Abstract
Introduction
Current drug–drug interaction (DDI) detection methods often miss the aspect of temporal plausibility, leading to false-positive disproportionality signals in spontaneous reporting system (SRS) databases.
Objective
This study aims to develop a method for detecting and prioritizing temporally plausible disproportionality signals of DDIs in SRS databases by incorporating co-exposure time in disproportionality analysis.
Methods
The method was tested in the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS). The CRESCENDDI dataset of positive controls served as the primary source of true-positive DDIs. Disproportionality analysis was performed considering the time of co-exposure. Temporal plausibility was assessed using the flex point of cumulative reporting of disproportionality signals. Potential confounders were identified using a machine learning method (i.e. Lasso regression).
Results
Disproportionality analysis was conducted on 122 triplets with more than three cases, resulting in the prioritization of 61 disproportionality signals (50.0%) involving 13 adverse events, with 61.5% of these included in the European Medicine Agency’s (EMA’s) Important Medical Event (IME) list. A total of 27 signals (44.3%) had at least ten cases reporting the triplet of interest, and most of them (n = 19; 70.4%) were temporally plausible. The retrieved confounders were mainly other concomitant drugs.
Conclusions
Our method was able to prioritize disproportionality signals with temporal plausibility. This finding suggests a potential for our method in pinpointing signals that are more likely to be furtherly validated.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-024-01430-8.
Key Points
| We have developed a novel method for detecting drug–drug interactions that integrates temporal plausibility into disproportionality analysis. |
| This method was applied to 27 disproportionality signals, revealing that the majority (70.4%) were temporally plausible. |
| Further investigation is needed to assess whether our novel method outperforms standard approaches in identifying and prioritizing safety signals. |
Introduction
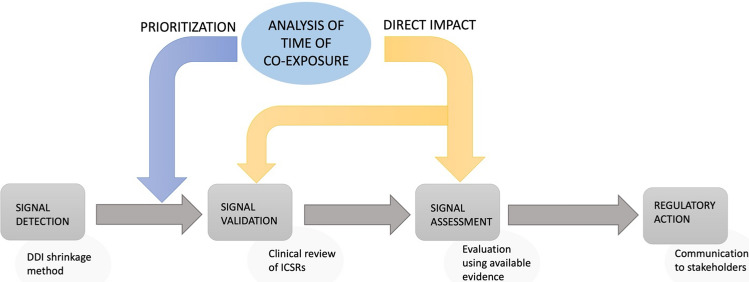
Drug–drug interactions (DDIs) pose a significant concern in healthcare, as the concomitant use of multiple medications can lead to adverse events (AEs) and compromised therapeutic outcomes [1]. To identify and assess DDIs, spontaneous reporting system (SRS) databases have become valuable resources [1]. Various approaches have been developed to uncover potential DDIs within these databases, with disproportionality analysis being a commonly used method [2]. Disproportionality analysis relies on statistical measures such as the reporting odds ratio (ROR), information component (IC), Bayesian confidence propagation neural network, or the gamma Poisson shrinker. Among these, the shrinkage method (Omega, Ω) proposed by Norén et al. [3] has emerged as the gold-standard method for detecting DDIs due to its ability to minimize false-positive disproportionality signals (see Fig. 1) [4]. However, despite their effectiveness, these methods lack consideration for key pharmacological principles such as biological and temporal plausibility [5] and further implementation decisions are needed once a potential alert is retrieved [6].
Fig. 1.
The importance of considering time of co-exposure at an early stage in the process once a potential alert is retrieved. DDI drug–drug interaction, ICSR individual case safety report
The omission of co-exposure time in current DDI detection methods can lead to the identification of disproportionality signals that are not substantiated by temporal relationships. Consequently, this may result in an increased number of false-positive signals and burden during signal validation [7].
Recognizing the significance of these principles, researchers found that the temporal interval of co-exposure is a crucial factor for validating signals of both pharmacokinetic and pharmacodynamic DDIs [1]. The assessment of temporal plausibility is essential for distinguishing temporally plausible DDI signals from spurious associations and prioritizing [8].
While some efforts have been made to incorporate temporal plausibility into signal detection methods by considering the time to onset of specific AEs or using overlapping treatment periods as a filter in the algorithm [5], to the best of our knowledge, none have comprehensively addressed the simultaneous inclusion of temporal plausibility factors in signal detection and prioritization of DDI detection. Therefore, there is a clear need to develop a novel approach that combines these crucial elements to enhance the accuracy and reliability of DDI signal detection and prioritization in SRS databases.
The objective of this study is to develop a novel method for signal detection and prioritization of DDIs in SRS databases by integrating temporal plausibility. By considering the temporal relationship between drug exposures and the onset of AEs, as well as leveraging known pharmacological mechanisms, we evaluated whether it is possible to identify disproportionality signals of DDIs that are already supported by temporal plausibility.
Methods
Data Source
The Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) was used as the data source. FAERS contains drug-related AEs that have been voluntarily reported by healthcare providers (e.g. physicians, pharmacists) and consumers (e.g. patients, family members, and lawyers) and recorded as individual case safety reports (ICSRs). FAERS collects the type of AEs that have occurred, plus any other available information, such as the age and gender of the individual and the drugs (reported as suspect or concomitant) that have been used, together with their indication for use and time of exposure.
Data Acquisition and Processing
American Standard Code for Information Interchange (ASCII) files of quarterly extraction of FAERS data were downloaded from the FDA website. We therefore retrieved our dataset from the third quarter (Q3) 2014 up to the fourth quarter (Q4) of 2022. Drugs reported as suspect, interacting, and concomitant with the exact exposure time (start date–end date) were included in the analysis. Drugs should also be started before the AE date. Duplicates for primary ID were removed. A local database was set up in R (version 4.1.2, R Development Core Team) by using as a linkage key the primary identifier of each ICSR, as described by Kass-Hout and colleagues [9].
True-Positive DDIs
The CRESCENDDI dataset of positive controls provided by Kontsioti et al. [10] was used as the primary source of true-positive DDIs. We focused our attention on those DDIs that are not fully contraindicated, which are labelled as “take into consideration” in the dataset by Kontsioti, because we expected that DDIs for which a contraindication exists would be less frequently recorded in an SRS database, and therefore, we did not consider them in this study. Within all the 10,286 available triplets drug A–drug B–AE, 2372 true positives were labelled as “take into consideration”. Of these, 68% of the triplets were pharmacodynamic (n = 879) and synergic (n = 740) DDIs.
Signal Detection of DDIs Considering Time of Co-exposure
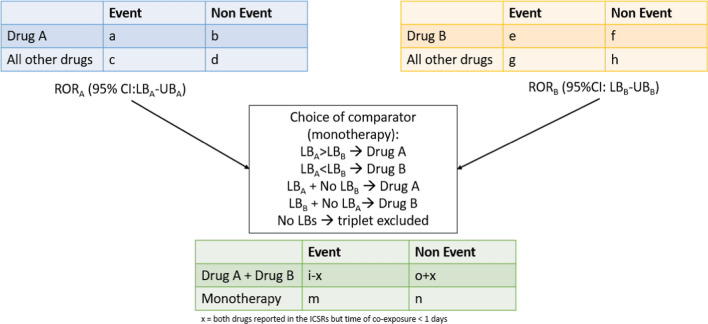
Considering two interacting drugs “drug A” and “drug B”, the ROR and the corresponding 95% confidence interval (CI) are calculated individually for each drug using 2 × 2 contingency tables [11, 12]. Subsequently, we combined the information from the contingency tables of the individual drugs into a new contingency table to compute the ROR and the 95% CI for the DDI. Specifically, for the computation of the ROR for the DDI, we considered as the “event” for the two interacting drugs “drug A + drug B” the number of cases where the AE of interest is reported together with drug A and drug B, but only when there was at least 1 day of co-exposure between drug A and drug B; if no days of co-exposure were reported, we did not consider the AEs related to drug A and drug B together, and these cases were considered as the “non-event”. We also propose a way for choosing the best comparator in disproportionality analyses, namely “monotherapy” between “drug A” and “drug B”, as described in Fig. 2. Specifically, we defined the drug to be considered as a comparator as the one with the highest lower bound of the ROR’s 95% CI in monotherapy. The ROR was therefore computed for each drug–event pair of the triplets. Triplets for which it was not possible to compute the ROR were excluded from the analysis.
Fig. 2.
Choice of comparator. CI confidence interval, ICSR individual case safety report, LB lower bound, ROR reporting odds ratio, UB upper bound
The triplets resulting in disproportionality signals (ROR lower limit of the 95% CI greater than 1) were further evaluated to assess if the AE was included in the Important Medical Event (IME) list provided by European Medicine Agency (EMA) [13]. As labelled by Kontsioti et al. [10], the type of interactions (theoretical/established/probable) and the severity of the AEs (minor/major) according to Micromedex were plotted using Venn diagrams. Each triplet was evaluated for pharmacological plausibility considering the time of co-exposure. Specifically, UpToDate [14], Medscape [15], and Drugs.com [16] were used for considering the type of pharmacological plausibility of each signal interaction.
Temporal Assessment Using the Time of Co-exposure
The time of co-exposure assessment was automized by developing a function in R that was able to calculate the temporal overlap by looking at the start and end dates of co-reported drugs (see “Code Availability” section).
In order to ensure a reasonable amount of data for data analysis, we decided to arbitrarily set a cut-off of ten cases involving the triplets (i.e. “event” in the contingency tables ≥ 10 cases) that were generated with the analysis described in Sect. 2.3.
A two-step process was used to assess the time of co-exposure in signal detections of DDIs:
Step A: The cumulative reporting of each disproportionality signal was calculated and plotted considering time of co-exposure. For each cumulative reporting plot, we assessed the derivative of the cumulative reporting function with the purpose of identifying its flex point. This flex point corresponded to the exponential increase in cumulative reporting, meaning that most cases retrieved in FAERS fell before that specific timing. The flex point of the cumulative reporting function was then evaluated with the information retrieved from the literature to determine whether the co-exposure time has to be considered temporally plausible. Mathematically, the flex point of a cumulative function represents the time at which the cumulative reporting function flexes. The method employed in this study to identify flex points in a cumulative distribution function (CDF) of ICSRs uses the derivative of the smoothed CDF to locate these flex points, which are identified as local maxima in the derivative. Moreover, the time of co-exposure assessed at the flex point represent the most representative time point among those reported in the ICSRs under investigation. In case of multiple flex points, we considered the first flex point, which empirically was related to the highest variation in the distribution (as a theoretical example, see Fig. 3). As an example, if most of the ICSRs indicated 1 day of co-exposure and it was found as the flex point of the cumulative reporting function, all the ICSRs with at least 1 day of co-exposure would be considered plausible if in the literature there was evidence of the occurrence of the AE after only 1 day of co-exposure. To showcase this approach, simulated data were generated using a progressively increasing rate. The rate was determined by the log2 transformation of time divided by 1, resulting in a slower increase rate over time. A single significant increase, corresponding to the flex point, was introduced at time point 5. Subsequently, for the remaining days, a smaller rate of increase was applied uniformly. The code of our simulations is provided in Electronic Supplementary Material 1 (ESM1).
Fig. 3.
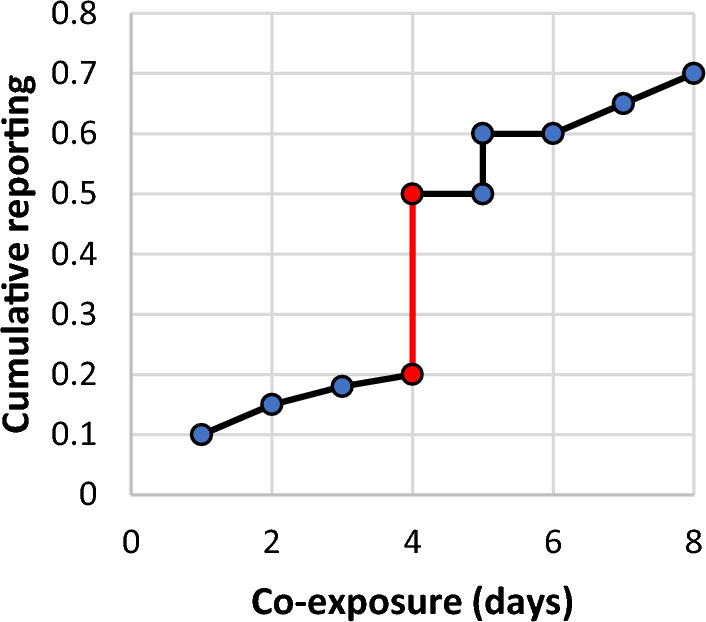
Example of flex point in cumulative reporting. The x-axis represents the co-exposure time of drug A and drug B expressed in days, while the y-axis represents the cumulative incidence of reporting a certain co-exposure time for a specific adverse event in each individual case safety report
If temporal plausibility was supported, under the assumption that the information reported was correct, the triplet was furtherly analysed:
Step B: Confounders in terms of age, sex, concomitant drugs, and therapeutic indications were analysed using a machine learning method (i.e. Lasso regression). We investigated the covariates among those described in the ICSRs that correlated with the time of co-exposure of the two drugs under the assumption that the time of co-exposure is correlated with the occurrence of the AE. Therefore, we assessed if the variables associated with the time of co-exposure could potentially be a source of reverse causation or risk factors for the AE. The variables, identified through Lasso regression, were further explored using a univariate analysis of the percentage of ICSRs with the covariate between cases and non-cases. Chi-square test for categorical variables and t test or Wilcoxon rank-sum test based on parametric assumptions for continuous variables were used to test the significant difference between groups.
The whole process is summarized in ESM2, Supplementary Fig. S1.
Statistical Software
All data processing and statistical analyses were performed using the R software (version 4.1.2) [17].
Results
The FAERS raw quarterly data contained 6,680,109 ICSRs, and after the cleaning procedure removing duplicates of the ICSRs, the final dataset consisted of 1,349,142 reports (20.2%). The FAERS raw quarterly data contained 17,441,164 drugs information, and after the cleaning procedure removing cases without active substance names and defined treatment duration, the final dataset consisted of 2,943,894 complete records (16.9%).
Signal Detection of DDIs Considering Time of Co-exposure
After the choice of the comparator, 122 (5.1%) out of 2372 triplets were eligible for the analysis and resulted in 61 disproportionality signals (50%) (ROR lower limit of the 95% CI greater than 1) involving 13 AEs (see ESM2, Supplementary Fig. S2); of these, eight AEs (61.5%) were included in the EMA’s IME list.
A general overview of the detected disproportionality signals is described in Table 1. Most of the interactions detected were synergic effects of the two drugs and 100% had pharmacological plausibility. Within the AEs detected with our methodology the most reported were the Medical Dictionary for Regulatory Activities (MedDRA®) Preferred Terms (PTs) “haemorrhage” and “hypotension”. Haemorrhage was reported mainly in ICSRs with a combination of platelet inhibitors, anticoagulant or fibrinolytic agents, non-steroidal anti-inflammatory drugs (NSAIDs), and antidepressants. DDIs related to hypotension were mostly described in ICSRs where there was an association with two antihypertensive drugs, or one antihypertensive drug associated with tricyclic antidepressants.
Table 1.
Disproportionality signals considering time of co-exposure
| AE | DDI | Event/non-event | ROR (95% CI) | Pharmacological actions | Type of interaction | UpToDate reliability rate |
|---|---|---|---|---|---|---|
| Acute kidney injury | Losartan ± ramipril | 9/32 | 2.91 (1.37–6.15) | ARB ± ACE inhibitor | PD | Excellent |
| Lisinopril ± valsartan | 8/23 | 3.42 (1.52–7.68) | ACE inhibitor ± ARB | PD | Excellent | |
| Bradycardia | Digoxin ± bisoprolol | 76/152 | 3.73 (2.70–5.18) | Cardiac glycoside ± β-blocker | PD | Good |
| Confusional state | Amitriptyline ± duloxetine | 14/71 | 3.87 (2.12–7.07) | TCA ± SNRI | Synergic effect | Good |
| QT prolonged | Paroxetine ± escitalopram | 9/26 | 32.27 (13.98–74.49) | SSRI ± SSRI | Synergic effect | Good |
| Clomipramine ± quetiapine | 20/71 | 4.51 (2.42–8.40) | TCA ± antipsychotic | Synergic effect | Fair | |
| Haloperidol ± fluoxetine | 4/39 | 4.38 (1.52–12.66) | Antipsychotic ± SSRI | Synergic effect | Fair | |
| Clomipramine ± venlafaxine | 12/64 | 2.39 (1.18–4.81) | TCA ± SNRI | Synergic effect | NA | |
| GI haemorrhage | Methylprednisolone ± dexketoprofen | 3/15 | 15.10 (4.33–52.59) | Corticosteroid ± NSAID | Synergic effect | Fair |
| Celecoxib ± dexamethasone | 6/115 | 3.30 (1.38–7.91) | NSAID ± corticosteroid | Synergic effect | Fair | |
| Haemorrhage | Ticagrelor ± escitalopram | 6/0.5 | 54.47 (3.04–976.58) | Platelet inhibitor ± SSRI | PD | Fair |
| Paroxetine ± clopidogrel | 3/9 | 21.97 (5.75–83.92) | SSRI ± platelet inhibitor | PD | Fair | |
| Duloxetine ± clopidogrel | 9/3 | 137.13 (36.40–516.58) | SNRI ± platelet inhibitor | PD | Fair | |
| Acenocoumarol ± paroxetine | 4/1 | 11.05 (1.22–99.74) | Anticoagulant ± SSRI | PD/PK | Fair | |
| Tinzaparin ± clopidogrel | 3/1 | 27.93 (2.87–271.52) | Anticoagulant ± platelet inhibitor | Synergic effect | Fair | |
| Enoxaparin ± ticagrelor | 34/50 | 5.51 (3.55–8.56) | Anticoagulant ± platelet inhibitor | Synergic effect | Fair | |
| Ticagrelor ± heparin | 45/69 | 3.11 (2.11–4.58) | Platelet inhibitor ± anticoagulant | Synergic effect | Fair | |
| Enoxaparin ± clopidogrel | 104/200 | 4.49 (3.51–5.75) | Anticoagulant ± platelet inhibitor | Synergic effect | Fair | |
| Apixaban ± ticagrelor | 13/3 | 12.10 (3.44–42.52) | Anticoagulant ± platelet inhibitor | Synergic effect | Fair | |
| Apixaban ± prasugrel | 11/5 | 6.14 (2.13–17.68) | Anticoagulant ± platelet inhibitor | Synergic effect | Fair | |
| Fondaparinux ± ticagrelor | 30/11 | 9.10 (4.46–18.57) | Anticoagulant ± platelet inhibitor | Synergic effect | Fair | |
| Fondaparinux ± clopidogrel | 23/14 | 5.17 (2.60–10.28) | Anticoagulant ± platelet inhibitor | Synergic effect | Fair | |
| Urokinase ± heparin | 5/5 | 26.00 (2.48–272.83) | Fibrinolytic agent ± anticoagulant | Synergic effect | Fair | |
| Alteplase ± heparin | 19/23 | 2.09 (1.12–3.88) | Fibrinolytic agent ± anticoagulant | Synergic effect | Fair | |
| Warfarin ± clopidogrel | 106/114 | 1.85 (1.41–2.42) | Anticoagulant ± platelet inhibitor | Synergic effect | Good | |
| Warfarin ± rivaroxaban | 48/64 | 1.47 (1.01–2.15) | Anticoagulant ± platelet inhibitor | Synergic effect | Fair | |
| Ticagrelor ± ibuprofen | 6/5 | 5.43 (1.66–17.87) | Platelet inhibitor ± NSAID | Synergic effect | Fair | |
| Ibuprofen ± clopidogrel | 11/31 | 6.19 (3.10–12.37) | NSAID ± platelet inhibitor | Synergic effect | Fair | |
| Diclofenac ± clopidogrel | 5/27 | 2.79 (1.07–7.30) | NSAID ± platelet inhibitor | Synergic effect | Fair | |
| Diclofenac ± paroxetine | 4/15 | 3.99 (1.32–12.09) | NSAID ± SSRI | Synergic effect | Good | |
| Naproxen ± clopidogrel | 5/17 | 4.06 (1.49–11.07) | NSAID ± platelet inhibitor | Synergic effect | Fair | |
| Celecoxib ± venlafaxine | 4/4 | 18.36 (4.55–74.13) | NSAID ± SNRI | PD | NA | |
| Celecoxib ± fluoxetine | 3/11 | 4.96 (1.37–17.97) | NSAID ± SSRI | PD | NA | |
| Meloxicam ± venlafaxine | 5/8 | 5.34 (1.72–16.61) | NSAID ± SNRI | Synergic effect | Fair | |
| Hyperkalaemia | Ramipril ± irbesartan | 3/8 | 8.69 (2.29–33.00) | ACE inhibitor ± ARB | PD | Excellent |
| Enalapril ± valsartan | 7/44 | 2.65 (1.17–6.01) | ACE inhibitor ± ARB | PD | Excellent | |
| Trimethoprim ± lisinopril | 18/66 | 7.77 (4.56–13.21) | Antibiotic ± ACE inhibitor | Synergic effect | Good | |
| Enalapril ± trimethoprim | 11/49 | 3.86 (1.95–7.63) | ACE inhibitor ± Antibiotic | Synergic effect | Good | |
| Hyponatraemia | Desmopressin ± fluoxetine | 3/1 | 11.71 (1.20–114.51) | synthetic analogue of ADH ± SSRI | Synergic effect | Fair |
| Duloxetine ± escitalopram | 10/25 | 10.45 (4.92–22.22) | SNRI ± SSRI | Synergic effect | NA | |
| Hypotension | Lercanidipine ± propranolol | 16/0.5 | 240.24 (14.23–4056.14) | CCB ± β-blocker | Synergic effect | NA |
| Irbesartan ± perindopril | 10/1 | 75.48 (9.60–593.19) | ARB ± ACE inhibitor | PD | Excellent | |
| Irbesartan ± lisinopril | 3/0.5 | 43.63 (2.18–874.51) | ARB ± ACE inhibitor | PD | Excellent | |
| Ramipril ± losartan | 12/29 | 2.87 (1.46–5.65) | ACE inhibitor ± ARB | PD | Excellent | |
| Sildenafil ± furosemide | 10/58 | 4.87 (2.38–10.00) | PDE5 inhibitor ± diuretic | Synergic effect | Fair | |
| Enalapril ± clomipramine | 4/2 | 24.99 (4.54–137.54) | ACE inhibitor ± TCA | Synergic effect | Fair | |
| Clomipramine ± oxepine | 3/10 | 4.21 (1.11–15.94) | TCA ± antipsychotic | Synergic effect | NA | |
| Amitriptyline ± propranolol | 13/31 | 22.26 (10.93–45.36) | TCA ± β-blocker | Synergic effect | Fair | |
| Tamsulosin ± propranolol | 9/5 | 17.65 (5.87–53.11) | α1 antagonist ± β-blocker | Synergic effect | Fair | |
| Nebivolol ± doxazosin | 15/11 | 7.86 (3.51–17.62) | β-blocker ± α1 antagonist | Synergic effect | Fair | |
| Bisoprolol ± alfuzosin | 13/19 | 5.67 (2.78–11.54) | β-blocker ± α1 antagonist | Synergic effect | Fair | |
| Bisoprolol ± doxazosin | 33/61 | 4.61 (2.99–7.11) | β-blocker ± α1 antagonist | Synergic effect | Fair | |
| Carvedilol ± doxazosin | 8/27 | 2.33 (1.04–5.20) | β-blocker ± α1 antagonist | Synergic effect | Fair | |
| Nifedipine ± doxazosin | 4/40 | 3.11 (1.06–9.13) | Ca2+ antagonist ± α1 antagonist | Synergic effect | Fair | |
| Nebivolol ± tamsulosin | 11/4 | 15.42 (4.82–49.37) | β-blocker ± α1 antagonist | Synergic effect | Fair | |
| Bisoprolol ± terazosin | 8/2 | 32.96 (6.98–155.51) | β-blocker ± α1 antagonist | Synergic effect | Good | |
| Myopathy | Dexamethasone ± atracurium | 4/360 | 3.34 (1.23–9.08) | Corticosteroid ± muscle relaxant | Synergic effect | Excellent |
| Orthostatic hypotension | Amitriptyline ± propranolol | 3/41 | 29.61 (7.16–122.49) | TCA ± β-blocker | PK | NA |
| Ototoxicity | Vancomycin ± gentamicin | 7/537 | 6.12 (2.46–15.23) | Antibiotic ± antibiotic | Synergic effect | Fair |
| Syncope | Valsartan ± enalapril | 8/43 | 4.04 (1.88–8.67) | ARB ± ACE inhibitor | PD | Excellent |
| Ramipril ± olmesartan | 4/9 | 4.49 (1.38–14.64) | ACE inhibitor ± ARB | PD | Excellent |
Event is the n° of AEs with co-exposure; non-event is the n° of other AEs with co-exposure.
ACE angiotensin-converting enzyme; ADH anti-diuretic hormone; AE adverse event; ARB angiotensin II receptor blocker; CCB calcium-channel blocker; CI confidence interval; DDI drug–drug interaction; GI gastrointestinal; NA not available; NSAID non-steroidal anti-inflammatory drug; PD pharmacodynamic interaction; PDE5 phosphodiesterase-5; PK pharmacokinetic interaction; ROR reporting odds ratio; SNRI serotonin and norepinephrine reuptake inhibitor; SSRI selective serotonin reuptake inhibitor; TCA tricyclic antidepressant
Less frequently reported AEs were related to PTs “acute kidney injury”, “hyperkalaemia”, and “syncope” with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs). The PT “QT prolonged” was mainly reported with the concomitant use of antipsychotics and antidepressants.
Severity of Signal Detection of DDIs Considering Time of Co-exposure
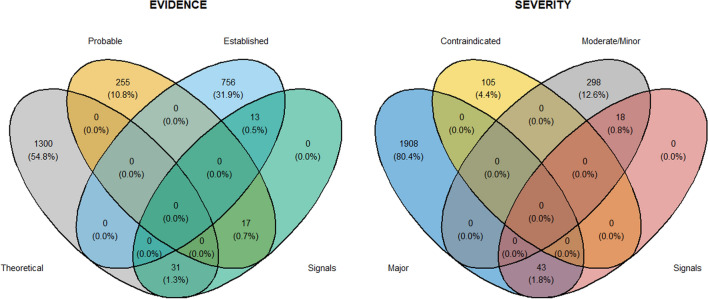
Out of 2372 triplets provided by Kontsioti et al. [10], our method was able to detect 31 theoretical signals (1.3%), 17 probable signals (0.7%), and 13 established signals (0.5%) (see Fig. 4). Considering the severity of the AEs, out of 2372 triplets screened, our method was able to detect 43 major AEs (1.8%) and 18 minor AEs (0.8%) (see Fig. 4).
Fig. 4.
Venn diagrams of type of triplets reported as frequencies (percentages) and retrieved considering those that were identified as a disproportionality signal for the temporal plausibility assessment using our method. Definitions of evidence and severity levels are those reported by Kontsioti et al. [10] in their work: evidence—evidence level associated with the drug–drug interaction as shown in Micromedex; severity—severity level associated with the drug–drug interaction as shown in Micromedex
Temporal Assessment Using the Time of Co-exposure
Twenty-seven (44.3%) out of 61 signals had at least ten cases reporting the triplet of interest and were assessed for temporal plausibility. After the assessment, 19 signals (70.4%) were temporally plausible after only 1 day of co-exposure (see ESM2, Supplementary Fig. S2). Below we have described in detail the approach to assess temporal plausibility for three PTs: “haemorrhage”, “hypotension”, and “hyperkalaemia”. This provides a clear example of how the flex point of the time of co-exposure can be used to identify true-positive and false-positive signals for DDIs in view of pharmacological plausibility. The complete analysis of all other MedDRA® PTs is reported in ESM2 (Supplementary Table S1 and S2).
Haemorrhage
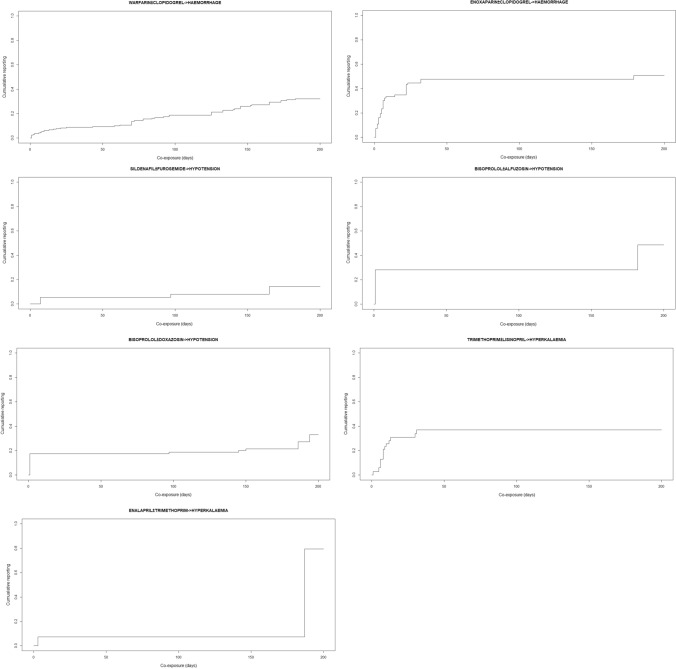
Haemorrhage was mainly reported with the concomitant use of more than one platelet inhibitor or anticoagulant/antithrombotic agent. According to the two steps described in Sect. 2.5, the flex point from the cumulative reporting was found to be 1 day of co-exposure time for each triplet, including for the DDIs enoxaparin/clopidogrel and warfarin/clopidogrel (see Fig. 5).
Fig. 5.
Cumulative reporting. Each graph represents the cumulative reporting of one triplet where drug A is analysed when reported (or not) in co-exposure with drug B. The x-axis represents the co-exposure time of drug A and drug B expressed in days, while the y-axis represents the cumulative incidence of reporting a certain co-exposure time for a specific adverse event in each individual case safety report
Step A: From the scientific literature, we assessed if haemorrhage was a temporal plausible event with at least 1 day of co-exposure of anticoagulant/antithrombotic agents. The short-term co-administration (even 1 day) is a common strategy in cardiology to ensure adequate anticoagulation in the transition period from one drug class to another, and it can be associated with the occurrence of bleeding [18]. Clinical guidelines and the Summary of Product Characteristics (SmPC) of drugs recommends clinical monitoring of bleedings even after 1 day of treatment [19, 20].
Step B: Among disproportionality signals involving platelet inhibitor or anticoagulant/antithrombotic agents, we used the signal of enoxaparin/clopidogrel as an example to emphasize how the machine learning method (i.e. Lasso regression) was able to identify potential confounders in our analysis. Lasso regression retrieved age and lorazepam to be related to the co-exposure time in the case of enoxaparin/clopidogrel and the occurrence of haemorrhage (see Table 2). Age is a known risk factor since major bleeding was found to increase steeply with age (≥ 75 years hazard ratio 3.10, 95% CI 2.27–4.24; p < 0.0001), in particular for fatal outcomes [21]. On the contrary, while other drug classes like antidepressants are known to increase the risk of bleeding [22], benzodiazepines seem not to interfere in this specific event. When age was examined in the univariate analysis between cases and non-cases, we found that individuals with the co-exposure were older (73.8 years vs. 71.9 years) than those without co-exposure in the ICSRs of those experiencing the AE of interest. This suggests that this signal could be potentially correlated to age. At the same time, the concomitant use of lorazepam was significantly less reported the ICSRs of those experiencing the AE of interest compared with those experiencing every other AEs (see Table 2).
Table 2.
Potential confounders in cases of haemorrhage (a) and hypotension (b)
| (a) | ||||
|---|---|---|---|---|
| Haemorrhage | ||||
| Enoxaparin ± clopidogrel | ||||
| Variable | AEs of interest | Other AEs | P value | |
| Lorazepam | No, n (%) | 104 (100) | 142 (85.0) | |
| Yes, n (%) | 0 (0) | 25 (9.2) | 0.001 | |
| Age | Mean (SD) | 73.8 (9.7) | 71.9 (11.2) | 0.161 |
| (b) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hypotension | |||||||||
| Bisoprolol ± alfuzosin | Bisoprolol ± doxazosin | ||||||||
| Variable | AEs of interest | Other AEs | P value | Variable | AEs of interest | Other AEs | P value | ||
| Apixaban | No, n (%) | 13 (100) | 9 (47) | Acetylsalicylic Acid | No, n (%) | 33 (100) | 51 (87) | ||
| Yes, n (%) | 0 (0) | 10 (53) | 0.006 | Yes, n (%) | 0 (0) | 8 (13) | 0.068 | ||
| Carbidopa/levodopa | No, n (%) | 9 (69) | 19 (100) | Cefalexin | No, n (%) | 33 (100) | 52 (88) | ||
| Yes, n (%) | 4 (31) | 0 (0) | 0.041 | Yes, n (%) | 0 (0) | 7 (12) | 0.099 | ||
| Cefuroxime | No, n (%) | 13 (100) | 9 (47) | Naproxen | No, n (%) | 33 (100) | 52 (88) | ||
| Yes, n (%) | 0 (0) | 10 (53) | 0.005 | Yes, n (%) | 0 (0) | 7 (12) | 0.099 | ||
| Dutasteride | No, n (%) | 9 (69) | 19 (100) | Warfarin | No, n (%) | 33 (100) | 47 (80) | ||
| Yes, n (%) | 4 (31) | 0 (0) | 0.041 | Yes, n (%) | 0 (0) | 12 (20) | 0.014 | ||
| Ezetimibe | No, n (%) | 13 (100) | 9 (47) | Age | Mean (SD) | 54.7 (23.5) | 66.5 (20.4) | 0.011 | |
| Yes, n (%) | 0 (0) | 10 (53) | 0.005 | ||||||
| Furosemide | No, n (%) | 13 (100) | 17 (89) | ||||||
| Yes, n (%) | 0 (0) | 2 (11) | 0.642 | ||||||
| Glyburide/Metformin | No, n (%) | 13 (100) | 17 (89) | ||||||
| Yes, n (%) | 0 (0) | 2 (11) | 0.642 | ||||||
| Hydrochlorothiazide /Olmesartan | No, n (%) | 13 (100) | 17 (89) | ||||||
| Yes, n (%) | 0 (0) | 2 (11) | 0.642 | ||||||
Chi-square test for categorical variables and t test or Wilcoxon rank-sum test based on parametric assumptions for continuous variables were used to test significant difference between groups
AE adverse event
Hypotension
Step A: DDIs related to hypotension were due to the association of two antihypertensive drugs or one antihypertensive drug associated with tricyclic antidepressants or α1 antagonists. The synergic effect of two drugs with cardiovascular effects might cause alterations in blood pressure even after 1 day of co-administration, which is why the flex time of 1 week (e.g. sildenafil ± furosemide) was temporally plausible (see Fig. 5 and ESM2, Supplementary Table S1).
Step B: Lasso regression was able to identify possible confounders concerning the DDIs of bisoprolol ± alfuzosin and bisoprolol ± doxazosin (see Table 2). From this analysis, it appeared that the disproportionality signal was confounded by age and co-reported drugs [23, 24].
Hyperkalaemia
Step A: Hyperkalaemia was also reported with the concomitant use of trimethoprim and ACE inhibitors. According to the scientific literature, a few days of co-exposure are not sufficient to induce hyperkalaemia [25]. Therefore, the flex point at 1 day was considered not temporally plausible (see Fig. 5).
As we did not find temporal plausibility in step A, no further analyses were performed for the signal and the signal was considered dismissed.
Discussion
In this study, we aimed at developing a signal detection and prioritization method that incorporated co-exposure time when assessing disproportionality for the DDIs. Our results suggest the ability of our method of detecting and prioritizing disproportionality signals with temporal plausibility for the DDIs. The inclusion of co-exposure time in disproportionality analysis of DDIs represents a significant advancement considering that current signal detection methods used for DDIs rely on a simplistic criterion: the co-reporting of two drugs within the same ICSRs. This assumption lacks pharmacological substantiation based on the established principles of DDIs [26]. Co-reporting alone does not provide conclusive evidence of an actual interaction between the drugs [27]. Pharmacological principles dictate that several factors need to be considered to establish the occurrence of a DDI. These include the pharmacokinetic properties of the drugs involved (such as metabolism and elimination pathways) and their potential interactions at the molecular level [28]. Furthermore, the dose, timing, and duration of drug co-exposure can significantly influence the likelihood and severity of a DDI [26, 28]. Neglecting these important factors and relying solely on the co-reporting of drugs within ICSRs can lead to misleading or inaccurate conclusions about the existence and clinical relevance of DDIs.
Another important result of the current study is that our method was able to prioritize 70.4% of disproportionality signals that were already temporally substantiated as they had a co-exposure time pharmacologically compatible with the observed AEs according to evidence from the scientific literature. Development of prioritization methods that incorporate co-exposure time is crucial in quantitative signal detection of DDIs considering the amount of generated disproportionality signals during routine signal detection activities and time-consuming and resource-intensive activities needed to dismiss these disproportionality signals based on a case-by-case assessment. The problem of inflation of disproportionality signals in assessing DDIs poses significant challenges in pharmacovigilance [4]. Furthermore, the feasibility and sustainability of current approaches for signal detection become increasingly problematic in the face of this signal inflation. The sheer volume of disproportionality signals requires substantial resources and expertise to evaluate and prioritize, which can strain pharmacovigilance systems. Therefore, finding robust automatable methods to prioritize disproportionality signals is crucial for ensuring timely assessment [29].
Overall, by strictly requiring availability of dates of reported drugs in ICSRs, we were able to identify only 50% of the disproportionality signals among true-positive DDIs. Ideally, a 100% detection rate for disproportionality signals was expected, considering the true-positive dataset as the starting point. It may appear tempting to assume that not prioritized disproportionality signals from our method lack evidence regarding temporality; however, it is crucial to recognize the inherent complexity of case series data. In scenarios where, for instance, 80% of cases lack crucial information such as dates, the remaining 20% may indeed comprise pivotal cases with robust temporal associations, warranting further scrutiny. However, a significant portion of data related to administration dates was missing. Therefore, it is not surprising that the power to detect disproportionality signals is reduced. In a real-world scenario, when having a large list of signals, our method can minimize the inflation using the ROR and only retrieve those for which there is sufficient information about dates to assess temporal plausibility. In practice, signals with sufficient information for validation are prioritized. It is therefore important to remember that the quality of the reporting has an important influence on the signal detection and that reporters should always fill reports with all relevant available information. The analysis of routinely collected health data could play a central role in the imputation of missing information, such as concomitant medications. At the same time, improving the completeness and quality of ICSRs, including accurate referencing of administration times, remains essential and it is therefore necessary to rethink the mandatory fields in the safety report submitted to SRS databases, including FAERS, which involve DDIs. Despite these obstacles, pharmacovigilance databases already enable the retrieval of information in the general population since they were created to collect AEs encompassing all age groups, types of treatments, and conditions. The collection of ICSRs still represents a valuable source of real-world data about the safety profile of specific drugs, and it allows comparison of therapeutic options, thus contributing to the pharmacological management of disorders and the prevention of AEs related to DDIs.
Strengths and Limitations
This study has several strengths. Firstly, we developed a method to detect and prioritize disproportionality signals with a temporal plausibility by looking at co-exposure time. Secondly, our method also implemented the analysis of co-exposure time of drugs that were reported as concomitant treatments, which are normally excluded in statistical signal detection. In this regard, it is worth mentioning that this problem is emphasized even by the reporter’s judgement, which may affect the results of signal detection if the potential drug is reported as “concomitant” and not as “suspect” [30]. Another important strength of our study is that we were able to consider as baseline the drug that is more frequently associated with the AEs, avoiding “masking” [31]. However, our approach is flexible to accommodate other choices for prioritizing the comparator. Another strength of our study is our choice of considering at least “1 day of co-exposure” when assessing DDIs. Drugs are strategically designed with a half-life falling within the range of 12 to 48 h to mitigate challenges related to drug accumulation and other pharmacokinetic considerations. Therefore, adopting a nominal value of 1 day as an “average” falls within the reasonable spectrum between the lower and upper bounds of the typical half-life range. This pragmatic approach aligns with established pharmaceutical design principles and serves as a practical means to address concerns related to exposure and the potential effects of treatments without direct overlap in co-exposure.
The study has also several limitations that are intrinsic to SRS databases: ICSRs are susceptible to the high variability of data quality, under-reporting, and incomplete reporting. This latter issue affected our analysis since many events were excluded because of a lack of specific information regarding the dates of reported drugs and their treatment duration. Indeed, it is essential to acknowledge the possibility that even though structured fields from FAERS lack certain details (i.e. dates), pertinent information might be available in free-text fields in the report. However, we did not consider free-text fields in our method. Another general limitation of all methods assessing DDI disproportionality signals, including ours, is that they are strongly influenced by the frequency of reporting, which directly or indirectly influences statistical power for detecting DDIs’ disproportionality signals. Finally, another limitation of our method is that we set a threshold that required a minimum count of “event” (the observed frequency) in the contingency tables to be greater than or equal to ten. This decision was made to provide a tangible demonstration of our method’s applicability, ensuring a sufficiently robust sample size for analysis. Future studies are needed to identify the optimal criteria for identifying the optimal cut-offs, as setting of such thresholds is inherently complex and dependent on various contextual factors, including the specific characteristics of the dataset, the nature of the research question, and statistical considerations.
Finally, in discussing the findings of this study, it is important to address other limitations. Firstly, the absence of comparison to a baseline method represents a notable limitation. While our proposed method has demonstrated promising results, the lack of comparative analysis may limit the ability to fully assess its effectiveness. Hence, it is imperative to acknowledge this limitation and consider it as a potential avenue for future research. Conducting comparisons with standard methods would provide valuable insights into the performance and efficacy of our approach. Secondly, another limitation arises from the exclusive analysis focused solely on the 27 triplets prioritized by our algorithm. Future studies may benefit from conducting comprehensive analyses that include all considered triplets, thereby enhancing the depth and breadth of insights gained from the methodology.
Future Work
In the future, we aim to furtherly investigate “indirect co-exposure” by exploring DDIs arising from the prolonged half-life of medications. Specifically, we aim to capture the cumulative impact of DDIs that may extend beyond the immediate co-administration period.
Conclusion
Our method was able to detect and prioritize signals with temporal plausibility, demonstrating its potential to enhance signal detection and prioritization during the signal management process. By efficiently retrieving signals that are more likely to be appropriately prioritized, it offers a valuable tool for optimizing resources and time, enabling pharmacovigilance professionals to concentrate their efforts on the most relevant signals and ultimately enhancing patient care and drug safety.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
VB is enrolled in a PhD in Experimental and Clinical Pharmacological Sciences, Università degli Studi di Milano, which supports her fellowship.
Declarations
Ethics approval
No ethical approval is required for studies using publicly available data from FAERS.
Data availability
The data sources are open access, and the datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Code is available from the corresponding author upon reasonable request.
Consent to participate
Not applicable. FAERS spontaneous reports are anonymous and publicly available.
Consent for publication
Not applicable. FAERS spontaneous reports are anonymous and publicly available.
Author contributions
VB, GP, AB, and MS conceived and designed the study. VB and MS designed and supervised the research and analysed the data. VB wrote the original draft, and MAB, CC, GP, AB, EC, and MS reviewed and edited, with inputs from all authors. VB, MC, MAB, GP, AB, CC, EC, and MS participated in the interpretation of data and revised and approved the final article as submitted.
Funding
Open access funding provided by Copenhagen University.
Conflict of interest
Greg Powell and Andrew Bate are both employees of GSK and hold stock and stock options. All the other authors have no conflict of interest to declare.
References
- 1.Strandell J, Norén GN, Hägg S. Key elements in adverse drug interaction safety signals: an assessment of individual case safety reports. Drug Saf. 2013;36:63–70. 10.1007/s40264-012-0003-9 [DOI] [PubMed] [Google Scholar]
- 2.Vilar S, Friedman C, Hripcsak G. Detection of drug–drug interactions through data mining studies using clinical sources, scientific literature and social media. Brief Bioinf. 2018;19:863–77. 10.1093/bib/bbx010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norén GN, Sundberg R, Bate A, Edwards IR. A statistical methodology for drug–drug interaction surveillance. Stat Med. 2008;27:3057–70. 10.1002/sim.3247 [DOI] [PubMed] [Google Scholar]
- 4.Noguchi Y, Yoshizawa S, Aoyama K, Kubo S, Tachi T, Teramachi H. Verification of the “Upward variation in the reporting odds ratio scores” to detect the signals of drug–drug interactions. Pharmaceutics. 2021;13:1531. 10.3390/pharmaceutics13101531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strandell J, Caster O, Hopstadius J, Edwards IR, Norén GN. The development and evaluation of triage algorithms for early discovery of adverse drug interactions. Drug Saf. 2013;36:371–88. 10.1007/s40264-013-0053-7 [DOI] [PubMed] [Google Scholar]
- 6.Bate A, Evans SJW. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18:427–36. 10.1002/pds.1742 [DOI] [PubMed] [Google Scholar]
- 7.Noguchi Y, Tachi T, Teramachi H. Comparison of signal detection algorithms based on frequency statistical model for drug–drug interaction using spontaneous reporting systems. Pharm Res. 2020;37:86. 10.1007/s11095-020-02801-3 [DOI] [PubMed] [Google Scholar]
- 8.Strandell J, Caster O, Bate A, Norén N, Edwards IR. Reporting patterns indicative of adverse drug interactions: a systematic evaluation in VigiBase. Drug Saf. 2011;34:253–66. 10.2165/11586990-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 9.Kass-Hout TA, Xu Z, Mohebbi M, Nelsen H, Baker A, Levine J, et al. OpenFDA: an innovative platform providing access to a wealth of FDA’s publicly available data. J Am Med Inf. 2016;23:596–600. 10.1093/jamia/ocv153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kontsioti E, Maskell S, Dutta B, Pirmohamed M. A reference set of clinically relevant adverse drug–drug interactions. Sci Data. 2022;9:72. 10.1038/s41597-022-01159-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agency EM. Guideline on good pharmacovigilance practices (GVP)–Annex I–Definitions (Rev 4). Heads Med Agencies; 2017. p. 1–33.
- 12.Sessa M, Kragholm K, Hviid A, Andersen M. Thromboembolic events in younger women exposed to Pfizer-BioNTech or Moderna COVID-19 vaccines. Expert Opin Drug Saf. 2021;20:1451–3. 10.1080/14740338.2021.1955101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Medicine Agency. MedDRA (Medical Dictionary for Regulatory Activities)—important medical event terms list, version 26.1; 2023.
- 14.Fox GN, Moawad NS. Up to date: a comprehensive clinical database. J Fam Pract. 2003;52:706–10. [PubMed] [Google Scholar]
- 15.Frishauf P. Medscape—the first 5 years, vol. 7. Medscape Gen Med. WebMD/Medscape Health Network; 2005. p. 5.
- 16.Plumb AL. Drugs.Com: drug information online 2004. Ref Rev. 2004;18:41. [Google Scholar]
- 17.Dessau RB, Pipper CB. ‘‘R”—project for statistical computing. Ugeskr Laeger. 2008;170:328–30. [PubMed] [Google Scholar]
- 18.Witt DM, Nieuwlaat R, Clark NP, Ansell J, Holbrook A, Skov J, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2:3257–91. 10.1182/bloodadvances.2018024893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e44S-e88S. 10.1378/chest.11-2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lidell C, Svedberg L-E, Lindell P, Bandh S, Job B, Wallentin L. Clopidogrel and warfarin: absence of interaction in patients receiving long-term anticoagulant therapy for non-valvular atrial fibrillation. Thromb Haemost. 2003;89:842–6. 10.1055/s-0037-1613471 [DOI] [PubMed] [Google Scholar]
- 21.Li L, Geraghty OC, Mehta Z, Rothwell PM. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet. 2017;390:490–9. 10.1016/S0140-6736(17)30770-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang K-H, Chen C-M, Wang C-L, Tu H-T, Huang Y-T, Wu H-C, et al. Major bleeding risk in patients with non-valvular atrial fibrillation concurrently taking direct oral anticoagulants and antidepressants. Front Aging Neurosci. 2022;14:791285. 10.3389/fnagi.2022.791285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhanu C, Nimmons D, Petersen I, Orlu M, Davis D, Hussain H, et al. Drug-induced orthostatic hypotension: a systematic review and meta-analysis of randomised controlled trials. PLoS Med. 2021;18: e1003821. 10.1371/journal.pmed.1003821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oelke M, Gericke A, Michel MC. Cardiovascular and ocular safety of α1-adrenoceptor antagonists in the treatment of male lower urinary tract symptoms. Expert Opin Drug Saf. 2014;13:1187–97. 10.1517/14740338.2014.936376 [DOI] [PubMed] [Google Scholar]
- 25.Crellin E, Mansfield KE, Leyrat C, Nitsch D, Douglas IJ, Root A, et al. Trimethoprim use for urinary tract infection and risk of adverse outcomes in older patients: cohort study. BMJ. 2018;360:k341. 10.1136/bmj.k341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu J, Straubinger RM, Mager DE. Pharmacodynamic drug–drug interactions. Clin Pharmacol Ther. 2019;105:1395–406. 10.1002/cpt.1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang H, Lin Y, Ren W, Fang Z, Liu Y, Tan X, et al. Adverse drug reactions and correlations with drug–drug interactions: a retrospective study of reports from 2011 to 2020. Front Pharmacol. 2022;13:923939. 10.3389/fphar.2022.923939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polaka S, Koppisetti HP, Tekade M, Sharma MC, Sengupta P, Tekade RK. Drug–drug interactions and their implications on the pharmacokinetics of the drugs. In: Polaka S, editor. Pharmacokinetics and toxicokinetic considerations. Amsterdam: Elsevier; 2022. p. 291–322. [Google Scholar]
- 29.Sartori D, Aronson JK, Norén GN, Onakpoya IJ. Signals of adverse drug reactions communicated by pharmacovigilance stakeholders: a scoping review of the global literature. Drug Saf. 2023;46:109–20. 10.1007/s40264-022-01258-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeiss R, Connemann BJ, Schönfeldt-Lecuona C, Gahr M. Risk of bleeding associated with antidepressants: impact of causality assessment and competition bias on signal detection. Front Psychiatry. 2021;12:727687. 10.3389/fpsyt.2021.727687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maignen F, Hauben M, Hung E, Van Holle L, Dogne JM. Assessing the extent and impact of the masking effect of disproportionality analyses on two spontaneous reporting systems databases. Pharmacoepidemiol Drug Saf. 2014;23:195–207. 10.1002/pds.3529 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sources are open access, and the datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.