Abstract
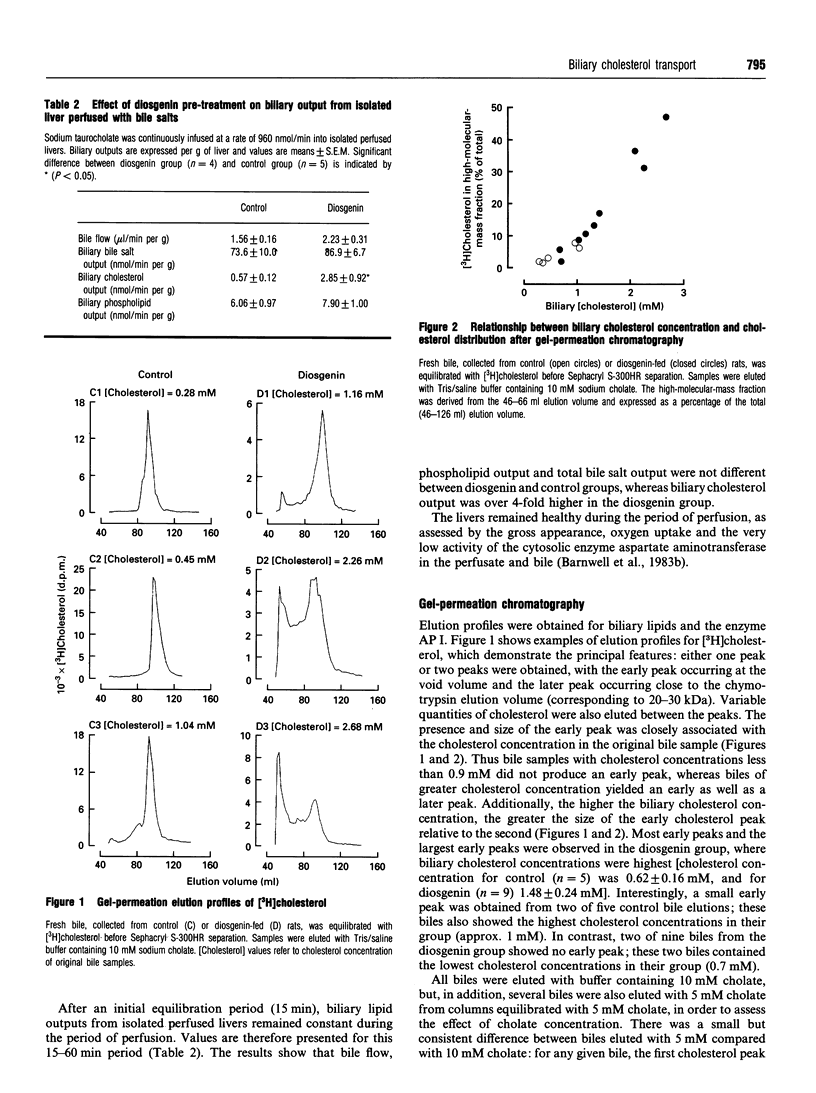
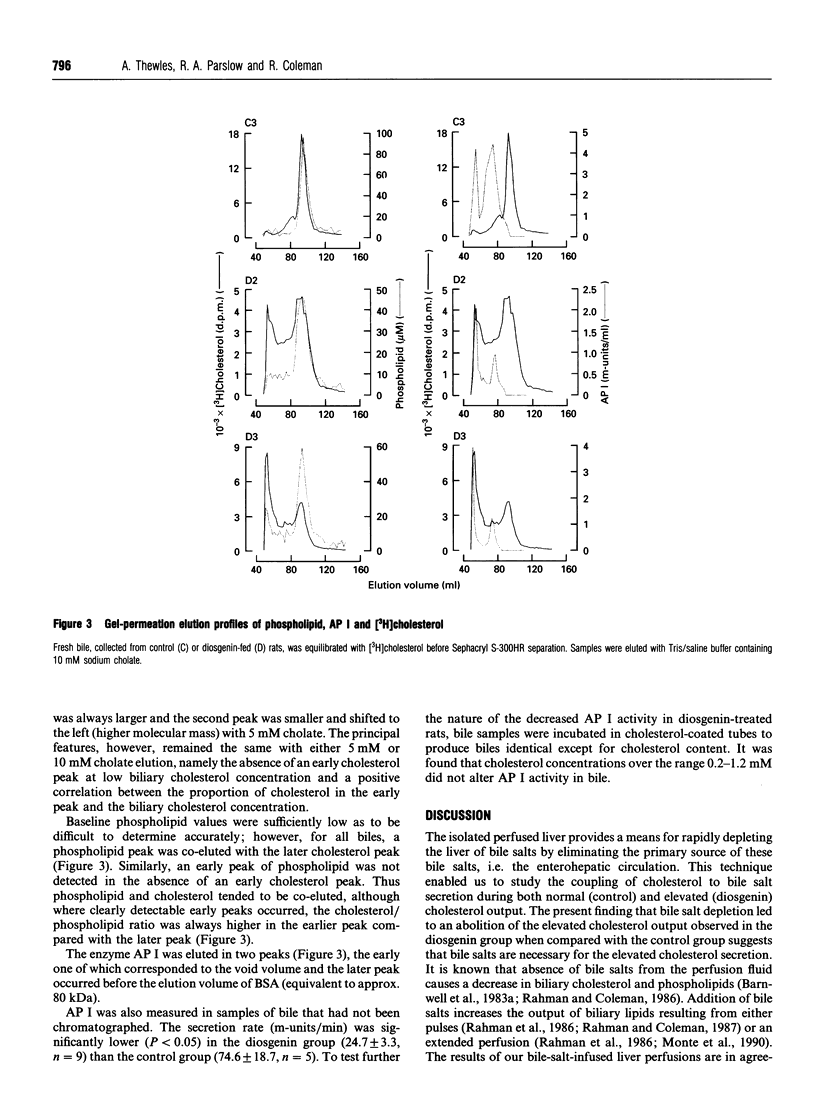
Biliary cholesterol output in rats was stimulated over 3-fold by feeding diosgenin for 5 days, whereas biliary outputs of phospholipid and bile salts were not changed by diosgenin feeding. Isolating and perfusing the liver without bile salts resulted in a rapid and substantial decrease in biliary bile salt output; bile salt depletion abolished the diosgenin-induced increment in biliary cholesterol output, showing that the diosgenin-elevated biliary cholesterol output was bile-salt-dependent. Diosgenin treatment also produced a significant decrease in biliary alkaline phosphodiesterase I. Fresh bile obtained from control and diosgenin-fed rats was subjected to gel-permeation chromatography in order to separate different-sized biliary cholesterol carriers. Two major peaks of cholesterol were eluted, with cholesterol also being eluted between the peaks. The cholesterol peak eluted at the lower molecular mass (20-30 kDa) was observed in all bile samples. The higher-molecular-mass peak, which was eluted at the void volume, was not observed in all biles; control biles contained very little high-molecular-mass form of cholesterol, whereas biles from the diosgenin group contained up to 47% of cholesterol in the high-molecular-mass fraction. Diosgenin treatment produced a range of elevated biliary cholesterol values which positively correlated with the proportion of cholesterol contained in the high-molecular-mass fraction (r = 0.98). The results show that diosgenin induced a marked bile-salt-dependent increase in biliary cholesterol output and a shift in biliary cholesterol transport to higher-molecular-mass structures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barnwell S. G., Godfrey P. P., Lowe P. J., Coleman R. Biliary protein output by isolated perfused rat livers. Effects of bile salts. Biochem J. 1983 Feb 15;210(2):549–557. doi: 10.1042/bj2100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayen M. N., Dvornik D. Effect of diosgenin on lipid metabolism in rats. J Lipid Res. 1979 Feb;20(2):162–174. [PubMed] [Google Scholar]
- Cederbaum A. I., Dicker E. Inhibition of microsomal oxidation of alcohols and of hydroxyl-radical-scavenging agents by the iron-chelating agent desferrioxamine. Biochem J. 1983 Jan 15;210(1):107–113. doi: 10.1042/bj2100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. Biochemistry of bile secretion. Biochem J. 1987 Jun 1;244(2):249–261. doi: 10.1042/bj2440249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Iqbal S., Godfrey P. P., Billington D. Membranes and bile formation. Composition of several mammalian biles and their membrane-damaging properties. Biochem J. 1979 Jan 15;178(1):201–208. doi: 10.1042/bj1780201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Rahman K. Lipid flow in bile formation. Biochim Biophys Acta. 1992 Apr 23;1125(2):113–133. doi: 10.1016/0005-2760(92)90036-u. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., Spady D. K. Measurement of rates of cholesterol synthesis using tritiated water. J Lipid Res. 1984 Dec 15;25(13):1469–1476. [PubMed] [Google Scholar]
- Godfrey P. P., Warner M. J., Coleman R. Enzymes and proteins in bile. Variations in output in rat cannula bile during and after depletion of the bile-salt pool. Biochem J. 1981 Apr 15;196(1):11–16. doi: 10.1042/bj1960011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Z., Dudley M. A., Kibe A., Lynn M. P., Breuer A. C., Holzbach R. T. Rapid vesicle formation and aggregation in abnormal human biles. A time-lapse video-enhanced contrast microscopy study. Gastroenterology. 1986 Apr;90(4):875–885. doi: 10.1016/0016-5085(86)90863-2. [DOI] [PubMed] [Google Scholar]
- Harvey P. R., Somjen G., Lichtenberg M. S., Petrunka C., Gilat T., Strasberg S. M. Nucleation of cholesterol from vesicles isolated from bile of patients with and without cholesterol gallstones. Biochim Biophys Acta. 1987 Sep 25;921(2):198–204. doi: 10.1016/0005-2760(87)90019-1. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F. Bile acid secretion, bile flow and biliary lipid secretion in humans. Hepatology. 1990 Sep;12(3 Pt 2):17S–25S. [PubMed] [Google Scholar]
- Holzbach R. T. Nucleation of cholesterol crystals in native bile. Hepatology. 1990 Sep;12(3 Pt 2):155S–161S. [PubMed] [Google Scholar]
- Kibe A., Holzbach R. T., LaRusso N. F., Mao S. J. Inhibition of cholesterol crystal formation by apolipoproteins in supersaturated model bile. Science. 1984 Aug 3;225(4661):514–516. doi: 10.1126/science.6429856. [DOI] [PubMed] [Google Scholar]
- Lowe P. J., Barnwell S. G., Coleman R. Rapid kinetic analysis of the bile-salt-dependent secretion of phospholipid, cholesterol and a plasma-membrane enzyme into bile. Biochem J. 1984 Sep 15;222(3):631–637. doi: 10.1042/bj2220631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte M. J., Parslow R. A., Coleman R. Inhibitory action of cyclobutyrol on the secretion of biliary cholesterol and phospholipids. Biochem J. 1990 Feb 15;266(1):165–171. doi: 10.1042/bj2660165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nervi F., Bronfman M., Allalón W., Depiereux E., Del Pozo R. Regulation of biliary cholesterol secretion in the rat. Role of hepatic cholesterol esterification. J Clin Invest. 1984 Dec;74(6):2226–2237. doi: 10.1172/JCI111649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nervi F., Marinović I., Rigotti A., Ulloa N. Regulation of biliary cholesterol secretion. Functional relationship between the canalicular and sinusoidal cholesterol secretory pathways in the rat. J Clin Invest. 1988 Dec;82(6):1818–1825. doi: 10.1172/JCI113797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled Y., Halpern Z., Baruch R., Goldman G., Gilat T. Cholesterol nucleation from its carriers in human bile. Hepatology. 1988 Jul-Aug;8(4):914–918. doi: 10.1002/hep.1840080435. [DOI] [PubMed] [Google Scholar]
- Rahman K., Coleman R. Biliary lipid secretion and its control. Effect of taurodehydrocholate. Biochem J. 1987 Jul 15;245(2):531–536. doi: 10.1042/bj2450531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K., Coleman R. Selective biliary lipid secretion at low bile-salt-output rates in the isolated perfused rat liver. Effects of phalloidin. Biochem J. 1986 Jul 1;237(1):301–304. doi: 10.1042/bj2370301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K., Hammond T. G., Lowe P. J., Barnwell S. G., Clark B., Coleman R. Control of biliary phospholipid secretion. Effect of continuous and discontinuous infusion of taurocholate on biliary phospholipid secretion. Biochem J. 1986 Mar 1;234(2):421–427. doi: 10.1042/bj2340421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriever C. E., Jüngst D. Association between cholesterol-phospholipid vesicles and cholesterol crystals in human gallbladder bile. Hepatology. 1989 Apr;9(4):541–546. doi: 10.1002/hep.1840090406. [DOI] [PubMed] [Google Scholar]
- Stone B. G., Larsen L. J., Knoll D. A., Bloomfield V. A., Duane W. C. Separation of bile vesicles and micelles by gel filtration chromatography: the importance of the intermicellar bile salt concentration. J Lab Clin Med. 1992 May;119(5):557–565. [PubMed] [Google Scholar]
- Sömjen G. J., Coleman R., Koch M. H., Wachtel E., Billington D., Towns-Andrews E., Gilat T. The induction of lamellar stacking by cholesterol in lecithin-bile salt model systems and human bile studied by synchrotron X-radiation. FEBS Lett. 1991 Sep 9;289(2):163–166. doi: 10.1016/0014-5793(91)81060-l. [DOI] [PubMed] [Google Scholar]
- Sömjen G. J., Gilat T. Changing concepts of cholesterol solubility in bile. Gastroenterology. 1986 Sep;91(3):772–775. doi: 10.1016/0016-5085(86)90652-9. [DOI] [PubMed] [Google Scholar]
- Sömjen G. J., Gilat T. Contribution of vesicular and micellar carriers to cholesterol transport in human bile. J Lipid Res. 1985 Jun;26(6):699–704. [PubMed] [Google Scholar]
- Sömjen G. J., Marikovsky Y., Wachtel E., Harvey P. R., Rosenberg R., Strasberg S. M., Gilat T. Phospholipid lamellae are cholesterol carriers in human bile. Biochim Biophys Acta. 1990 Jan 16;1042(1):28–35. doi: 10.1016/0005-2760(90)90052-y. [DOI] [PubMed] [Google Scholar]
- Sömjen G. J., Rosenberg R., Gilat T. Gel filtration and quasielastic light scattering studies of human bile. Hepatology. 1990 Sep;12(3 Pt 2):123S–129S. [PubMed] [Google Scholar]
- Ulloa N., Garrido J., Nervi F. Ultracentrifugal isolation of vesicular carriers of biliary cholesterol in native human and rat bile. Hepatology. 1987 Mar-Apr;7(2):235–244. doi: 10.1002/hep.1840070206. [DOI] [PubMed] [Google Scholar]


