trends. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences . 2010;365(1554):2897–2912. doi: 10.1098/rstb.2010.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghelichpour M., Mirghaed A. T. Effects of sublethal exposure to new pesticides lufenuron and flonicamid on common carp, Cyprinus carpio, hydromineral balance to further saltwater exposure. International Journal of Aquatic Biology . 2019;7(4):195–201. [Google Scholar]
- 8.Ghelichpour M., Taheri Mirghaed A., Hoseini S. M., Perez Jimenez A. Plasma antioxidant and hepatic enzymes activity, thyroid hormones alterations and health status of liver tissue in common carp (Cyprinus carpio) exposed to lufenuron. Aquaculture . 2020;516734634 [Google Scholar]
- 9.Barton B. A., Morgan J. D., Vijayan M. M. Physiological and condition-related indicators of environmental stress in fish. In: Adams S. M., editor. Biological Indicators of Aquatic Ecosystem Stress . Bethesda: American Fisheries Society; 2002. pp. 111–148. [Google Scholar]
- 10.Hoseini S. M., Gharavi B., Mirghaed A. T., Hoseinifar S. H., Van Doan H. Effects of dietary phytol supplementation on growth performance, immunological parameters, antioxidant and stress responses to ammonia exposure in common carp, Cyprinus carpio (Linnaeus 1758) Aquaculture. 2021;545737151 [Google Scholar]
- 11.Hoseinifar S. H., Shakouri M., Doan H. V., et al. Dietary supplementation of lemon verbena (Aloysia citrodora) improved immunity, immune-related genes expression and antioxidant enzymes in rainbow trout (Oncorrhyncus mykiss) Fish & Shellfish Immunology . 2020;99:379–385. doi: 10.1016/j.fsi.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Liu X., Shi H., Liu Z., Kang Y., Wang J., Huang J. Effect of heat stress on heat shock protein 30 (Hsp30) mrna expression in rainbow trout (Oncorhynchus mykiss) Turkish Journal of Fisheries and Aquatic Sciences . 2019;19(8):681–688. [Google Scholar]
- 13.Cui Y., Liu B., Xie J., Xu P., Habte-Tsion H.-M., Zhang Y. Effect of heat stress and recovery on viability, oxidative damage, and heat shock protein expression in hepatic cells of grass carp (Ctenopharyngodon idellus) Fish Physiology and Biochemistry . 2014;40(3):721–729. doi: 10.1007/s10695-013-9879-2. [DOI] [PubMed] [Google Scholar]
- 14.Mozaffarian V. A Dictionary of Iranian Plant Names . Tehran: Farhang Moaser; 1996. [Google Scholar]
- 15.Hosseinzadeh H., Amel S. Antinociceptive effects of the aerial parts of Perovskia abrotanoides extracts in mice. 2001.
- 16.Sairafianpour M., Christensen J., Staerk D., et al. Leishmanicidal, antiplasmodial, and cytotoxic activity of novel diterpenoid 1, 2-quinones from Perovskia abrotanoides: new source of tanshinones. Journal of Natural Products . 2001;64(11):1398–1403. doi: 10.1021/np010032f. [DOI] [PubMed] [Google Scholar]
- 17.Jaafari M. R., Hooshmand S., Samiei A., Hossainzadeh H. Evaluation of-leishmanicidal effect of Perovskia abrotanoides karel root extract by in vitro leishmanicidal assay using promastigotes of leishmania major. Pharmacol Online . 2007;1(1):299–303. [Google Scholar]
- 18.Mahboubi M., Kazempour N. The antimicrobial activity of essential oil from Perovskia abrotanoides karel and its main components. Indian Journal of Pharmaceutical Sciences . 2009;71(3):343–7. doi: 10.4103/0250-474X.56016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moallem S. A., Niapour M. Study of embryotoxicity of Perovskia abrotanoides, an adulterant in folk-medicine, during organogenesis in mice. Journal of Ethnopharmacology . 2008;117(1):108–114. doi: 10.1016/j.jep.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Kumar G. P., Gupta S., Murugan M. P., Singh S. B. Ethnobotanical studies of Nubra Valley-a cold arid zone of Himalaya. Ethnobotanical Leaflets . 2009;2009(6):p. 9. [Google Scholar]
- 21.Tareen R. B., Bibi T., Khan M. A., Ahmad M., Zafar M., Hina S. Indigenous knowledge of folk medicine by the women of Kalat and Khuzdar regions of Balochistan, Pakistan. Pakistan Journal of Botany . 2010;42(3):1465–1485. [Google Scholar]
- 22.Nasiriasl M., Parvardeh S., Niapour M., Hosseinzadeh H. Antinociceptive and anti-inflammatory effects of Perovskia abrotanoides aerial part extracts in mice and rats. 2002.
- 23.Morteza-Semnani K. The essential oil composition of Perovskia abrotanoides from Iran. Pharmaceutical Biology . 2004;42(3):214–216. [Google Scholar]
- 24.Chakraborty S. B., Hancz C. Application of phytochemicals as immunostimulant, antipathogenic and antistress agents in finfish culture. Reviews in Aquaculture . 2011;3(3):103–119. [Google Scholar]
- 25.Yousefi M., Hoseini S. M., Kulikov E. V., et al. Effects of dietary Hyssop, Hyssopus officinalis, extract on physiological and antioxidant responses of rainbow trout, Oncorhynchus mykiss, juveniles to thermal stress. Frontiers in Veterinary Science . 2022;9 doi: 10.3389/fvets.2022.1042063.1042063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar D. S., Banji D., Harani A., et al. Screening of polyphenolic compounds in Echinochloa crusgalli Roxb extracts by various analytical techniques. Asian Journal of Chemistry . 2013;25(17):9848–9852. doi: 10.14233/ajchem.2013.15505. [DOI] [Google Scholar]
- 27.Alamdari E. G. GC-MS analysis of the essential oil composition and antioxidant activity of Perovskia abrotanoides Kar. from different growth stages. Indian Journal of Natural Products and Resources (IJNPR) . 2021;12(2):230–237. [Google Scholar]
- 28.Yousefi M., Adineh H., Reverter M., et al. Protective effects of black seed (Nigella sativa) diet supplementation in common carp (Cyprinus carpio) against immune depression, oxidative stress and metabolism dysfunction induced by glyphosate. Fish & Shellfish Immunology . 2021;109:12–19. doi: 10.1016/j.fsi.2020.11.032. [DOI] [PubMed] [Google Scholar]
- 29.Ashaf-Ud-Doulah M., Mamun A. A., Rahman M. L., et al. High temperature acclimation alters upper thermal limits and growth performance of Indian major carp, rohu, Labeo rohita (Hamilton, 1822) Journal of Thermal Biology . 2020;93 doi: 10.1016/j.jtherbio.2020.102738.102738 [DOI] [PubMed] [Google Scholar]
- 30.Dawood M. A. O., Eweedah N. M., Elbialy Z. I., Abdelhamid A. I. Dietary sodium butyrate ameliorated the blood stress biomarkers, heat shock proteins, and immune response of Nile tilapia (Oreochromis niloticus) exposed to heat stress. Journal of Thermal Biology . 2020;88 doi: 10.1016/j.jtherbio.2019.102500.102500 [DOI] [PubMed] [Google Scholar]
- 31.Adineh H., Naderi M., Yousefi M., Khademi Hamidi M., Ahmadifar E., Hoseini S. M. Dietary licorice (Glycyrrhiza glabra) improves growth, lipid metabolism, antioxidant and immune responses, and resistance to crowding stress in common carp, Cyprinus carpio. Aquaculture Nutrition . 2021;27(2):417–426. doi: 10.1111/anu.13194. [DOI] [Google Scholar]
- 32.Adineh H., Naderi M., Khademi Hamidi M., Harsij M. Biofloc technology improves growth, innate immune responses, oxidative status, and resistance to acute stress in common carp (Cyprinus carpio) under high stocking density. Fish & Shellfish Immunology . 2019;95:440–448. doi: 10.1016/j.fsi.2019.10.057. [DOI] [PubMed] [Google Scholar]
- 33.Rungruangsak-Torrissen K., Rustad A., Sunde J., et al. In vitro digestibility based on fish crude enzyme extract for prediction of feed quality in growth trials. Journal of the Science of Food and Agriculture . 2002;82(6):644–654. [Google Scholar]
- 34.Cahu C. L., Infante J. Z., Quazuguel P., Le Gall M. M. Protein hydrolysate vs. fish meal in compound diets for 10-day old sea bass Dicentrarchus labrax larvae. Aquaculture . 1999;171(1-2):109–119. [Google Scholar]
- 35.Langlois A., Corring T., Fevrier C. Effects of wheat bran on exocrine pancreas secretion in the pig. Reproduction Nutrition Development . 1987;27(5):929–939. doi: 10.1051/rnd:19870705. [DOI] [PubMed] [Google Scholar]
- 36.Iijima N., Tanaka S., Ota Y. Purification and characterization of bile salt-activated lipase from the hepatopancreas of red sea bream, Pagrus major. Fish Physiology and Biochemistry . 1998;18(1):59–69. [Google Scholar]
- 37.Walter H. E. Proteases and their inhibitors. 1984. pp. 270–277. 2. 15. 2 Method with haemoglobin, casein and azocoll as substrate. Methods of Enzymatic Analysis.
- 38.Demers N. E., Bayne C. J. The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Developmental and Comparative Immunology . 1997;21(4):363–373. doi: 10.1016/s0145-305x(97)00009-8. [DOI] [PubMed] [Google Scholar]
- 39.Sunyer J. O., Tort L. Natural hemolytic and bactericidal activities of sea bream Sparus aurata serum are effected by the alternative complement pathway. Veterinary Immunology and Immunopathology . 1995;45(3-4):333–345. doi: 10.1016/0165-2427(94)05430-z. [DOI] [PubMed] [Google Scholar]
- 40.Yeh S.-P., Chang C.-A., Chang C.-Y., Liu C.-H., Cheng W. Dietary sodium alginate administration affects fingerling growth and resistance to Streptococcus sp. and iridovirus, and juvenile non-specific immune responses of the orange-spotted grouper, Epinephelus coioides. Fish & Shellfish Immunology . 2008;25(1-2):19–27. doi: 10.1016/j.fsi.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Siwicki A. I., Anderson D. P. Nonspecific defense mechanisms assay in fish. II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin (Ig) level in serum. In: Siwicki A. K., Anderson D. P., Waluga J., editors. Fish Diseases Diagnosis and Preventions Methods . Olsztyn: Wydawnictwo Instytutu Rybactwa Strodladowego; 1993. pp. 105–111. [Google Scholar]
- 42.Hoseini S. M., Paolucci M., Arghideh M., et al. Effects of dietary glycine administration on biochemical responses to ammonia toxicity in common carp, Cyprinus carpio. Aquaculture Research . 2022;53(6):2185–2194. doi: 10.1111/are.15737. [DOI] [Google Scholar]
- 43.Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clinica Chimica Acta . 1991;196(2-3):143–151. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 44.Citarasu T. Herbal biomedicines: a new opportunity for aquaculture. Aquaculture International . 2010;18(3):403–414. [Google Scholar]
- 45.Jana P., Karmakar S., Roy U., Paul M., Bera A. K. S. Phytobiotics in aquaculture health management: a review. Journal of Entomology and Zoology Studies . 2018;6(4):1422–1429. [Google Scholar]
- 46.Caipang C. M. A. Phytogenics in aquaculture: a short review of their effects on gut health and microflora in. The Philippine Journal of Fisheries . 2020;27(2):11–22. [Google Scholar]
- 47.Mohiseni M. Medicinal herbs, strong source of antioxidant in aquaculture:a mini review. Modern Applications in Pharmacy & Pharmacology . 2017;1 [Google Scholar]
- 48.Elumalai P., Kurian A., Lakshmi S., Faggio C., Esteban M. A., Ringø E. Herbal immunomodulators in aquaculture. Reviews in Fisheries Science & Aquaculture . 2020;29(1):33–57. [Google Scholar]
- 49.Tadese D. A., Song C., Sun C., et al. The role of currently used medicinal plants in aquaculture and their action mechanisms: a review. Reviews in Aquaculture . 2022;14(2):816–847. [Google Scholar]
- 50.Yılmaz S., Ergün S. Trans-cinnamic acid application for rainbow trout (Oncorhynchus mykiss): I. Effects on haematological, serum biochemical, non-specific immune and head kidney gene expression responses. Fish & Shellfish Immunology . 2018;78:140–157. doi: 10.1016/j.fsi.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 51.Ghafarifarsani H., Hoseinifar S. H., Adorian T. J., Ferrigolo F. R. G., Raissy M., Van Doan H. The effects of combined inclusion of Malvae sylvestris, Origanum vulgare, and Allium hirtifolium boiss for common carp (Cyprinus carpio) diet: growth performance, antioxidant defense, and immunological parameters. Fish & Shellfish Immunology . 2021;119:670–677. doi: 10.1016/j.fsi.2021.10.014. [DOI] [PubMed] [Google Scholar]
- 52.Cheng C., Park S. C., Giri S. S. Effect of Pandanus tectorius extract as food additive on oxidative stress, immune status, and disease resistance in Cyprinus carpio. Fish & Shellfish Immunology . 2022;120:287–294. doi: 10.1016/j.fsi.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Hosseini H., Pooyanmehr M., Foroughi A., Esmaeili M., Ghiasi F., Lorestany R. Remarkable positive effects of figwort (Scrophularia striata) on improving growth performance, and immunohematological parameters of fish. Fish & Shellfish Immunology . 2022;120:111–121. doi: 10.1016/j.fsi.2021.11.020. [DOI] [PubMed] [Google Scholar]
- 54.Baba E., Acar Ü., Öntaş C., Kesbiç O. S., Yilmaz S. The use of Avena sativa extract against Aeromonas hydrophila and its effect on growth performance, hematological and immunological parameters in common carp (Cyprinus carpio) Italian Journal of Animal Science . 2016;15(2):325–333. [Google Scholar]
- 55.Xue S., Xia B., Zou Y., et al. Dandelion extract on growth performance, immunity, stress and infection resistance in common carp. Aquaculture Reports . 2022;26101330 [Google Scholar]
- 56.Raissy M., Ghafarifarsani H., Hoseinifar S. H., El-Haroun E. R., Naserabad S. S., Van Doan H. The effect of dietary combined herbs extracts (oak acorn, coriander, and common mallow) on growth, digestive enzymes, antioxidant and immune response, and resistance against Aeromonas hydrophila infection in common carp, Cyprinus carpio. Aquaculture . 2022;546737287 [Google Scholar]
- 57.Jung U. J., Lee M. K., Park Y. B., Kang M. A., Choi M. S. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. The International Journal of Biochemistry & Cell Biology . 2006;38(7):1134–1145. doi: 10.1016/j.biocel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Touiss I., Khatib S., Bekkouch O., Amrani S., Harnafi H. Phenolic extract from Ocimum basilicum restores lipid metabolism in Triton WR-1339-induced hyperlipidemic mice and prevents lipoprotein-rich plasma oxidation. Food Science and Human Wellness . 2017;6(1):28–33. [Google Scholar]
- 59.Sari W. F., Suwondo A. A literature review of effect of Moringa oleifera leaf extract toward lipid profile level in hyperlipidemia patients. International Journal of Nursing and Health Services (IJNHS) . 2022;5(3):294–303. [Google Scholar]
- 60.Ahmadifar E., Pourmohammadi Fallah H., Yousefi M., et al. The gene regulatory roles of herbal extracts on the growth, immune system, and reproduction of fish. Animals . 2021;11(8) doi: 10.3390/ani11082167.2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poli G., Albano E., Dianzani M. U. The role of lipid peroxidation in liver damage. Chemistry and Physics of Lipids . 1987;45(2–4):117–142. doi: 10.1016/0009-3084(87)90063-6. [DOI] [PubMed] [Google Scholar]
- 62.Mirghaed A. T., Paknejad H., Mirzargar S. S. Hepatoprotective effects of dietary Artemisia (Artemisia annua) leaf extract on common carp (Cyprinus carpio) exposed to ambient ammonia. Aquaculture . 2020;527735443 [Google Scholar]
- 63.Paray B. A., Hoseini S. M., Hoseinifar S. H., Van Doan H. Effects of dietary oak (Quercus castaneifolia) leaf extract on growth, antioxidant, and immune characteristics and responses to crowding stress in common carp (Cyprinus carpio) Aquaculture . 2020;524735276 [Google Scholar]
- 64.Yousefi M., Zahedi S., Reverter M., et al. Enhanced growth performance, oxidative capacity and immune responses of common carp, Cyprinus carpio fed with Artemisia absinthium extract-supplemented diet. Aquaculture . 545737167 [Google Scholar]
- 65.Jiang X., Dong S., Liu R., et al. Effects of temperature, dissolved oxygen, and their interaction on the growth performance and condition of rainbow trout (Oncorhynchus mykiss) Journal of Thermal Biology . 2021;98 doi: 10.1016/j.jtherbio.2021.102928. 102928. [DOI] [PubMed] [Google Scholar]
- 66.Hoseini S. M., Aydın B., Hoseinifar S. H., Moonmanee T., Van Doan H. Dietary Artemisia, Artemisia annua, supplementation improves common carp welfare under high stocking density. Aquaculture Research . 2022;53(9):3494–3503. doi: 10.1111/are.15855. [DOI] [Google Scholar]
- 67.Adineh H., Naderi M., Jafaryan H., Khademi Hamidi M., Yousefi M., Ahmadifar E. Effect of stocking density and dietary protein level in biofloc system on the growth, digestive and antioxidant enzyme activities, health, and resistance to acute crowding stress in juvenile common carp (Cyprinus carpio) Aquaculture Nutrition . 2022;2022:12. doi: 10.1155/2022/9344478.9344478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yilmaz S., Ergün S., Çelik E., Banni M., Ahmadifar E., Dawood M. A. O. The impact of acute cold water stress on blood parameters, mortality rate and stress-related genes in Oreochromis niloticus, Oreochromis mossambicus and their hybrids. Journal of Thermal Biology . 2021;100 doi: 10.1016/j.jtherbio.2021.103049.103049 [DOI] [PubMed] [Google Scholar]
- 69.Liu B., Xie J., Ge X., et al. Effects of anthraquinone extract from Rheum officinale Bail on the growth performance and physiological responses of Macrobrachium rosenbergii under high temperature stress. Fish & Shellfish Immunology . 2010;29(1):49–57. doi: 10.1016/j.fsi.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 70.Mazeaud M. M., Mazeaud F., Donaldson E. M. Primary and secondary effects of stress in fish: some new data with a general review. Transactions of the American Fisheries Society . 1977;106(3):201–212. [Google Scholar]
- 71.Barton B. A., Iwama G. K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annual Review of Fish Diseases . 1991;1:3–26. [Google Scholar]
- 72.Mommsen T. P., Vijayan M. M., Moon T. W. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Reviews in Fish Biology and Fisheries . 1999;9(3):211–268. [Google Scholar]
- 73.Liu B., Xie J., Ge X., et al. Comparison study of the effects of anthraquinone extract and emodin from Rheum officinale Bail on the physiological response, disease resistance of Megalobrama amblycephala under high temperature stress. Turkish Journal of Fisheries and Aquatic Sciences . 2012;12(4) [Google Scholar]
- 74.Rodríguez-Quiroga J. J., Otero-Rodiño C., Suárez P., et al. Differential effects of exposure to parasites and bacteria on stress response in turbot Scophthalmus maximus simultaneously stressed by low water depth. Journal of Fish Biology . 2017;91(1):242–259. doi: 10.1111/jfb.13338. [DOI] [PubMed] [Google Scholar]
- 75.Yılmaz S., Ergun S., Şanver Çelik E., Yigit M., Bayizit C. Dietary trans-cinnamic acid application for rainbow trout (Oncorhynchus mykiss): II. Effect on antioxidant status, digestive enzyme, blood biochemistry and liver antioxidant gene expression responses. Aquaculture Nutrition . 2019;25(6):1207–1217. doi: 10.1111/anu.12935. [DOI] [Google Scholar]
- 76.Er A., Dik B. The effects of florfenicol on the values of serum tumor necrosis factor-and other biochemical markers in lipopolysaccharide-induced endotoxemia in brown trout. Mediators of inflammation. 2014. [DOI] [PMC free article] [PubMed]
- 77.Rankouhi S. E. R., Nejad M. M., Langrodi H. F., Moghadam A. A. Effects of dietary supplementation of Kakuti, Ziziphora clinopodioides, extract on enzymatic and antioxidant parameters of the plasma of common carp Cyprinus carpio. Iranian Journal of Fisheries Sciences . 2021;20(2):385–395. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author upon reasonable request.


