Abstract
The Sindbis virus variant NE2G216 is a PE2-containing host range mutant that is growth restricted in cultured mosquito cells (C6/36) due to inefficient release of virions from this cell type. The maturation defect of NE2G216 has been linked to the structures of N-linked oligosaccharides synthesized by arthropod cells. Analysis of C6/36 cells infected with NE2G216 by transmission electron microscopy revealed the presence of dense virus aggregates within cytoplasmic vacuoles and virus aggregates adhered to the cell surface. The virus aggregation phenotype of NE2G216 was reproduced in vertebrate cells (Pro-5) by the addition of 1-deoxymannojirimycin, an inhibitor of carbohydrate processing which limits the processing of N-linked oligosaccharides to structures that are structurally similar, albeit not identical, to those synthesized in C6/36 cells. We conclude that defective maturation of NE2G216 in mosquito cells is due to virion aggregation and retention on the cell surface and that this phenotype is directly linked to the carbohydrate-processing properties of these cells.
Most alphaviruses (family Togaviridae) are transmitted to their vertebrate host through the bites of infected mosquitoes, and productive replication within the vertebrate and mosquito host is required for the maintenance of these viruses in nature. E1 and E2 glycoproteins synthesized in cultured mosquito cells (C6/36) are attached to N-linked oligosaccharides in the form of Man3GlcNAc2, Man5GlcNAc2, or Man7–9GlcNAc2 (10), whereas those synthesized in vertebrate cell lines contain high mannose, hybrid, and complex-type oligosaccharides (11, 15). These cellular differences were recently linked to a host range phenotype displayed by a Sindbis virus mutant designated NE2G216, which displays a maturation defect when grown in C6/36 cells (2). NE2G216 encodes a PE2 glycoprotein that is not cleaved by the furin endoprotease due to the presence of an additional N-linked oligosaccharide that restricts access to the PE2 cleavage site. Consequently, NE2G216 retains PE2 in place of E2 within its virion structure (8, 20). NE2G216 replicates to nearly normal levels in vertebrate cell lines but is severely restricted in C6/36 cells due to the inefficiency with which virions are released from the host cell (2).
Alphavirus morphogenesis in vertebrate cells has been reported to occur exclusively by virus budding at the plasma membrane (see references 18 and 28 for reviews). Alphaviruses also mature at the plasma membrane of cultured mosquito cells; however, virion budding into cytoplasmic vacuoles has also been described, and intracellular budding appears to be the primary mode of virus maturation for some alphaviruses in this cell type (7, 19, 21, 22, 26). The site(s) at which NE2G216 buds in C6/36 cells has not been defined, nor is the location of the virions believed to be retained in these cells known. The purpose of this study was to further characterize the NE2G216 maturation defect by analyzing virus maturation at the ultrastructural level using transmission electron microscopy (TEM).
To determine if the host range phenotype of NE2G216 is specific for C6/36 cells or if it extends to other cells of mosquito origin, virus maturation and virion release were compared in three Aedes albopictus mosquito cell lines (C6/36, C7-10, and u4.4), each of which is thought to have been derived from a different larval tissue (12, 19, 24). The PE2 cleavage-competent parental virus, TRSB (17), and three additional PE2-containing mutant viruses were included in these studies (Table 1). Like NE2G216, the N6R1 mutant was selected in BHK-21 cells and is markedly restricted in C6/36 cells (2, 8). The NS-4 and NS-7 mutants were selected based on their efficient maturation in C6/36 cells, and each replicates to wild-type levels in these cells (2).
TABLE 1.
Genetic and phenotypic properties of parental and mutant Sindbis viruses
| Virus | Mutation(s) relative to TRSB | Glycoprotein compositiona | Cell line used for selection of mutant |
|---|---|---|---|
| Parental | |||
| TRSB (wild type) | E1/E2 | ||
| TRSB-N (non-infectious)b | E2 residue 1 Arg to Asnc | E1/PE2 | |
| Infectious mutants | |||
| NE2G216 | E2 residue 1 Arg to Asn | E1/PE2 | BHK-21 |
| E2 residue 216 Glu to Glyd | |||
| N6R1 | E2 residue 1 Arg to Asn | E1/PE2 | BHK-21 |
| E3 residue 25 Cys to Argd | |||
| NS-4 | E2 residue 1 Arg to Asn | E1/PE2 | C6/36 |
| E3 residue 34 Leu to Hisd | |||
| NS-7 | E2 residue 1 Arg to Asn | E1/PE2 | C6/36 |
| E3 residue 46 Tyr to Hisd |
As determined by electrophoretic analysis of purified virions.
For a description of this virus, see reference 8.
This mutation generates a signal for N-linked glycosylation at E2 residue 1 and is individually lethal.
This mutation suppresses the lethal effects of oligosaccharide addition at E2 residue 1.
Cells were infected with each virus, and virus released into the medium and virus that remained cell-associated were quantified separately at 12 and 20 h postinfection essentially as described (2) except that infections were initiated with free virus at a multiplicity of infection (MOI) of 5 PFU/cell instead of by electroporation with viral transcripts. The extracellular titers of NE2G216 and N6R1 measured at 12 and 20 h postinfection were markedly reduced in each cell line compared to those of TRSB (Fig. 1a and c). NS-4 and NS-7 produced extracellular titers that were similar to or greater than those of TRSB in all three cell lines (Fig. 1a and c). The titers of cell-associated virus were very similar between the viruses on all three cell lines (Fig. 1b and d). Most significantly, the cell-associated titers of NE2G216 and N6R1 were 20 to 390% as high as those obtained for TRSB (Fig. 1b and d). These results indicate that the host range phenotype of NE2G216 and N6R1 is not specific for C6/36 cells but extends to additional mosquito cell lines and indicate that these viruses are not inherently defective for virion budding in mosquito cells but instead appear to be defective in their ability to be released from this cell type.
FIG. 1.
Comparisons between extracellular and cell-associated virus titers from infected mosquito cell lines. Titers are presented as a percentage of that obtained for TRSB (100%). Extracellular virus titers (a and c) and cell-associated virus titers (b and d) were determined at 12 h (a and b) and 20 h (c and d) postinfection as described in the text. Virus used for infection is shown on the x axis. Results represent the averages of values obtained in two independent experiments.
To investigate the process of virion morphogenesis and release at the ultrastructural level, C6/36 cells infected with each of the five viruses were analyzed by TEM. Mildly adherent monolayers of C6/36 cells grown in 75-cm2 flasks were infected with each virus at an MOI of 5 PFU/cell. At 20 h postinfection, cells were collected by gentle scraping, pelleted, washed three times with phosphate-buffered saline (PBS), and fixed in Ito's fixative (1.25% formaldehyde, 2.5% glutaraldehyde, 0.03% trinitrophenol, 0.03% CaCl2 in 0.05 M cacodylate buffer [pH 7.3]) (13). Cells were washed in cacodylate buffer and postfixed in 1% OsO4 for 1 h at room temperature. Cell pellets were washed in cacodylate buffer, stained en bloc in 1.0% uranyl acetate in 0.1 M maleate buffer (pH 5.2), dehydrated in ethanol, processed through propylene oxide, and embedded in Poly/Bed 812 (Polysciences, Warrington, Pa.). Sections were cut on a Reichert Ultracut S ultramicrotome (Leica, Deerfield, Ill.), stained with uranyl acetate and lead citrate, and examined in a Philips 410 or CM 100 electron microscope (Philips Electron Optics, Eindhoven, The Netherlands) at 60 kV.
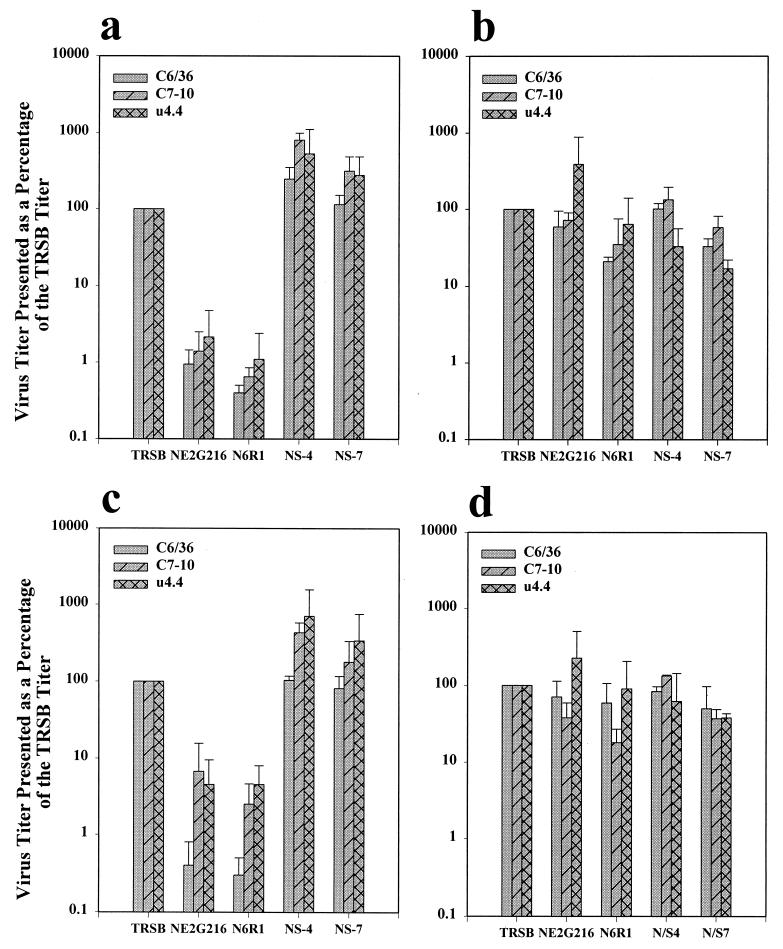
TEM analysis revealed obvious maturation differences between the viruses. In cells infected with TRSB, virions budded at the plasma membrane, and the surface of these cells contained only individual and small groups of virions (Fig. 2a). Virions were also present within cytoplasmic vacuoles (Fig. 2b). These observations were generally consistent with those described by Miller and Brown (19), who used TEM to study the maturation of a different strain of Sindbis virus (HR [3]) in the same three mosquito cell lines used here. However, intracellular maturation of TRSB virions was not as extensive as in the earlier study, and the plasma membrane appeared to be the primary site of virus maturation for TRSB (Fig. 2e). This difference between the studies may be due to the fact that in the earlier work, cells were infected for a longer period before being processed for TEM (30 to 50 h). Cells in this study were infected for 20 h before being processed to limit the amplification of PE2-cleaving revertants that are selected for in NE2G216-infected C6/36 cells (9). Alternatively, the differences may be authentic and could be attributed to dissimilar glycoprotein functions linked to one or more of the seven glycoprotein residues that differ between TRSB and HR (17, 23, 27) (data not shown). Maturation of NS-4 and NS-7 was similar to that of TRSB, with most virions budding at the plasma membrane (Fig. 2c and d).
FIG. 2.
C6/36 cells infected with TRSB (a, b, and e) and NS-4 (c and d). In cells infected with these viruses, virions were most commonly seen attached to the cell surface or in the process of budding at this site (a, c, d, and e). Virions were also seen within cytoplasmic vacuoles (b). Cells were processed for TEM analysis at 20 h postinfection. Bar, 100 nM (a to d) or 0.5 μM (e). Magnifications: ×78,375 (a and b), ×57,750 (c and d), and ×24,475 (e).
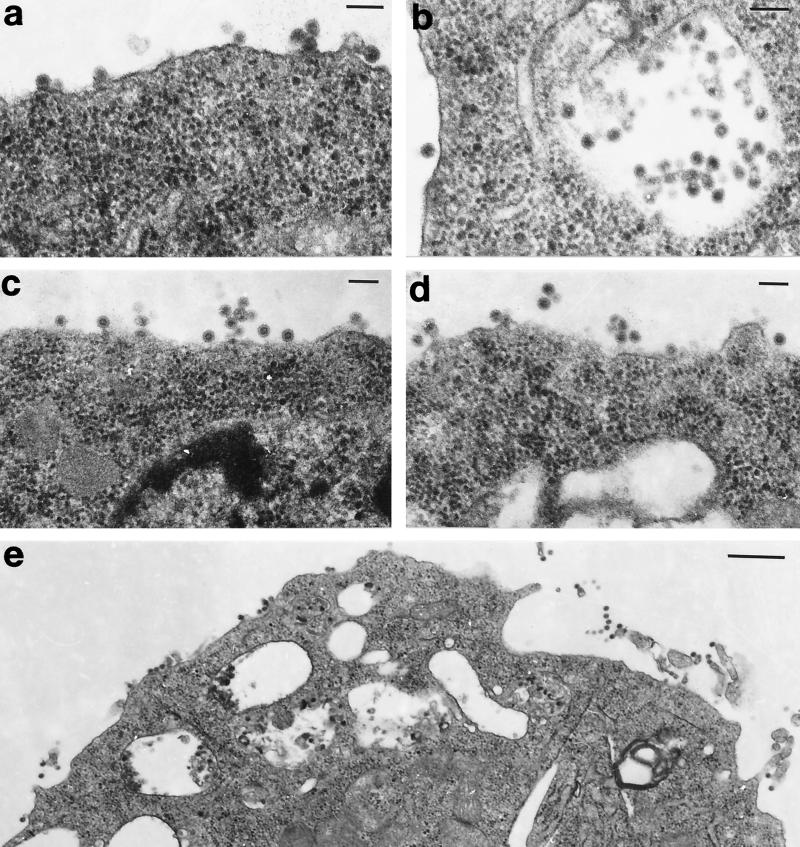
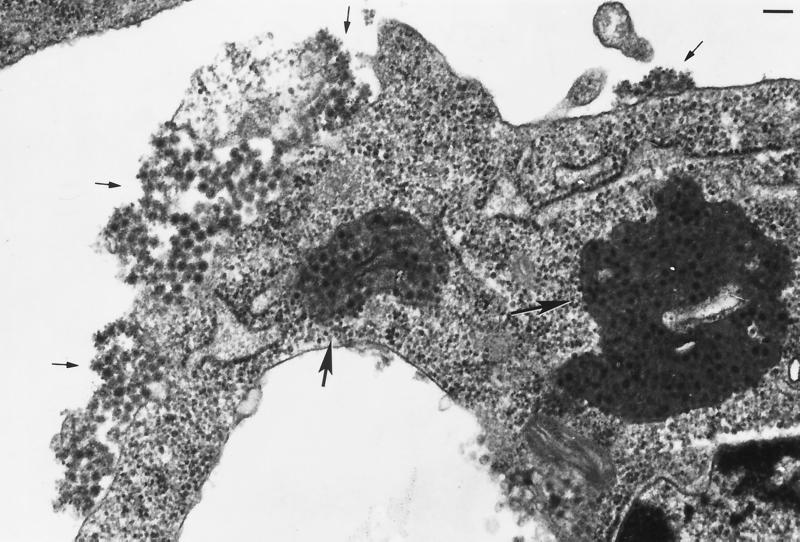
Cells infected with NE2G216 and N6R1 displayed two remarkable features that were unique to these samples. First, vacuoles filled with dense masses of virions were observed only in these cells (Fig. 3, large arrows). Most of the virions contained within the vacuoles appeared to be associated with a dense, amorphous matrix material. The presence of virions and/or nucleocapsids bound within a matrix material has been described in earlier studies using the HR strain of Sindbis virus (7, 19); however, based on the photographs presented in these reports, considerably less of the material was present than in samples infected with NE2G216 and N6R1, and in contrast to our findings, the association of virions within a matrix was the exception and not the norm and was confined to virions within cytoplasmic vacuoles. As stated above, the differences observed in these studies may be related to alternative properties of the viruses used (HR versus NE2G216 and N6R1), to the stage of the infection at the time of cell harvest (30 to 50 h postinfection versus 20 h postinfection) (19), or to the mosquito cell line used (u4.4 versus C6/36) (7). Second, large aggregates of virions were observed in globular patches or in mats covering large areas of the cell surface (Fig. 3, small arrows, and Fig. 4). Aggregated virions at the cell surface appeared to result from fusion of virus-filled vacuoles with the plasma membrane, as surface budding of NE2G216 and N6R1 was only rarely observed in C6/36 cells, and virus-filled vacuoles which appeared to be in the process of fusing with the plasma membrane and depositing virions onto the cell surface were observed in several samples (Fig. 4c, arrow). The virions within the surface aggregates also appeared to be associated with a matrix substance which caused the boundaries between virions to be much less defined than the boundaries between surface-bound virions of TRSB, NS-4, and NS-7. NE2G216 and N6R1 virions appear to associate with the matrix material within the cytoplasmic vacuoles; however, our results do not exclude the possibility that the virions which mature at the plasma membrane of C6/36 cells associate with the matrix material concomitantly with the budding process. Although the composition of the matrix material was not addressed in this study, it seems likely that the association between the virions and this material may restrict the release of virions from the surface of the host cell. Affinity between virions may also contribute to aggregate formation and virion retention.
FIG. 3.
C6/36 cell infected with N6R1. Virions are present at a high density within cytoplasmic vacuoles (large arrows) and within virus aggregates adhered to the plasma membrane (small arrows). Cells were processed for TEM analysis at 20 h postinfection. Bar, 100 nM. Magnification, ×62,700.
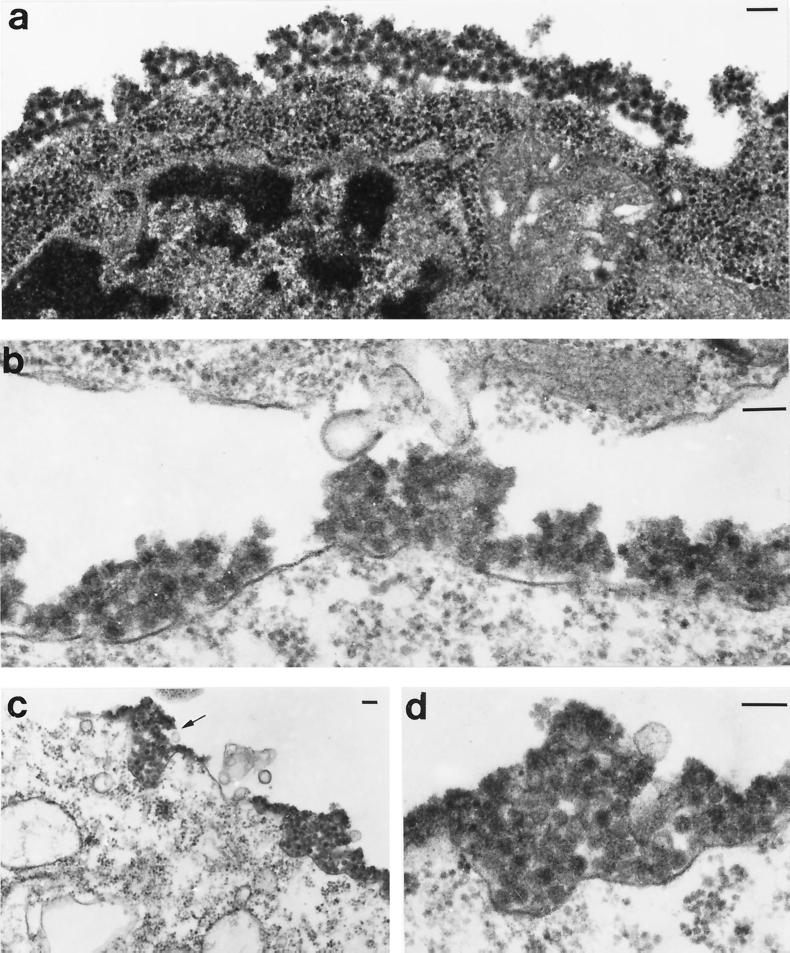
FIG. 4.
C6/36 cells infected with NE2G216 (a, c, and d), and N6R1 (b). Virion aggregates are seen covering large areas of the infected cell and appear to be associated with a matrix material. A vacuole filled with virions appears to be in the final stage of fusion with the plasma membrane (arrow in panel c). Panel d presents a higher-power magnification of a surface-bound virus aggregate from panel c (aggregate on right). Cells were processed for TEM analysis at 20 h postinfection. Bar, 100 nM. Magnifications: ×62,700 (a), ×94,000 (b and d), and ×31,550 (c).
The retention of virions on the surface of infected C6/36 cells could account for the low titers of extracellular NE2G216 and N6R1 virions and the relatively high titers of cell-associated virus, as seen in Fig. 1. To address this issue, a simple cell agitation experiment was performed in which virus titers were determined prior to and following physical agitation (but not lysis) of infected C6/36, C7-10, and u4.4 cells. Briefly, mildly adherent monolayers of cells in 75-cm2 flasks were infected with each virus as described (2). At 20 h postinfection, samples of culture medium were collected, clarified by microcentrifugation, and stored at −70°C (preagitation samples). Cells were then detached from the flask by gentle scrapping, passed slowly three times through a 5.0-ml pipette, and pelleted. Prior to the final pelleting, a sample of cell suspension was viewed under an inverted microscope to assess cell integrity (intact versus lysed). Clarified samples were collected and stored at −70°C until assayed by plaque titration on BHK-21 cells (postagitation samples). Agitation had the least effect on the viral titer of culture medium from cells infected with TRSB, NS-4, and NS-7, as this treatment increased titers by a maximum of 3.8-fold (NS-7 on u4.4 cells). Agitation enhanced the viral titer of the culture medium from NE2G216- and N6R1-infected C6/36 cells by an average of 150- and 25-fold, respectively (Fig. 5). These results indicate that the aggregated material consists largely of infectious virions which can be dislodged by mechanical means. It is unlikely that all surface-bound virions were removed by the agitation treatment, and many of the virions dislodged in this way would likely remain in aggregate form. These virions would have a reduced specific infectivity, and thus, the postagitation virus titers are probably a low-end estimate of the actual number of infectious virions contained within the aggregated material.
FIG. 5.
Effect of cell agitation on extracellular virus titers. Extracellular virus titers were determined prior to and following mechanical agitation of infected mosquito cells as described in the text. Results are presented as the fold enhancement of virus titers as a result of cell agitation. Virus used for infection is shown on the x axis. Results represent the averages of values obtained in two independent experiments.
The results of the agitation assay suggest that the low extracellular virus titers from NE2G216- and N6R1-infected mosquito cells (Fig. 1a and c) is due at least in part to the inefficiency with which virions are released from the surface of the host mosquito cell. These results also indicate that the titers reported for cell-associated NE2G216 and N6R1 viruses (Fig. 1b and d), are likely to be artificially low because cells were scraped and washed extensively to remove extracellular virions, and much of the surface-bound virus would have been removed by this treatment prior to quantification. Together, the virus titration assays and the TEM analysis demonstrate that the glycoprotein mutations present in NE2G216 and N6R1 do not significantly affect the formation of PE2-E1 heterodimers, the transport of heterodimers to sites of intracellular virus budding, or the capsid-glycoprotein interactions that drive the budding process. Instead, these mutations appear to induce virus morphogenesis and aggregation within cytoplasmic vacuoles and to restrict the release of aggregated virions from the surface of the host mosquito cell.
In a previous study, we demonstrated that the extracellular titers of NE2G216 grown in a vertebrate cell line (Pro-5) could be reduced by a factor of approximately 102 by treating the cells with 1-deoxymannojirimycin (1-dMM) (2). This treatment did not affect the extracellular titers of TRSB and actually enhanced the extracellular titers of NS-4 and NS-7. 1-dMM is an inhibitor of the Golgi α-mannosidase I enzyme and restricts the processing of N-linked oligosaccharides to Man8–9GlcNAc2 structures which approximate, albeit not exactly, the structures of carbohydrates synthesized in C6/36 cells (1, 6). These results suggested that the maturation defect of NE2G216 in C6/36 cells is linked to the carbohydrate-processing phenotypes of the mosquito cell. From the TEM results, we predicted that the reduction in NE2G216 and N6R1 titers produced in the prior study was due to virion aggregation on the surface of the 1-dMM-treated Pro-5 cells. To test this prediction, TEM analysis was performed on Pro-5 cells infected with TRSB, NE2G216, and NS-4 and grown in the presence (2.5 mM) or absence of 1-dMM. Briefly, monolayers of subconfluent Pro-5 cells were grown in 60-mm petri dishes, infected with TRSB, NE2G216, or NS-4 at an MOI of 5 PFU/cell, and maintained in growth medium alone or in growth medium supplemented with 1-dMM (2.5 mM). At 14 h postinfection, samples of cell culture medium were harvested and clarified by microcentrifugation, and viruses were quantified in plaque assays on BHK-21 cells. Cells were washed twice with PBS, fixed in Ito's fixative, collected by gentle scrapping, and prepared for TEM as described above.
The presence of 1-dMM increased the titers of TRSB and NS-4 by factors of 2 and 10, respectively, and decreased the titer of NE2G216 by a factor of 63, confirming the potency of the 1-dMM used. TEM analysis of TRSB-infected cells revealed extensive virus budding at the plasma membrane of 1-dMM-treated and untreated cells and no evidence of virus aggregation (Fig. 6a and b). In contrast to an earlier report in which 1-dMM was shown to induce intracellular maturation of the Sindbis group alphavirus S.A.AR86 in BHK-21 cells (16), intracellular maturation of TRSB virions was not observed in 1-dMM-treated Pro-5 cells. This finding was supported by the results of an assay that compared the virus titers of culture medium alone with those of culture medium plus cell lysates under conditions in which 1-dMM was included or excluded from the culture medium as described (16). The inclusion of 1-dMM did not markedly enhance the fraction of TRSB virions that were associated with the host cells (Table 2). In contrast, the inclusion of 1-dMM enhanced the fraction of cell-associated NE2G216 virions by greater than 2 log10. The differences between the two studies may be attributed to the alternative cell lines used and/or to significant genetic and phenotypic differences that have been identified between TRSB and the S.A.AR86 virus (25).
FIG. 6.
Pro-5 cells infected with TRSB (a and b) and NE2G216 (c, d, and e). Cells were infected and maintained in the absence of 1-dMM (a and c) or in the presence of 2.5 mM 1-dMM (b, d, and e). TRSB and NE2G216 virions were observed budding individually at the plasma membrane in the absence of 1-dMM (a and c) and in TRSB-infected cells maintained in the presence of 1-dMM (b). NE2G216 virions formed aggregates at the plasma membrane of Pro-5 cells in the presence of 1-dMM, and budding virions appeared to assemble into aggregates concomitantly with the budding process (d and e, arrows). Cells were processed for TEM analysis at 14 h postinfection. Bar, 100 nM. Magnifications: ×85,250 (a and b), ×90,000 (c and d), and ×94,000 (e).
TABLE 2.
Effects of 1-dMM on virus replication in Pro-5 cells
| Virus sample | Virus titera (PFU/ml) in the absence of 1-dMM | Ratiob | Virus titer (PFU/ml) in the presence of 2.5 mM 1-dMM | Ratio |
|---|---|---|---|---|
| TRSB | ||||
| Culture medium and cellsc | 6.3 × 108 | 4.2 | 1.1 × 109 | 4.4 |
| Culture mediumd | 1.5 × 108 | 2.5 × 108 | ||
| NE2G216 | ||||
| Culture medium and cells | 4.7 × 107 | 2.8 | 3.1 × 107 | 397 |
| Culture medium | 1.7 × 107 | 7.8 × 104 |
Virus titers were determined by plaque assay on monolayers of BHK-21 cells.
Virus titer obtained for culture medium and cells divided by the virus titer obtained for culture medium only.
Cells were scraped from dishes and collected together with culture medium, then frozen at −70°C to lyse the cells.
Sample of culture medium clarified of cells by microcentrifugation.
NE2G216 displayed extensive budding at the plasma membrane in the absence of 1-dMM, and virus aggregation on the surface of these cells was never seen (Fig. 6c). In contrast, NE2G216 formed extensive surface aggregates similar to those seen in NE2G216-infected C6/36 cells in the presence of 1-dMM (Fig. 6d and e). As was observed in C6/36 cells, the virions within these aggregates appeared to be associated with a matrix-like material which blurred the boundaries between individual virions. In contrast to what was observed in C6/36 cells, surface-bound aggregates of NE2G216 virions appeared to form concomitantly with virus budding at the plasma membrane (Fig. 6e, arrows), and no evidence of intracellular virion budding and/or exocytosis of virus-filled vacuoles was observed under these conditions. The aggregates of NE2G216 virions produced under these conditions would explain the marked increase in virus titer that was obtained when scraped and lysed cells from the 1-dMM-positive samples were included with the culture medium (Table 2). NS-4 budded at the plasma membrane in the absence of 1-dMM, and no virion aggregation was observed. NS-4 also budded at the plasma membrane in the presence of 1-dMM, and some virus aggregates were observed. Aggregates of NS-4 were smaller and less dense than those of NE2G216, and less of the matrix material appeared to be present.
These results support the hypothesis that the host range phenotype of NE2G216 and N6R1 is linked to the inability of mosquito cells to process N-linked oligosaccharides beyond low- and high-mannose forms (see reference 14 for a review). Oligosaccharide processing is known to influence glycoprotein structure, function, and transport properties (see references 4 and 5 for reviews); however, we cannot yet explain at the molecular level precisely how low- or high-mannose oligosaccharide structures affect the maturation of NE2G216 and N6R1 in the way that we have observed. If the oligosaccharides themselves were directly responsible for the virion aggregation phenotype, perhaps through an affinity between these structures and cellular protein or carbohydrate components, we would not expect this effect to be as virus specific as it is. More likely, oligosaccharides in these forms probably affect the structural properties of the NE2G216 and N6R1 glycoproteins, which in turn affect a glycoprotein functional property(s) that results in the virion aggregation phenotype observed. These putative glycoprotein structural and functional changes appear to be sensitive to subtle genetic differences between the viruses, and even NE2G216 and N6R1 do not appear to be affected equally. The virion aggregation phenotype appeared to be similar for NE2G216 and N6R1, based on TEM; however, differences were noted between these viruses in the cell agitation assay. Specifically, agitation of infected mosquito cells had a much greater effect on the extracellular titer of NE2G216 than on that of N6R1. Virus-specific differences in the affinity among virions within the aggregated material could account for these results. The physical nature of the viral material released by agitation was not determined; however, this model would predict that NE2G216 virions are bound more tightly within the aggregated material and fewer infectious units (individual or clumps of virions) would be released by agitation and quantified in subsequent plaque assays. In contrast, NE2G216 virions may be bound less tightly within the aggregates, and agitation would be expected to release a greater number of quantifiable infectious units. Finally, our model would predict that the E3 mutations present within the NS-4 and NS-7 viruses prevent the glycoproteins from folding into the restrictive conformation or somehow suppress the restrictive property assumed by the alternatively folded proteins. This prediction is consistent with the fact that the NS-4 and NS-7 viruses were isolated under conditions that selected for efficient maturation and release from C6/36 cells (2). Further evidence that the virion aggregation phenotype is specific for defined genotypes is the observation that placement of the NS-4 mutation (His for Leu at E3 residue 34) into the genetic background of NE2G216 did not suppress the host range phenotype (2).
In conclusion, the results presented here indicate that the restricted growth of NE2G216 in cultured mosquito cells is not due to an inherent inability to form virions by budding, but instead is due to the propensity of NE2G216 virions to assemble into aggregates that appear to form within cytoplasmic vesicles and which eventually are deposited and retained on the surface of the infected cell. Furthermore, the virus aggregation phenotype displayed by NE2G216 is linked to structural and functional properties of the viral glycoproteins which are dependent upon the carbohydrate-processing phenotypes of the cultured mosquito cell.
Acknowledgments
We thank Dennis T. Brown for his kind gift of the C7-10 and u4.4 cell lines. We also acknowledge Violet C. Han and Julie W. Wen for their excellent technical assistance in TEM procedures.
This work was supported by grant R29 AI40937 from the National Institutes of Health.
REFERENCES
- 1.Bischoff J, Liscum L, Kornfeld R. The use of 1-deoxymannojirimycin to evaluate the role of various α-mannosidases in oligosaccharide processing in intact cells. J Biol Chem. 1986;261:4766–4774. [PubMed] [Google Scholar]
- 2.Boehme K W, Williams J C, Johnston R E, Heidner H W. Linkage of an alphavirus host-range restriction to the carbohydrate processing phenotypes of the host cell. J Gen Virol. 2000;81:161–170. doi: 10.1099/0022-1317-81-1-161. [DOI] [PubMed] [Google Scholar]
- 3.Burge B W, Pfefferkorn E R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966;30:204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- 4.Doms R W, Lamb R A, Rose J K, Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993;193:545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- 5.Fiedler K, Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- 6.Fuhrmann U, Bause E, Legler G, Ploegh H. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature (London) 1984;307:755–758. doi: 10.1038/307755a0. [DOI] [PubMed] [Google Scholar]
- 7.Gliedman J B, Smith J F, Brown D T. Morphogenesis of Sindbis virus in cultured Aedes albopictus cells. J Virol. 1975;16:913–926. doi: 10.1128/jvi.16.4.913-926.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heidner H W, McKnight K L, Davis N L, Johnston R E. Lethality of PE2 incorporation into Sindbis virus can be suppressed by second-site mutations in E3 and E2. J Virol. 1994;68:2683–2692. doi: 10.1128/jvi.68.4.2683-2692.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidner H W, Knott T A, Johnston R E. Differential processing of Sindbis virus glycoprotein PE2 in cultured vertebrate and arthropod cells. J Virol. 1996;70:2069–2073. doi: 10.1128/jvi.70.3.2069-2073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh P, Robbins P W. Regulation of asparagine-linked oligosaccharide processing: oligosaccharide processing in Aedes albopictus mosquito cells. J Biol Chem. 1984;259:2375–2382. [PubMed] [Google Scholar]
- 11.Hsieh P, Rosner M R, Robbins P W. Host-dependent variation of asparagine-linked oligosaccharides at individual glycosylation sites of Sindbis virus glycoproteins. J Biol Chem. 1983;258:2548–2554. [PubMed] [Google Scholar]
- 12.Igarashi A. Isolation of a Singh Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978;40:531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- 13.Ito S, Rikihisa Y. Techniques for electron microscopy of rickettsiae. In: Burgdorfer W, Anacker R L, editors. Rickettsiae and rickettsial diseases. New York, N.Y: Academic Press, Inc.; 1981. pp. 213–227. [Google Scholar]
- 14.März L, Altmann F, Staudacher E, Kubelka V. Protein glycosylation in insects. In: Montreuil J, Vliegenthart J F G, Schachter H, editors. Glycoproteins. Amsterdam, The Netherlands: Elsevier; 1995. pp. 543–563. [Google Scholar]
- 15.Mayne J T, Bell J R, Strauss E G, Strauss J H. Pattern of glycosylation of Sindbis virus envelope proteins synthesized in hamster and chicken cells. Virology. 1985;142:121–133. doi: 10.1016/0042-6822(85)90427-1. [DOI] [PubMed] [Google Scholar]
- 16.McDowell W, Romero P A, Datema R, Schwarz R T. Glucose trimming and mannose trimming affect different phases of the maturation of Sindbis virus in infected BHK cells. Virology. 1987;161:37–44. doi: 10.1016/0042-6822(87)90168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKnight K L, Simpson D A, Lin S C, Knott T A, Polo J M, Pence D F, Johannsen D B, Heidner H W, Davis N L, Johnston R E. Deduced consensus sequence of Sindbis virus strain AR339: mutations contained in laboratory strains which affect cell culture and in vivo phenotypes. J Virol. 1996;70:1981–1989. doi: 10.1128/jvi.70.3.1981-1989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller M L, Brown D T. Alphavirus infection in cultured tissue cells. Adv Dis Vector Res. 1991;8:107–142. [Google Scholar]
- 19.Miller M L, Brown D T. Morphogenesis of Sindbis virus in three subclones of Aedes albopictus (mosquito) cells. J Virol. 1992;66:4180–4190. doi: 10.1128/jvi.66.7.4180-4190.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paredes A M, Heidner H W, Thuman-Commike P, Prasad B V V, Johnston R E, Chiu W. Structural localization of the E3 glycoprotein in attenuated Sindbis virus mutants. J Virol. 1998;72:1534–1541. doi: 10.1128/jvi.72.2.1534-1541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raghow R S, Davey M W, Dalgarno L. The growth of Semliki Forest virus in cultured mosquito cells: ultrastructural observations. Arch Gesamte Virusforsch. 1973;43:165–168. doi: 10.1007/BF01249360. [DOI] [PubMed] [Google Scholar]
- 22.Raghow R S, Grace T D C, Filshie B K, Barley W, Dalgarno L. Ross River virus replication in cultured mosquito and mammalian cells: virus growth and correlated ultrastructural changes. J Gen Virol. 1973;21:109–122. doi: 10.1099/0022-1317-21-1-109. [DOI] [PubMed] [Google Scholar]
- 23.Rice C M, Strauss J H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci USA. 1981;78:2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarver N, Stollar V. Sindbis virus-induced cytopathic effect in clones of Aedes albopictus (Singh) cells. Virology. 1977;80:390–400. doi: 10.1016/s0042-6822(77)80014-7. [DOI] [PubMed] [Google Scholar]
- 25.Simpson D A, Davis N L, Lin S C, Russell D, Johnston R E. Complete nucleotide sequence and full-length cDNA clone of S.A.AR86, a South African alphavirus related to Sindbis. Virology. 1996;222:464–469. doi: 10.1006/viro.1996.0445. [DOI] [PubMed] [Google Scholar]
- 26.Stollar V, Harrap K, Thomas V, Sarver N. Observations related to cytopathic effect in Aedes albopictus cells infected with Sindbis virus. In: Kurstak E, editor. Arctic and tropical arboviruses. New York, N.Y: Academic Press, Inc.; 1979. pp. 277–296. [Google Scholar]
- 27.Strauss E G, Rice C M, Strauss J H. Complete nucleotide sequence of the genomic RNA of Sindbis virus. Virology. 1984;133:92–110. doi: 10.1016/0042-6822(84)90428-8. [DOI] [PubMed] [Google Scholar]
- 28.Strauss J H, Strauss E G, Kuhn R J. Budding of alphaviruses. Trends Microbiol. 1995;3:346–350. doi: 10.1016/s0966-842x(00)88973-8. [DOI] [PubMed] [Google Scholar]