Abstract
In adenovirus-infected cells, binding of E1B-55kDa and E4orf6 to the tumor suppressor protein p53 inhibits its transcriptional activity and causes rapid turnover of the protein. To investigate the requirements of the E1B-E4orf6 complex to modulate p53 function, we generated an E4orf6 mutant that failed to associate functionally and physically with E1B-55kDa but still interacted with p53. We confirm that E4orf6 and E1B-55kDa reduce p53 transactivation individually and show that their combined inhibition is additive rather than synergistic. Furthermore, we found that downregulation of p53's expression level, but not transcriptional inhibition of p53, depends on a functional E1B-E4 complex. A functional interaction of E1B-55kDa with p53, on the other hand, is a prerequisite for both transcriptional repression and downregulation of p53. The separation of these two functions will enable further dissection of the requirements for oncogenicity by the E4orf6 protein.
The phosphoprotein p53 is a cellular transcription factor that regulates cell cycle progression and induction of apoptosis in response to stress and DNA damage (2, 17). Inactivation of p53 function renders the cell susceptible to unregulated proliferation and is the single most common event in human cancer (13). Elimination of functional p53 is also important for replication of DNA tumor viruses which require entry into the S phase of the cell cycle. Many DNA viruses encode specific oncoproteins that bind to p53 and modulate its normal biological function. Human adenovirus type 5 (Ad5) expresses genes from three different regions of the viral genome that modulate p53 function. These are the gene products of early region 1A (E1A), the 55-kDa product of the E1B region (E1B-55kDa), and a 34-kDa product encoded by open reading frame 6 of early region 4 (E4orf6). The E1A proteins stabilize p53, leading to nuclear accumulation and induction of apoptosis (8, 18). E1B-55kDa blocks p53-mediated transcriptional activation by binding directly to its amino-terminal transactivation domain (4, 14, 19, 34, 35), thus inhibiting both p53-induced growth arrest and apoptosis (8). The third adenovirus protein shown to inhibit p53-mediated transactivation is E4orf6 (10, 21). There are, however, conflicting reports in which expression of E4orf6 alone was unable to inhibit p53 activation (26, 31). The E4 protein can also block p53-dependent apoptosis (20) and can cooperate with E1A to transform primary rodent cells (20, 21).
E4orf6 forms a physical and functional complex with E1B-55kDa (5, 27). Association with E4orf6 targets E1B-55kDa to the nucleus (24), and it has been suggested that the resulting complex shuttles between the two cellular compartments and serves as a nucleocytoplasmic transporter for viral mRNAs (9, 32). Both E1B-55kDa and E4orf6 bind independently to p53, and concomitant expression of the two oncogenes leads to rapid turnover of p53 in 293 cells (12, 20) and in Ad5-infected cells (11, 25, 30). It is unclear whether individual interactions of both E4orf6 and E1B-55kDa with p53 are necessary to affect the transcriptional activity and stability of p53. In this report, we examined the interactions of E4orf6 with E1B-55kDa and p53 and the requirements for modulating p53 function.
Mutation of the RXL motif disrupts the E4orf6–E1B-55kDa complex.
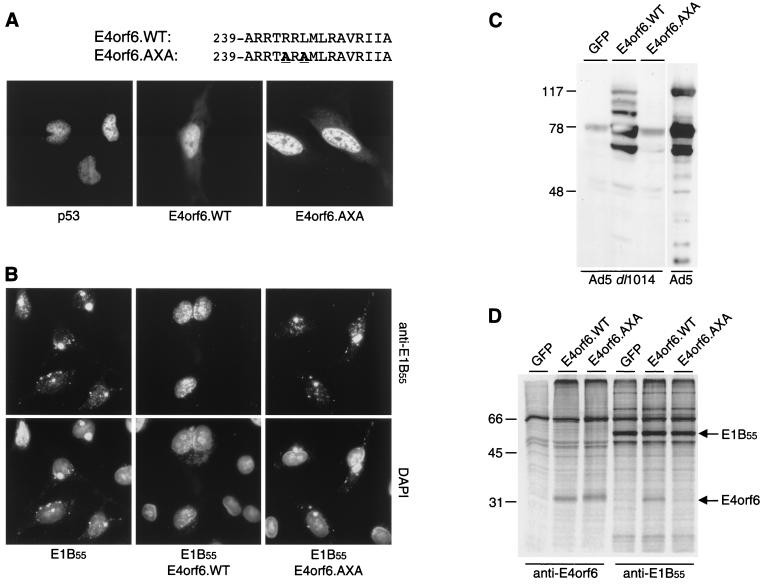
The carboxyl terminus of E4orf6 contains an amphipathic α-helix that has been suggested to be critical for the formation of a functional E1B-E4 complex (23, 32). We recently uncovered a link between expression of E4orf6 and arrest of the cell cycle and noted within this same region a putative RXL motif that might mediate interactions with cyclin A and associated kinases (1, 12). The E4orf6.AXA mutant contains two alanine substitutions (R243A and L245A) disrupting this putative RXL motif (Fig. 1A, top). We used this mutant to examine whether a mutation in this region affects binding to E1B-55kDa and p53. Expression and subcellular localization of the E4orf6 proteins were analyzed by indirect immunofluorescence (Fig. 1A) and immunoblotting (Fig. 2B). Both wild-type E4orf6 and E4orf6.AXA were expressed at similar levels and localized predominantly in the nucleus.
FIG. 1.
The E4orf6.AXA mutant fails to associate functionally and physically with the E1B-55kDa protein. (A) Cellular localization of wild-type and mutant E4orf6 proteins. Expression plasmids pSV2.p53, pRK5.E4orf6.WT, and pRK5.E4orf6.AXA were transfected into Saos-2 or HeLa cells, respectively, and proteins were detected by indirect immunofluorescence with an antibody directed against p53 (FL-393; Santa Cruz Biotechnology) or E4orf6 (MAb M45). The amino acid sequence of the C-terminal α-helix of E4orf6 is indicated on top; substitutions to create the mutant are highlighted in boldface and underlined. (B) Relocalization of E1B-55kDa by E4orf6. E1B-55kDa was transiently expressed in HeLa cells in the absence or presence of coexpressed wild-type or mutant E4orf6, as indicated below the panels. Localization of E1B-55kDa was determined by indirect immunofluorescence with antibody 2A6 (upper panels). Nuclei were located by costaining cellular DNA with 4′,6′-diamidino-2-phenylindole. Merged pictures are shown in the lower panels. (C) Complementation assay for Ad5 late protein expression. 293T cells were transfected with expression plasmids encoding either GFP, wild-type E4orf6, or E4orf6.AXA and subsequently infected with E4-deleted Ad5 dl1014 at a multiplicity of infection of 10 PFU/cell. Late protein expression was assessed after 48 h by immunoblotting of cell lysates with a polyclonal antibody generated to Ad5 particles (16). As a control, purified Ad5 particles (10 μg) were loaded in the far-right lane. Positions of the molecular mass markers (in kilodaltons) are indicated on the left. (D) Coimmunoprecipitation assay for E1B-55kDa and E4orf6. 293T cells were transfected with expression plasmids encoding either GFP, wild-type E4orf6, or E4orf6.AXA. Cells were metabolically labeled with [35S]Met-Cys, and proteins were immunoprecipitated with antibodies to E4orf6 (MAb M45) and E1B-55kDa (2A6). Positions of the molecular mass markers (in kilodaltons) are shown on the left, and the respective positions of E4orf6 and E1B-55kDa are indicated on the right.
FIG. 2.
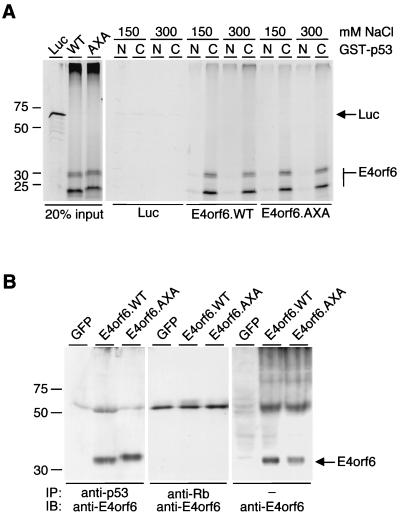
The E4orf6.AXA mutant retains the ability to bind p53 in vitro and in vivo. (A) In vitro analysis of protein-protein interactions by GST pull-downs. GST-p53 fusion proteins, as indicated on top (N, amino-terminal segment, residues 1 to 219; C, carboxyl-terminal segment, residues 220 to 393), were assayed for their ability to capture in vitro-translated wild-type E4orf6 or E4orf6.AXA in the presence of 150 or 300 mM NaCl, respectively. The lanes on the left show 20% of the input amount of in vitro-translated protein added to each pull-down experiment. Positions of the molecular mass markers (in kilodaltons) are indicated on the left, and the positions of E4orf6 and luciferase (Luc) are indicated on the right. (B) Coimmunoprecipitation analysis to detect in vivo interactions. 293T cells were transfected with expression plasmids encoding either GFP, wild-type E4orf6, or E4orf6.AXA. Proteins were immunoprecipitated with antibodies to p53 (Ab-6; Calbiochem) or Rb (IF8; Santa Cruz Biotechnology), and E4orf6 was detected by immunoblotting with either RSA3 or MAb M45. Positions of the molecular mass markers are shown on the left, and the position of E4orf6 is indicated on the right.
We employed two assays to determine whether the E4orf6 mutant functionally interacts with the E1B-55kDa protein. In the absence of E4orf6, E1B-55kDa is detected in cytoplasmic perinuclear bodies. Coexpression of E4orf6 recruits E1B-55kDa to the nucleus where it localizes to characteristic nuclear speckles (24). HeLa cells were transfected with an E1B-55kDa-expressing plasmid (pcDL.E1B55) either alone or in combination with plasmids encoding wild-type E4orf6 (pRK5.E4orf6.WT) or the E4orf6 mutant (pRK5.E4orf6.AXA). Subcellular localization of E1B-55kDa was determined by indirect immunofluorescence (Fig. 1B), as previously described (33). Coexpression of E4orf6.WT recruited E1B-55kDa to the nucleus, whereas E4orf6.AXA failed to relocalize E1B-55kDa. In the second assay, we analyzed the ability of the E4orf6 proteins to complement an E4-deleted mutant adenovirus for late protein expression (15). 293T cells were transfected with plasmids expressing green fluorescent protein (GFP), E4orf6.WT, or E4orf6.AXA and subsequently infected with the E4 mutant virus, Ad5 dl1014 (6). Cell lysates were assessed for production of late adenovirus proteins by being immunoblotted with a polyclonal serum (Fig. 1C) as previously described (7). Expression of E4orf6.WT was sufficient to complement Ad5 dl1014 for late protein expression, but E4orf6.AXA had lost this activity. The results of these two assays show that the E4orf6.AXA mutant has lost the ability to interact functionally with E1B-55kDa.
We next investigated whether the lack of activity of the E4orf6 mutant was due to disruption of the physical association with E1B-55kDa by coimmunoprecipitation analysis with metabolically labeled cell extracts. 293T cells were transfected with plasmids expressing GFP, E4orf6.WT, or E4orf6.AXA, and proteins were labeled with [35S]Met-Cys for 8 h. Cell lysates were immunoprecipitated with monoclonal antibodies (MAbs) M45 (22) to E4orf6 or MAb 2A6 to E1B-55kDa (29), and proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1D), as previously described (28). The E4orf6 antibody precipitated E4orf6.WT and E4orf6.AXA, indicating that the two proteins were expressed at comparable levels. We did not detect coprecipitation of E1B-55kDa because MAb M45 recognizes the N terminus of E4orf6, the region that also interacts with E1B-55kDa (27). On the other hand, E4orf6.WT could be isolated by coimmunoprecipitation with the E1B-55kDa antibody, whereas the E4orf6 mutant could not. This demonstrates that the inability of E4orf6.AXA to interact functionally with E1B-55kDa is due to the lack of a physical association.
The E4orf6.AXA mutant retains the ability to bind p53 in vitro and in vivo.
The mechanism by which E4orf6 abrogates transcriptional activation is not well understood. It has been proposed that E4orf6 binding to the carboxyl-terminal regulatory domain of p53 blocks the ability of the amino-terminal activation domain to recruit TAFII31, a component of TFIID (10). We therefore analyzed whether the introduced mutations affect the interaction of E4orf6.AXA with human p53. The interaction was studied in vitro by comparing the ability of p53 fusion proteins to capture radiolabeled E4orf6 proteins (Fig. 2A). As previously observed (10), in vitro-translated E4orf6 runs in a multiple-banding pattern with the slowest-migrating band running at about the expected position of 34 kDa. Glutathione S-transferase (GST) fusion proteins, containing either an amino-terminal (GST-p53N) or a carboxyl-terminal (GST-p53C) segment of p53, were overexpressed in bacteria and purified using glutathione-Sepharose beads. Purified proteins remained on the beads and were stored in phosphate-buffered saline. Consistent with previous results (10), the GST-p53C fusion protein was able to capture wild-type E4orf6 at two different salt concentrations (150 or 300 mM NaCl; 20 mM HEPES-KOH [pH 7.9]; 1 mM EDTA; 0.1 mM MgCl2; 0.1 mM ZnCl2; 0.05% Tween 20; 0.2% Triton X-100; 1 mM dithiothreitol), whereas an interaction between GST-p53N and E4orf6 was barely detectable. Likewise, GST-p53C, but not GST-p53N, was able to pull down E4orf6.AXA efficiently. In a control reaction, interaction of in vitro-translated luciferase with either GST-p53 segment was barely detectable. We also investigated whether the E4orf6 mutant physically associates with p53 in vivo. 293T cells were transfected with plasmids expressing GFP, E4orf6.WT, or E4orf6.AXA, and cell lysates were immunoprecipitated with antibodies directed against p53 or retinoblastoma protein. Proteins were separated by SDS-PAGE, and E4orf6 was detected by immunoblotting (Fig. 2B), as described previously (12). The retinoblastoma protein control antibody did not precipitate E4orf6, but both E4orf6.WT and E4orf6.AXA were coprecipitated by the p53 antibody. These results demonstrate that both E4orf6.WT and the E4orf6.AXA mutant can interact with p53 in vitro and in vivo.
Interaction of E1B-55kDa and E4orf6 is necessary for downregulation of p53 but not for transcriptional inhibition.
Since concomitant expression of E1B-55kDa and E4orf6 was shown to shorten the half-life of p53 (11, 20, 25, 30), we used two different ratios of expression plasmids encoding p53, E1B-55kDa, and E4orf6 to discriminate between transcriptional inhibition and changes in steady-state levels of p53.
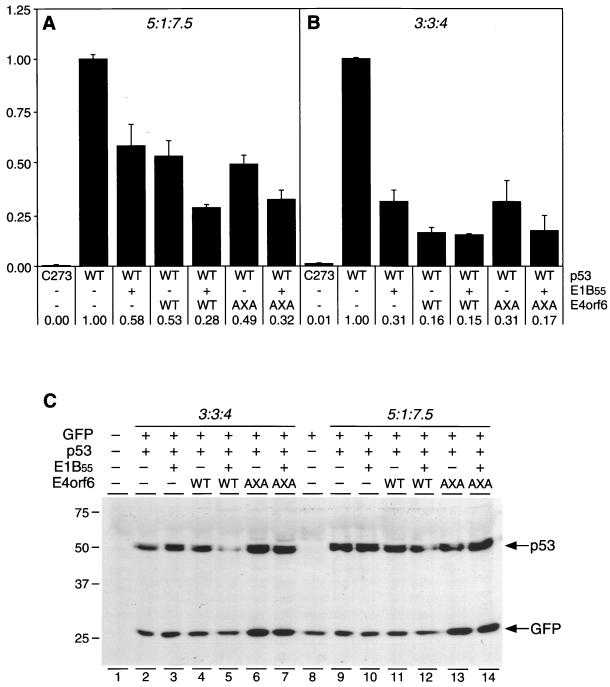
We used transient-transfection assays to test whether E4orf6.AXA was able to affect p53-mediated transcription. Human p53-deficient Saos-2 cells were transfected with 1 μg of a reporter plasmid containing 13 copies of the p53-binding site upstream of a luciferase reporter gene (pPG13.Luc) and plasmids expressing p53 (pSV2.p53.WT), E1B-55kDa, and E4orf6 by calcium phosphate precipitation. The ratios of included plasmids were as follows: p53:E1B-55kDa:E4orf6 = 5:1:7.5 (Fig. 3A) and 3:3:4 (Fig. 3B), respectively, corresponding to a ratio (in micrograms) of 1.0:0.2:1.5 and 0.9:0.9:1.2. For all experiments, transfection efficiency was assessed by including 1 μg of plasmid pCMVβ (Stratagene) in the transfection mixture. Total DNA quantity was kept constant by adding empty pcDNA vector (Invitrogen). Cells were harvested 32 h posttransfection, and lysates were analyzed for luciferase and β-galactosidase activities, as previously described (7, 12). As shown in Fig. 3A, cotransfection of the p53 expression plasmid with the reporter enhanced luciferase expression by a factor of 200, as compared to transfection with a plasmid encoding a p53 DNA-binding mutant (pSV2.p53.C273). Inclusion of E1B-55kDa reduced p53 transactivation to 58% of wild-type activity, whereas coexpression of E4orf6 decreased p53 activity to 53% of that of the wild type. Cotransfection of both E4orf6 and E1B-55kDa expression plasmids revealed an additive effect, reducing the transcriptional activity of p53 to 28%. Expression of E4orf6.AXA alone (49%) or in combination with E1B-55kDa (32%) led to an inhibition of p53 similar to that observed for wild-type E4orf6. Since the E4orf6.AXA mutant does not interact with E1B-55kDa, combined repression of p53 by E4orf6 and E1B-55kDa is additive and does not depend on an interaction between the two viral oncoproteins. A combination of higher expression levels of E1B-55kDa and slightly lower levels of p53 led to a more pronounced inhibition of p53 activity by both E1B-55kDa and E4orf6 (Fig. 3B).
FIG. 3.
Downregulation of p53's expression level but not transcriptional inhibition requires a functional complex of E4orf6 and E1B-55kDa. (A and B) Inhibition of p53 transcriptional activity in the presence of E1B-55kDa and E4orf6 proteins. Saos-2 cells were transfected with the p53-responsive reporter plasmid pPG13.Luc and one of two different ratios of expression plasmids for p53, E1B-55kDa, and E4orf6.WT or E4orf6.AXA as indicated below the columns. Ratios were 5:1:7.5 for results shown in panel A and 3:3:4 for those shown in panel B, as indicated on top. Transfection efficiency was assessed by including plasmid pCMVβ (Stratagene) in the transfection mixture. After 32 h, cells were harvested in RLB buffer (Promega), and luciferase and β-galactosidase activities were determined with a luminometer. Individual experiments were repeated at least twice in duplicate. Measured light units are indicated as relative activity compared to that of wild-type p53 alone. Numbers below the columns and error bars reflect the relative transactivation and the standard deviation of a representative experiment performed in duplicate. (C) Steady-state levels of transiently expressed p53 in the presence or absence of E1B-55kDa and E4orf6 proteins. Saos-2 cells were transfected with expression plasmids for p53, E1B-55kDa, and E4orf6 at ratios of 3:3:4 and 5:1:7.5, as indicated on the top. The transfection mixture also contained plasmids pCMV.Luc to normalize for transfection efficiency and pEGFP (Clontech) as an internal standard. After 32 h, cells were harvested and lysates were subjected to immunoblot analysis with antibodies to p53 (Ab-6) and GFP (MAb 2510). In lane 1, a mock-transfected lysate of Saos-2 was applied. Positions of the molecular mass markers (in kilodaltons) are shown on the left, and the respective proteins are indicated with arrows.
There have been conflicting reports of the effect of E4orf6 on p53-mediated transactivation. Although inhibition has been previously reported (10), other groups have been unable to detect repression by E4orf6 alone (26, 31). We have observed that the p53:E4orf6 ratio as well as the absolute expression level of E4orf6 is critical for repression. Inhibition is easily observed if moderate levels of p53 expression (as from pSV2.p53.WT) are combined with high levels of E4orf6 expression derived from transcripts that include an upstream splice donor-acceptor site (as from pRK5.E4orf6.WT). However, when we used expression vectors that lack a splice donor-acceptor site (e.g., pcDNA3.1), we obtained a lower level of E4orf6 expression and inhibition was barely detectable, even with high concentrations of input plasmid (T. Cathomen and M. D. Weitzman, unpublished observations).
We then analyzed whether there are detectable alterations in steady-state levels of p53 under the conditions used in the reporter assays. Confluent Saos-2 cells were transfected with Effectene (Qiagen) with wild-type p53 expression plasmid either alone or in combination with plasmids expressing E4orf6 and E1B-55kDa. The same plasmid ratios were used as for the reporter assays, corresponding to ratios (in micrograms) of 0.37:0.07:0.56 and 0.3:0.3:0.4, respectively. Plasmids pCMV.Luc and pEGFP-N3 (Clontech) were cotransfected to normalize for transfection efficiency and as an internal standard (0.1 μg each). Cells were harvested 32 h after transfection and lysed in RIPA-CoIP buffer (50 mM Tris [pH 7.5]) 150 mM NaCl; 5 mM EDTA; 0.05% bovine serum albumin; 0.2% NP-40). Luciferase activity was determined with a luminometer, and lysates corresponding to equal amounts of luciferase activity were subjected to SDS-PAGE. Expression levels of p53 and GFP were determined by immunoblot analysis with antibody Ab-6 (Calbiochem) and MAb 2510 (Chemicon), as previously described (12). As shown in Fig. 3C, expression of the internal standard, GFP, was not significantly affected by expression of E1B-55kDa or E4orf6. Also, p53 levels were not affected by coexpression of the adenovirus proteins at a plasmid DNA ratio of p53:E1B-55kDa:E4orf6 = 5:1:7.5 (Fig. 3C, lanes 9 to 14), suggesting that the repression shown in Fig. 3A was not due to alterations in steady-state levels of p53. Analysis of cell lysates transfected at a ratio of 3:3:4 (lanes 2 to 7) revealed that coexpression of either oncogene did not affect p53 stability (lanes 2 to 4); however, the combined expression of E4orf6 and E1B-55kDa significantly reduced the steady-state levels of p53 (lane 5). In contrast, concomitant expression of E1B with E4orf6.AXA did not downregulate p53 (lane 7). This implies that, as opposed to transcriptional repression, downregulation of p53 requires a functional association between E4orf6 and E1B-55kDa. The data also suggest that E1B-55kDa levels are crucial for p53 stability, since downregulation was only observed with a higher ratio of E1B to p53.
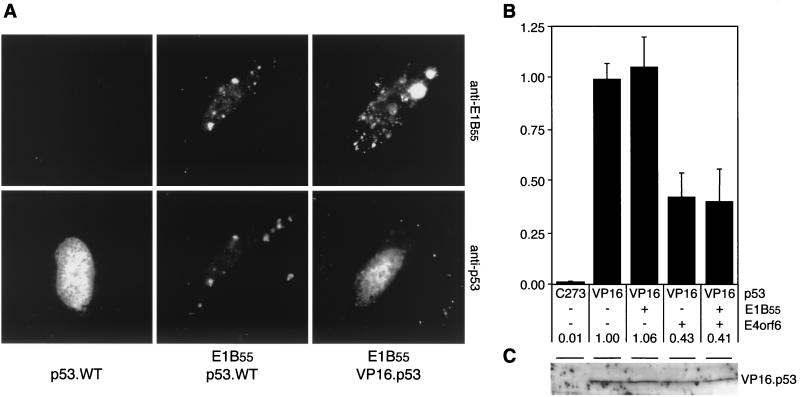
To analyze whether a functional interaction of E1B-55kDa with p53 is a requirement for both transcriptional repression and decrease of steady-state levels, we used a construct in which the p53 activation domain was replaced by the heterologous VP16 activation domain (gift of T. Halazonetis). Coexpression of E1B-55kDa recruits wild-type p53 to cytoplasmic perinuclear bodies, as shown by indirect immunofluorescence analysis of transfected Saos-2 cells (Fig. 4A). Since subcellular localization of the chimeric p53 protein was not affected by concomitant expression of E1B, we concluded that the chimeric proteins did not functionally interact with E1B-55kDa. A reporter assay of Saos-2 cells revealed that the chimeric VP16.p53 protein retained the potential to transactivate p53-responsive reporters (Fig. 4B). Although VP16.p53 was still repressed by E4orf6, coexpression of E1B-55kDa did not repress its transcriptional activity. Moreover, stability of VP16.p53 was only marginally affected by the presence of the E1B-E4 complex, as determined by immunoblot analysis (Fig. 4C) with a polyclonal anti-p53 antibody (FL-393; Santa Cruz Biotechnology). We therefore conclude that a functional association of E1B-55kDa with p53 is a prerequisite for both transcriptional repression and downregulation of p53.
FIG. 4.
A functional interaction of E1B-55kDa with p53 is required for both transcriptional repression and downregulation of p53. (A) The chimeric VP16.p53 protein is not relocalized to perinuclear bodies by E1B-55kDa. Wild-type and chimeric p53 proteins were transiently expressed in Saos-2 cells in the absence or presence of E1B-55kDa, as indicated below the panels. The localization of E1B-55kDa and p53 proteins was determined by indirect immunofluorescence with antibody 2A6 (upper panel) and FL-393 (lower panel). Nuclear localization was confirmed by costaining cellular DNA with 4′,6′-diamidino-2-phenylindole (results not shown). (B) Transcriptional activity of VP16.p53 is repressed by E4orf6 but not by E1B-55kDa. Saos-2 cells were transfected with plasmids pPG13.Luc, pSV2.VP16.p53, pcDL.E1B55, pRK5.E4orf6.WT (at a ratio of 5:1:7.5), and pCMVβ. After 32 h, cells were harvested and processed as indicated in the legend to Fig. 3. Measured light units are indicated as relative activity compared to VP16.p53 alone. The numbers below the columns and error bars reflect the relative transactivation and the standard deviation of at least two experiments performed in duplicate. (C) Steady-state levels of transiently expressed VP16.p53 are not affected by E1B-55kDa and E4orf6. Saos-2 cells were transfected with expression plasmids for VP16.p53, E1B-55kDa, and E4orf6.WT (at a ratio of 3:3:4), as indicated on top. After 32 h, cells were harvested and lysates were subjected to immunoblot analysis with a polyclonal antibody to p53 (FL-393). In lane 1, a mock-transfected lysate of Saos-2 cells was applied. The position of VP16.p53 is indicated.
In summary, we have addressed the requirements for regulation of the expression level of p53 and p53 transcriptional activity by the E1B-55kDa and E4orf6 adenovirus oncoproteins. Our results show that E1B-55kDa and E4orf6 can each inhibit p53 independently and that their combined effect is additive rather than synergistic. On the other hand, efficient downregulation of p53 expression levels requires direct binding of E1B-55kDa to p53 and association with E4orf6.
The E4orf6.AXA mutant fails to associate physically with E1B-55kDa, and it is unable to form a functional complex with E1B-55kDa. Recent reports have suggested that an arginine-faced, amphipathic α-helix, encompassing residues 239 through 253, is critical for a functional interaction with E1B-55kDa (23, 32). Our results support these findings, as the amino acid substitutions introduced in E4orf6.AXA (R243A and L245A) may lead to a disorganization of this α-helical structure and thus could account for the disruption of the E4orf6–E1B-55kDa complex. This supports the suggestion that a physical association is required to recruit E1B-55kDa to the nucleus and also to complement late protein expression during adenovirus infection. Alternatively, as both E1B-55kDa and E4orf6 have been shown to be phosphoproteins, phosphorylation of either protein might be important for their association. The putative RXL motif in E4orf6 raises the possibility that E4orf6 could be a target substrate of cdk2. A mutation in the RXL motif could result in an altered phosphorylation pattern and thus abolish the association between E4orf6 and E1B-55kDa. It is also possible that the RXL region interacts with a cellular protein that is necessary for the association between the two viral proteins.
Our ability to separate inhibition of p53 transactivation from downregulation of p53's expression level will enable further dissection of the requirements for oncogenicity by the E4orf6 protein.
Acknowledgments
We thank Tom Hope for use of the microscope and Joanne Chory for use of the luminometer. We are grateful to T. Halazonetis, W. El-Deiry, and A. Berk for plasmids; G. Ketner for E4-deleted Ad5 dl1014; K. F. Kozarsky for rabbit polyclonal antibody to Ad5 particles; P. Hearing and T. Shenk for E4orf6-specific antibodies MAb M45 and RSA3; and A. J. Levine for antibody 2A6. We also thank Wendy Cordier and Betty Gilbert for technical assistance and T. Halazonetis, Mirta Grifman, and Travis Stracker for helpful discussions and comments on the manuscript.
This work was supported by a fellowship from the Swiss National Science Foundation (T.C.), a grant from the N.I.H. (M.D.W.), an Innovation Grant from the President's Club of the Salk Institute (M.D.W.), and gifts from the Oracle Corporate Giving Program and Odette Wurzburger (M.D.W.).
REFERENCES
- 1.Adams P D, Sellers W R, Sharma S K, Wu A D, Nalin C M, Kaelin W G., Jr Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amundson S A, Myers T G, Fornace A J., Jr Roles for p53 in growth arrest and apoptosis: putting on the brakes after genotoxic stress. Oncogene. 1998;17:3287–3299. doi: 10.1038/sj.onc.1202576. [DOI] [PubMed] [Google Scholar]
- 3.Boivin D, Morrison M R, Marcellus R C, Querido E, Branton P E. Analysis of synthesis, stability, phosphorylation, and interacting polypeptides of the 34-kilodalton product of open reading frame 6 of the early region 4 protein of human adenovirus type 5. J Virol. 1999;73:1245–1253. doi: 10.1128/jvi.73.2.1245-1253.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braithwaite A W, Blair G E, Nelson C C, McGovern J, Bellett A J. Adenovirus E1b-58 kD antigen binds to p53 during infection of rodent cells: evidence for an N-terminal binding site on p53. Oncogene. 1991;6:781–787. [PubMed] [Google Scholar]
- 5.Bridge E, Ketner G. Interaction of adenoviral E4 and E1b products in late gene expression. Virology. 1990;174:345–353. doi: 10.1016/0042-6822(90)90088-9. [DOI] [PubMed] [Google Scholar]
- 6.Bridge E, Ketner G. Redundant control of adenovirus late gene expression by early region 4. J Virol. 1989;63:631–638. doi: 10.1128/jvi.63.2.631-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cathomen T, Collete D, Weitzman M D. A chimeric protein containing the N terminus of the adeno-associated virus Rep protein recognizes its target site in an in vivo assay. J Virol. 2000;74:2372–2382. doi: 10.1128/jvi.74.5.2372-2382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debbas M, White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 9.Dobbelstein M, Roth J, Kimberly W T, Levine A J, Shenk T. Nuclear export of the E1B 55-kDa and E4 34-kDa adenoviral oncoproteins mediated by a rev-like signal sequence. EMBO J. 1997;16:4276–4284. doi: 10.1093/emboj/16.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Blockage by adenovirus E4orf6 of transcriptional activation by the p53 tumor suppressor. Science. 1996;272:1470–1473. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- 11.Grand R J, Grant M L, Gallimore P H. Enhanced expression of p53 in human cells infected with mutant adenoviruses. Virology. 1994;203:229–240. doi: 10.1006/viro.1994.1480. [DOI] [PubMed] [Google Scholar]
- 12.Grifman M, Chen N N, Gao G P, Cathomen T, Wilson J M, Weitzman M D. Overexpression of cyclin A inhibits augmentation of recombinant adeno-associated virus transduction by the adenovirus E4orf6 protein. J Virol. 1999;73:10010–10019. doi: 10.1128/jvi.73.12.10010-10019.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollstein M, Rice K, Greenblatt M S, Soussi T, Fuchs R, Sorlie T, Hovig E, Smith-Sorensen B, Montesano R, Harris C C. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994;22:3551–3555. [PMC free article] [PubMed] [Google Scholar]
- 14.Kao C C, Yew P R, Berk A J. Domains required for in vitro association between the cellular p53 and the adenovirus 2 E1B 55K proteins. Virology. 1990;179:806–814. doi: 10.1016/0042-6822(90)90148-k. [DOI] [PubMed] [Google Scholar]
- 15.Ketner G, Bridge E, Virtanen A, Hemstrom C, Pettersson U. Complementation of adenovirus E4 mutants by transient expression of E4 cDNA and deletion plasmids. Nucleic Acids Res. 1989;17:3037–3048. doi: 10.1093/nar/17.8.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozarsky K F, Jooss K, Donahee M, Strauss III J F, Wilson J M. Effective treatment of familial hypercholesterolaemia in the mouse model using adenovirus-mediated transfer of the VLDL receptor gene. Nat Genet. 1996;13:54–62. doi: 10.1038/ng0596-54. [DOI] [PubMed] [Google Scholar]
- 17.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 18.Lowe S W, Ruley H E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993;7:535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 19.Martin M E, Berk A J. Adenovirus E1B 55K represses p53 activation in vitro. J Virol. 1998;72:3146–3154. doi: 10.1128/jvi.72.4.3146-3154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore M, Horikoshi N, Shenk T. Oncogenic potential of the adenovirus E4orf6 protein. Proc Natl Acad Sci USA. 1996;93:11295–11301. doi: 10.1073/pnas.93.21.11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nevels M, Rubenwolf S, Spruss T, Wolf H, Dobner T. The adenovirus E4orf6 protein can promote E1A/E1B-induced focus formation by interfering with p53 tumor suppressor function. Proc Natl Acad Sci USA. 1997;94:1206–1211. doi: 10.1073/pnas.94.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obert S, O'Connor R J, Schmid S, Hearing P. The adenovirus E4–6/7 protein transactivates the E2 promoter by inducing dimerization of a heteromeric E2F complex. Mol Cell Biol. 1994;14:1333–1346. doi: 10.1128/mcb.14.2.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orlando J S, Ornelles D A. An arginine-faced amphipathic alpha helix is required for adenovirus type 5 E4orf6 protein function. J Virol. 1999;73:4600–4610. doi: 10.1128/jvi.73.6.4600-4610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ornelles D A, Shenk T. Localization of the adenovirus early region 1B 55-kilodalton protein during lytic infection: association with nuclear viral inclusions requires the early region 4 34-kilodalton protein. J Virol. 1991;65:424–429. doi: 10.1128/jvi.65.1.424-429.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Querido E, Marcellus R C, Lai A, Charbonneau R, Teodoro J G, Ketner G, Branton P E. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J Virol. 1997;71:3788–3798. doi: 10.1128/jvi.71.5.3788-3798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth J, Konig C, Wienzek S, Weigel S, Ristea S, Dobbelstein M. Inactivation of p53 but not p73 by adenovirus type 5 E1B 55-kilodalton and E4 34-kilodalton oncoproteins. J Virol. 1998;72:8510–8516. doi: 10.1128/jvi.72.11.8510-8516.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubenwolf S, Schütt H, Nevels M, Wolf H, Dobner T. Structural analysis of the adenovirus type 5 E1B 55-kilodalton–E4orf6 protein complex. J Virol. 1997;71:1115–1123. doi: 10.1128/jvi.71.2.1115-1123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruffner H, Verma I M. BRCA1 is a cell cycle-regulated nuclear phosphoprotein. Proc Natl Acad Sci USA. 1997;94:7138–7143. doi: 10.1073/pnas.94.14.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarnow P, Sullivan C A, Levine A J. A monoclonal antibody detecting the adenovirus type 5-E1B–58Kd tumor antigen: characterization of the E1b–58Kd tumor antigen in adenovirus-infected and -transformed cells. Virology. 1982;120:510–517. doi: 10.1016/0042-6822(82)90054-x. [DOI] [PubMed] [Google Scholar]
- 30.Steegenga W T, Riteco N, Jochemsen A G, Fallaux F J, Bos J L. The large E1B protein together with the E4orf6 protein target p53 for active degradation in adenovirus infected cells. Oncogene. 1998;16:349–357. doi: 10.1038/sj.onc.1201540. [DOI] [PubMed] [Google Scholar]
- 31.Steegenga W T, Shvarts A, Riteco N, Bos J L, Jochemsen A G. Distinct regulation of p53 and p73 activity by adenovirus E1A, E1B, and E4orf6 proteins. Mol Cell Biol. 1999;19:3885–3894. doi: 10.1128/mcb.19.5.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weigel S, Dobbelstein M. The nuclear export signal within the E4orf6 protein of adenovirus type 5 supports virus replication and cytoplasmic accumulation of viral mRNA. J Virol. 2000;74:764–772. doi: 10.1128/jvi.74.2.764-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weitzman M D, Fisher K J, Wilson J M. Recruitment of wild-type and recombinant adeno-associated virus into adenovirus replication centers. J Virol. 1996;70:1845–1854. doi: 10.1128/jvi.70.3.1845-1854.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yew P R, Berk A J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992;357:82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- 35.Yew P R, Liu X, Berk A J. Adenovirus E1B oncoprotein tethers a transcriptional repression domain to p53. Genes Dev. 1994;8:190–202. doi: 10.1101/gad.8.2.190. [DOI] [PubMed] [Google Scholar]