Abstract
The human T-cell leukemia virus type 1 Tax protein activates the expression of cellular immediate early genes controlled by the serum response element (SRE), which contains both the serum response factor (SRF) binding element (CArG box) and the ternary complex factor (TCF) binding element (Ets box). We show that TCF binding is necessary for Tax activation of the SRE and that Tax directly interacts with TCFs in vitro. In addition, Tax interactions with CREB binding protein (CBP) and p300- and CBP-associated factor were found to be essential for Tax activation of SRF-mediated transcription.
Human T-cell leukemia virus type 1 (HTLV-1) encodes a 40-kDa trans-regulatory protein, Tax, that was shown to activate c-fos transcription by interacting directly with the serum response factor (SRF) (5–7), which binds the central CC(A/T)6GG (CArG) sequence of the serum response element (SRE). Once SRF occupies the CArG element, the ternary complex factor (TCF) establishes protein interaction with SRF and binds DNA at the upstream GGA(A/T) site. Sap-1 or Sap-1a, Sap-2 or Sap-1b, and Elk-1, which are members of the Ets binding protein family, have been identified as TCFs (3, 4, 11, 12, 16, 18). It is known that signal-induced activation of c-fos expression requires SRF, TCF, and the CREB binding protein (CBP) (13, 14, 19). We have asked whether other cellular factors, in addition to SRF, are necessary for Tax-mediated activation of the SRE.
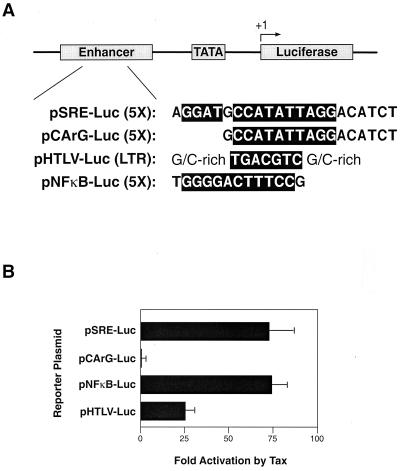
As an initial test of the role of TCFs in Tax-mediated induction of SRF-directed transcriptional activity, we compared the abilities of Tax proteins to activate reporter gene expression controlled by promoters containing either a full-length enhancer with a CArG box and a 5′-end-proximal TCF binding site (pSRE-Luc) or an enhancer with only the CArG box (pCArG-Luc) (Fig. 1A). Jurkat cells were transfected with each reporter plasmid in combination with a Tax expression plasmid (pRS-HTax1C) or an empty vector (pBS-KS-RSPA). Tax activated luciferase expression from pSRE-Luc by almost 70-fold (Fig. 1B). In contrast, pCArG-Luc, which lacks a TCF binding site, was not activated by Tax (Fig. 1B). For comparison, cotransfections were also performed with pHTLV-Luc, which contains the HTLV-1 promoter, or pNF-κB-Luc, which contains NF-κB binding sites. Tax activated luciferase expression directed by pHTLV-Luc and NF-κB-Luc by approximately 25-fold and 70-fold, respectively (Fig. 1B). Thus, the TCF binding site is essential for Tax activation of the SRE.
FIG. 1.
Cotransfection of luciferase reporter plasmids and Tax in Jurkat cells. (A) The transcription factor recognition sequences of the four luciferase reporter plasmids are highlighted. The plasmid pSRE-Luc contains five copies of the SRE element, which includes the CArG box and TCF binding site, pCArG-Luc contains five copies of the CArG box, pHTLV-Luc contains the HTLV-1 LTR with its three CRE sites, and pNF-κB-Luc contains five copies of the NF-κB DNA binding site. (B) Luciferase assay in which 2 μg of pRS-HTax1C or pBS-RSPA (nonspecific Rous sarcoma virus promoter-containing plasmid) was cotransfected with 2 μg of the indicated reporter plasmids in 2 × 106 Jurkat T cells using Superfect (Qiagen) in a six-well plate. The reporter plasmids are shown on the y axis, and the Tax activation levels normalized for background luciferase expression are presented on the x axis. All experiments were performed at least three times, and the error bars represent the standard deviations.
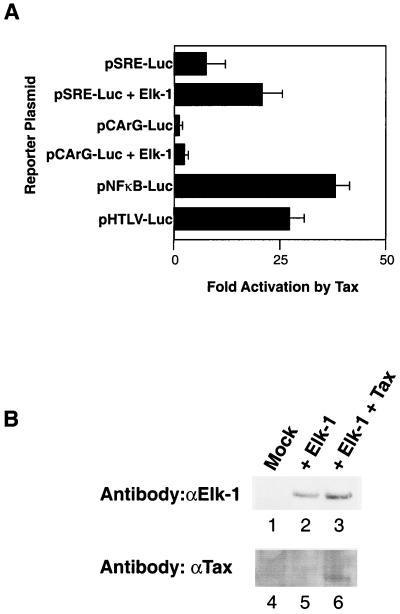
A similar pattern of Tax transactivation of reporter plasmids was observed in transfections of human 293 cells with the difference that pSRE-Luc was activated only fivefold by Tax (Fig. 2A). Ectopic expression of Elk-1 increased Tax activation of pSRE-Luc approximately 16-fold; however, Elk-1 expression alone had no effect on pCArG-Luc activity (Fig. 2A). Elk-1 protein was detected in 293 cells which had been transfected with an Elk-1 expression plasmid but not in untransfected cells (Fig. 2B). We performed the same transactivation assay with expression of Sap-1 instead of Elk-1, and Sap-1 increased Tax activation of pSRE-Luc but not of pCArG (data not shown). Therefore, Tax activation of the SRE in 293 cells may be augmented by ectopic expression of a TCF in this cell line.
FIG. 2.
Cotransfection of Tax with luciferase reporter plasmids in 293 cells. (A) Luciferase assay in which 0.25 μg of pRS-HTax1C was cotransfected with 0.25 μg of the indicated reporter plasmids in 5 × 104 293 cells with FuGene6 reagent (Roche) in 24-well plates. In experiments using Elk-1, either the Elk-1 expression plasmid (pElk-1-RSPA) or pBS-RSPA was transfected at 0.25 μg per well. The reporter plasmids are shown on the y axis, and activation levels by Tax are represented on the x axis. The experiment was repeated at least three times, and the error bars represent the standard deviations. (B) Expression level of Elk-1 in 293 cells as shown by Western blotting. Lysates are labeled at the top. Lanes 1 and 4 were loaded with mock-transfected lysates, lanes 2 and 5 were loaded with lysates from cells transfected with pElk-1-RSPA, and lanes 3 and 6 were loaded with lysates from cells transfected with both Elk-1 and Tax expression plasmids. Lysates were analyzed on 4 to 12% Bis-Tris gels (Novex), probed with a 1:1,000 dilution of rabbit anti-Elk-1 (αElk-1) (New England Biolabs) and rabbit anti-Tax antibodies, and visualized by chemiluminescence (New England Biolabs).
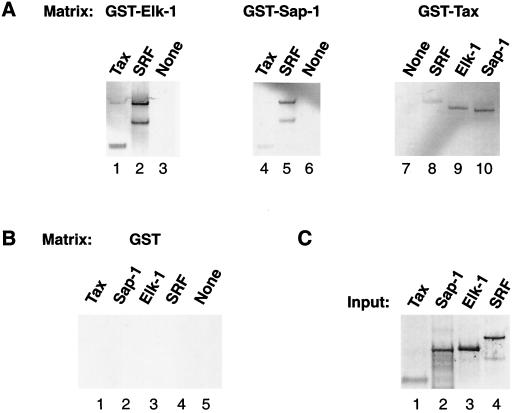
We next asked whether Tax directly interacts with either Sap-1a or Elk-1 proteins in vitro. Glutathione S-transferase (GST)–Sap-1, GST–Elk-1, and GST were immobilized on glutathione-Sepharose beads and incubated with in vitro-translated 35S-labeled Tax using the GST pull-down protocol previously described (21). The 35S-labeled Tax was retained on GST–Elk-1 and GST–Sap-1 beads but did not bind GST control beads (compare Fig. 3A, lanes 1 and 4, with Fig. 3B, lane 1). As a positive control, 35S-labeled SRF was incubated with GST–Elk-1 and GST-Sap1 and was retained on both matrices (Fig. 3A, lanes 2 and 5) but did not bind to GST (Fig. 3B, lane 4). A 50-kDa SRF polypeptide, which was synthesized in vitro from an alternative AUG start site located at nucleotide 312 of the cDNA (17) also bound the immobilized TCFs (Fig. 3A, lanes 2 and 5, lower band). In a reciprocal experiment, 35S-labeled SRF, Elk-1, and Sap-1 were incubated with GST-Tax, and all three proteins were retained on the GST-Tax matrix (Fig. 3A, lanes 8 to 10). The 35S-labeled input proteins for all GST pull-down assays are shown in Fig. 3C. Our results show that Tax and TCFs directly interact in vitro and suggest that Tax activation of the SRE in vivo may depend on Tax-TCF and Tax-SRF interactions.
FIG. 3.
GST pull-down assays reveal Tax-TCF interactions. (A) Two-microgram quantities of GST–Elk-1, GST–Sap-1 (both GST-TCF constructs were the gifts of Andrew Sharrocks, University of Manchester), and GST-Tax (a gift from Susan Marriott, Baylor University), which were bound to a 50% glutathione-Sepharose slurry, used to pull down the indicated 35S-labeled proteins. Lanes denoted by “None” contain the GST matrix alone; no lysate was incubated with the matrix. Bound proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiographed. (B) Results of the GST pull-down assay using the negative-control GST matrix are shown. (C) Full-length SRF, Elk-1, Sap-1a, and Tax were translated in vitro in a rabbit reticulocyte lysate system (Promega TNT system). Ten microliters (10% of total lysate) of input protein was used to indicate the amount of 35S-labeled lysate that was loaded onto the GST-bound matrix.
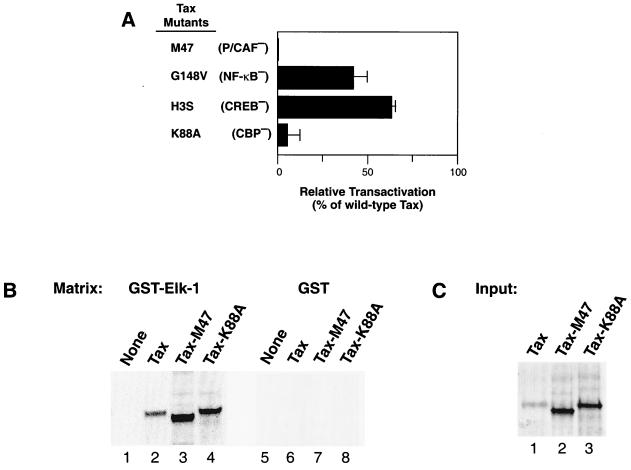
Mutant Tax proteins with specific transactivation phenotypes provide important tools for determining Tax function, particularly in the characterization of protein-protein interaction domains. To identify other components necessary for Tax activation of the SRE, we cotransfected Jurkat cells with a series of mutant Tax proteins and pSRE-Luc. Tax-M47, which contains two amino acid changes (L319R and L320S), cannot activate viral gene expression from the HTLV-1 long terminal repeat (LTR) due to the inability of Tax-M47 to interact with the p300- and CBP-associated factor (PCAF) (15, 22). No activation of pSRE-Luc was seen with Tax-M47 (Fig. 4A). Tax with the G148V mutation (Tax-G148V), which is analogous to Tax-M22 (T130S and L131A), can activate the CREB and SRF transcriptional pathways but cannot activate NF-κB due to a defect in associating with IKKγ (2, 8, 9, 22, 23). Tax-G148V retained almost 50% of wild-type Tax activity on pSRE-Luc (Fig. 4A). Both Tax-H3S and Tax-K88A are incapable of activating the HTLV-1 promoter, but they differ in their functional defects: Tax-H3S is unable to interact with the bZIP domain of CREB (1), whereas Tax-K88A is unable to bind CBP (10). Tax-H3S activated the SRE enhancer by almost 70% in Jurkat cells compared to the level of enhancement by wild-type Tax, whereas Tax-K88A was unable to activate pSRE-Luc (5% of the wild-type level of Tax) (Fig. 4A). To make certain that the defect in SRE activation was not due to a defect in TCF binding, we performed a GST pull-down assay with Tax-M47 and Tax-K88A. Compared to wild-type Tax (Fig. 4B, lane 2), Tax-M47 (Fig. 4B, lane 3) and Tax-K88A (Fig. 4B, lane 4) were not impaired in their ability to bind Elk-1. However, the inability of Tax-M47 and Tax-K88A to interact with PCAF and CBP, respectively, was detrimental to Tax transactivation of pSRE-Luc. None of the proteins bound the GST-only matrix (Fig. 4B, lanes 6 to 8), and the input proteins (Fig. 4C, lanes 1 to 3) are shown for comparison of the amounts of protein retained on GST–Elk-1 beads. The data show that both the CBP and PCAF binding domains of Tax are required for Tax activation of transcription from the SRE enhancer and that these Tax motifs are not important for Elk-1 binding in vitro.
FIG. 4.
Functional analysis of SRE transactivation phenotypes of mutant Tax proteins. (A) Jurkat cells were cotransfected with pSRE-Luc and plasmids bearing genes encoding the indicated mutant Tax proteins as described for Fig. 1. The levels of transactivation are reported relative to the levels of activation of Tax by the wild type. (B) Results of a GST pull-down assay to determine whether Tax-M47 and -K88A retain their ability to interact with GST–Elk-1 are shown. The GST matrix is shown at the top of each gel, and the 35S-labeled proteins are shown immediately above each lane. The experiments were performed as described for Fig. 3. (C) The 35S-labeled proteins Tax, Tax-M47, and Tax-K88A are shown to indicate the amounts of proteins loaded onto the matrices.
We have presented the results of functional experiments showing that Tax activation of SRF-dependent transcription is dependent on TCF binding to the SRE. Furthermore, transient transfections with mutant Tax proteins revealed that CBP and PCAF are essential for Tax activation of SRF- and TCF-dependent transcription. We also demonstrated that Tax interacts directly with TCFs in vitro, which suggests that Tax activation of the SRE requires contacts with TCFs as well as SRF. We are currently analyzing the composition and assembly of complexes formed on the SRE in the presence of Tax using nuclear extracts and recombinant proteins.
The relative importance of various cellular transcription factor pathways to HTLV-1-mediated T-cell transformation has been examined using the mutant proteins Tax-M47 and -M22 (20, 22). The former is defective for activation of the CREB and SRF pathways, while the latter is unable to activate NF-κB. In the past, the effects of these mutations have been interpreted in the context of a CREB–versus–NF-κB dichotomy. However, neither mutation can discriminate between the CREB and SRF pathways and no mutant Tax proteins that specifically target SRF or TCF interactions have been identified. At present only Tax-H3S, which is specifically defective for CREB interactions, is informative in this regard and is now being tested in T-cell transformation assays.
Acknowledgments
We thank Andrew Sharrocks for his generous gifts of plasmids pAS535 and pAS600 and Susan Marriott for her gift of plasmid pGEX-Tax. We also thank A. Sharrocks, S. Marriott, and Ralf Janknecht for helpful discussions and advice and Huey-Jane Liao for critically reading the manuscript.
REFERENCES
- 1.Adya N, Zhao L J, Huang W, Boros I, Giam C Z. Expansion of CREB's DNA recognition specificity by Tax results from interaction with Ala-Ala-Arg at positions 282–284 near the conserved DNA-binding domain of CREB. Proc Natl Acad Sci USA. 1994;91:5642–5646. doi: 10.1073/pnas.91.12.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu Z L, Shin Y A, Yang J M, DiDonato J A, Ballard D W. IKKγ mediates the interaction of cellular IκB kinases with the tax transforming protein of human T cell leukemia virus type 1. J Biol Chem. 1999;274:15297–15300. doi: 10.1074/jbc.274.22.15297. [DOI] [PubMed] [Google Scholar]
- 3.Dalton S, Marais R, Wynne J, Treisman R. Isolation and characterization of SRF accessory proteins. Philos Trans R Soc Lond B. 1993;340:325–332. doi: 10.1098/rstb.1993.0074. [DOI] [PubMed] [Google Scholar]
- 4.Dalton S, Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992;68:597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- 5.Fujii M, Chuhjo T, Minamino T, Masaaki N, Miyamoto K, Seiki M. Identification of the Tax interaction region of serum response factor that mediates the aberrant induction of immediate early genes through CArG boxes by HTLV-1 Tax. Oncogene. 1995;11:7–14. [PubMed] [Google Scholar]
- 6.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 7.Fujii M, Tsuchiya H, Chuhjo T, Minamino T, Miyamoto K, Seiki M. Serum response factor has functional roles both in indirect binding to the CArG box and in the transcriptional activation function of human T-cell leukemia virus type I Tax. J Virol. 1994;68:7275–7283. doi: 10.1128/jvi.68.11.7275-7283.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geleziunas R, Ferrell S, Lin X, Mu Y, Cunningham E T, Jr, Grant M, Connelly M A, Hambor J E, Marcu K B, Greene W C. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase alpha (IKKα) and IKKβ cellular kinases. Mol Cell Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harhaj E W, Sun S C. IKKγ serves as a docking subunit of the IκB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J Biol Chem. 1999;274:22911–22914. doi: 10.1074/jbc.274.33.22911. [DOI] [PubMed] [Google Scholar]
- 10.Harrod R, Tang Y, Nicot C, Lu H S, Vassilev A, Nakatani Y, Giam C Z. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol Cell Biol. 1998;18:5052–5061. doi: 10.1128/mcb.18.9.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hipskind R A, Rao V N, Mueller C G, Reddy E S, Nordheim A. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature. 1991;354:531–534. doi: 10.1038/354531a0. [DOI] [PubMed] [Google Scholar]
- 12.Janknecht R, Nordheim A. Gene regulation by Ets proteins. Biochim Biophys Acta. 1993;1155:346–356. doi: 10.1016/0304-419x(93)90014-4. [DOI] [PubMed] [Google Scholar]
- 13.Janknecht R, Nordheim A. MAP kinase-dependent transcriptional coactivation by Elk-1 and its cofactor CBP. Biochem Biophys Res Commun. 1996;228:831–837. doi: 10.1006/bbrc.1996.1740. [DOI] [PubMed] [Google Scholar]
- 14.Janknecht R, Nordheim A. Regulation of the c-fos promoter by the ternary complex factor Sap-1a and its coactivator CBP. Oncogene. 1996;12:1961–1969. [PubMed] [Google Scholar]
- 15.Jiang H, Lu H, Schiltz R L, Pise-Masison C A, Ogryzko V V, Nakatani Y, Brady J N. PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner. Mol Cell Biol. 1999;19:8136–8145. doi: 10.1128/mcb.19.12.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 17.Norman C, Runswick M, Pollock R, Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988;55:989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- 18.Price M A, Rogers A E, Treisman R. Comparative analysis of the ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET) EMBO J. 1995;14:2589–2601. doi: 10.1002/j.1460-2075.1995.tb07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez S, Ait-Si-Ali S, Robin P, Trouche D, Harel-Bellan A, Ait Si Ali S. The CREB-binding protein (CBP) cooperates with the serum response factor for transactivation of the c-fos serum response element. J Biol Chem. 1997;272:31016–31021. doi: 10.1074/jbc.272.49.31016. . (Erratum, 274:18140, 1999.) [DOI] [PubMed] [Google Scholar]
- 20.Ross T M, Narayan M, Fang Z Y, Minella A C, Green P L. Human T-cell leukemia virus type 2 tax mutants that selectively abrogate NFκB or CREB/ATF activation fail to transform primary human T cells. J Virol. 2000;74:2655–2662. doi: 10.1128/jvi.74.6.2655-2662.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shore P, Sharrocks A D. The transcription factors Elk-1 and serum response factor interact by direct protein-protein contacts mediated by a short region of Elk-1. Mol Cell Biol. 1994;14:3283–3291. doi: 10.1128/mcb.14.5.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith M R, Greene W C. Identification of HTLV-1 tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. . (Errata, 5:150, 1991, and 9:2324, 1995.) [DOI] [PubMed] [Google Scholar]
- 23.Yamaoka S, Inoue H, Sakurai M, Sugiyama T, Hazama M, Yamada T, Hatanaka M. Constitutive activation of NF-κB is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type I Tax protein. EMBO J. 1996;15:873–887. [PMC free article] [PubMed] [Google Scholar]