Abstract
Introduction
Available data regarding the safety and efficacy of sotrovimab in pregnant patients remain limited due to their exclusion from clinical trials.
Methods
The COVID-19 International Drug Pregnancy Registry (COVID-PR) was established to gather comprehensive safety data from pregnant women who have received monoclonal antibody (mAb) or antiviral treatments for mild, moderate, or severe coronavirus disease 2019 (COVID-19) during pregnancy. Participants actively contributed self-reported data concerning their COVID-19 symptoms, in addition to sociodemographic and health-related characteristics. Obstetric, neonatal, and infant outcomes were also documented, with follow-up extending up to 12 months after childbirth.
Results
As of 30 November 2023, sotrovimab was administered to 39 participants enrolled in the COVID-PR. At the time of this report, 26 participants had given birth, with nine deliveries performed via cesarean section. The infants’ birthweight ranged from 2381 g to 4762 g, with a mean of 3439.91 g. Twenty-five infants were born at ≥37 weeks. A total of 31 adverse events (AEs) were reported by 12 participants. The most frequently reported AE was gestational hypertension, observed in three participants. COVID-19 re-infection, fatigue, gestational diabetes, headache, and morning sickness were each reported by two participants. Of the reported AEs, eight (in five participants) were classified as serious, including four AEs (prolonged labor, pre-eclampsia, polyhydramnios, premature labor) that affected pregnancy. Seven of these eight serious AEs (SAEs) were found to be unrelated to sotrovimab, with one event (urinary retention) not assessable. A total of 44 AEs were reported in 19 delivered infants or in utero fetuses. The most common were COVID-19 (n = 6 events), ear infection (n = 5 events), neonatal dyspnea (n = 3 events), and respiratory syncytial virus infection (n = 3 events). Sixteen AEs (in 11 infants/fetuses) were classified as serious, including one report each of fetal cardiac disorder, congenital ankyloglossia, persistent right umbilical vein, and congenital hydronephrosis; the latter was considered a major congenital malformation. For all assessable SAEs, causality of sotrovimab treatment was ruled out based on lack of a temporal relationship alone or in combination with absence of a plausible mechanism.
Conclusion
A sizable proportion of sotrovimab-treated participants in the COVID-PR had underlying medical conditions associated with an increased risk of severe COVID-19. None of the assessable SAEs were considered to be related to sotrovimab treatment.
Key Points
| Most participants had underlying medical conditions associated with an increased risk of severe coronavirus disease 2019 (COVID-19). |
| None of the assessable serious adverse events reported were considered to be related to sotrovimab treatment. |
Introduction
Monoclonal antibody (mAb) therapies have emerged as standard treatments for diverse inflammatory and autoimmune disorders [1]. Nevertheless, concerns about their use during pregnancy and the potential fetal effects have been raised [1, 2]. Consequently, the utilization of mAbs during pregnancy has been largely limited [1]. The extent of placental transfer and fetal exposure to mAbs varies, influenced by factors such as drug structure, half-life, dose, and timing concerning gestational age [1]. In the first trimester, minimal transfer occurs, primarily through placental diffusion. As pregnancy advances, maternal immunoglobulin (Ig) G antibodies are increasingly actively transported, peaking after 36 weeks of gestation [3]. This holds significance, particularly for women of reproductive potential who might conceive after receiving a long half-life mAb (e.g., sotrovimab [4]), as fetal exposure may coincide with the formation of the mature placenta, typically occurring after 14 weeks of gestation, a time frame when evidence suggests IgG-based therapies can cross the placenta [3]. Among IgG subclasses, IgG1 exhibits the highest transfer efficiency, whereas other subclasses cross at lower rates. Cord blood mAb concentrations are inversely linked to the time elapsed since the last maternal dose [1]. At birth, infant mAb levels may surpass maternal levels but usually wane by approximately 6 months of age [1].
Sotrovimab, an Fc-engineered, dual-action, human IgG1κ mAb derived from the parental mAb S309, was developed in response to the coronavirus disease 2019 (COVID-19) pandemic, effectively neutralizing the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein [5–8]. In addition, sotrovimab has been shown to have potent effector functions in vitro that may result in immune-mediated viral clearance [5]. In the randomized COMET-ICE clinical trial (NCT04545060), a single intravenous 500 mg dose of sotrovimab significantly reduced the risk of all-cause hospitalization lasting longer than 24 h or death due to any cause compared with placebo in high-risk patients with mild-to-moderate COVID-19 at risk of progressing to severe disease [9, 10]. This study was conducted during the early stages of the pandemic, when the original ‘wild-type’ variant predominated. Sotrovimab has been granted a marketing authorization in the European Union, Norway, Iceland, Bahrain, Australia (conditional), United Kingdom (conditional), Saudi Arabia (conditional), and Switzerland (conditional). In Japan, a Special Approval in Emergency has been granted. In addition, sotrovimab currently has a temporary/emergency authorization Canada, and the United Arab Emirates. An Emergency Use Authorization was issued by the US FDA in May 2021. However, the sotrovimab Emergency Use Authorization was deauthorized in all US regions on 5 April 2022 due to a decrease in in vitro neutralization of sotrovimab against circulating Omicron BA.2 SARS-CoV-2 variants.
The emergence of a novel pathogen such as SARS-CoV-2 raises important questions about pregnancy as a risk factor for infection or developing severe disease [11]. Available data are insufficient to determine whether pregnancy increases SARS-CoV-2 susceptibility [12]. However, comparisons between pregnant and non-pregnant women of reproductive age suggest pregnancy itself poses a risk for severe COVID-19 [13, 14]. Pregnancy was categorized as a qualifying high-risk condition for sotrovimab use in the US in May 2021, followed by other countries [15]. As is common practice, pregnant patients were excluded from sotrovimab clinical trials; data on the comprehensive safety and efficacy of sotrovimab in pregnant patients are therefore limited.
The COVID-19 International Drug Pregnancy Registry (COVID-PR; NCT05013632) is an international, non-interventional, postmarketing cohort study aimed at collecting safety data for pregnant and recently pregnant (enrolled within 30 days post-pregnancy) women treated with mAbs or antiviral drugs for mild, moderate, or severe COVID-19 during pregnancy [16]. In this report, we present data from participants who received sotrovimab up to 30 November 2023.
Methods
The study included pregnant or recently pregnant women aged 18 years or older with mild-to-moderate COVID-19 during pregnancy, and who were administered the antiviral agents remdesivir or molnupiravir, or the mAbs casirivimab/imdevimab or sotrovimab. In the case of mAbs, the exposure period also incorporated the 90 days preceding the commencement of the last menstrual period to account for preconceptional exposure given the long half-life of mAbs (the half-life of sotrovimab is approximately 56.5 days [17]). Participants contributed self-reported data on COVID-19 symptoms, in addition to sociodemographic and health characteristics. Obstetric, neonatal, and infant outcomes were also documented (Table 1), with follow-up extending over 12 months after childbirth. Furthermore, an online data collection system streamlined the retrieval of de-identified medical records from participants. The study followed FDA and European Medicines Agency (EMA) guidance for pregnancy registries for regulatory reporting of adverse events (AEs) [18, 19]. AEs were defined as any untoward medical occurrence in a patient administered a medicinal product and which does not necessarily have a causal relationship with this treatment. An AE could therefore be any unfavorable and unintended sign (e.g., an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medicinal product, whether or not considered related to the medicinal product. Causality assessment was performed by the COVID-PR safety physician based on the totality of the available evidence. Serious AEs (SAEs) were defined as any untoward medical occurrence that follows treatment (regardless of causal relationship with the therapy) that resulted in death, was life-threatening, required inpatient hospitalization or prolongation of hospitalization, resulted in persistent or significant disability/incapacity (substantial disruption of the ability to conduct normal life functions), resulted in congenital anomaly/birth defect, or required intervention to prevent permanent impairment or damage. As above, causality assessment was performed by the COVID-PR safety physician based on the totality of the available evidence.
Table 1.
Obstetric, neonatal, and infant outcomes assessed by the COVID-PR
| Obstetrica | Neonatal (aged < 29 days)a | Infant (aged 29 days–12 months) |
|---|---|---|
| Spontaneous abortion | Major congenital malformations | Developmental milestones (motor, cognitive, language, social-emotional, and mental health skills) at 6, 9, and 12 months of ageb |
| Gestational diabetes | Low birth weight | Height |
| Gestational hypertension | Neonatal death | Weight |
| Intrauterine growth restriction | Neonatal infections | Failure to thrive |
| Postpartum hemorrhage | Preterm birth | |
| Cesarean delivery | Small for gestational age | |
| Stillbirth |
The COVID-PR study was expected to span 5 years from study start; it was conducted in any country where the drugs of interest were available and where Pregistry, the study sponsor, had obtained research ethics clearance or approval. The original aim was to enroll a minimum of 200 pregnancies for each medication, including 100 cases where the respective treatment was administered during the first trimester of pregnancy. Recruitment for sotrovimab was initiated on 1 December 2021; however, no patients have been recruited into the study since May 2023, largely due to the deauthorization of sotrovimab in the US in April 2022. The data encapsulated within this report encompass data collected up to 30 November 2023.
The COVID-PR was conducted in compliance with the International Society for Pharmacoepidemiology ‘Guidelines for Good Epidemiology Practices for drug, device, and vaccine research in the United States’ [20], the EMA ‘Guideline on good pharmacovigilance practices’ [21], the FDA regulatory requirements including the draft guidance ‘Postapproval pregnancy safety studies: guidance for industry’ [22], and the ethical principles of the Declaration of Helsinki [23].
Results
As of 30 November 2023, 39 women in the COVID-PR received sotrovimab treatment. Most participants were from the US, with additional enrollments from Germany and Canada. Baseline characteristics are presented in Table 2. The participants’ ages ranged from 24 to 40 years, with a mean age of 32.38 (standard deviation 4.15) years. The majority of participants identified as White, had college-level education, did not use tobacco or alcohol, regularly took vitamin supplements, and considered themselves to be in good health. Among those with available data, half (n = 14/28) had at least one comorbidity during pregnancy onset, with polycystic ovary syndrome and asthma being the most prevalent conditions (six participants each). Additionally, 19 participants (48.7%) with available data had a pre-pregnancy body mass index indicative of overweight or obesity. Twenty-five women (64.1%) received sotrovimab treatment during their second or third trimester of pregnancy.
Table 2.
Baseline characteristics of COVID-PR participants treated with sotrovimab (N = 39)
| Characteristic | N | % |
|---|---|---|
| Age, years | ||
| 21–25 | 3 | 7.7 |
| 26–30 | 11 | 28.2 |
| 31–35 | 13 | 33.3 |
| 36–40 | 12 | 30.8 |
| Ethnicity | ||
| White/Caucasian | 33 | 84.6 |
| Black/African American | 1 | 2.6 |
| Middle Eastern/North African | 1 | 2.6 |
| Missing data | 4 | 10.3 |
| Tobacco use | ||
| None | 25 | 64.1 |
| Yes, before this pregnancy | 6 | 15.4 |
| Yes, before and during this pregnancy | 1 | 2.6 |
| Missing data | 7 | 17.9 |
| Alcohol use during pregnancy | ||
| Never | 29 | 74.4 |
| Once per month | 2 | 5.1 |
| Once per week | 1 | 2.6 |
| Missing data | 7 | 17.9 |
| Gestational timing of treatment | ||
| First trimester | 14 | 35.9 |
| Second trimester | 17 | 43.6 |
| Third trimester | 8 | 20.5 |
| Vitamin use during pregnancy | ||
| Daily | 24 | 61.5 |
| 4–6 times/week | 8 | 20.5 |
| Missing data | 7 | 17.9 |
| Pre-pregnancy body mass index | ||
| 18.5–25.9 | 15 | 38.5 |
| 26.0–30.0 | 8 | 20.5 |
| > 30.1 | 11 | 28.2 |
| Missing data | 5 | 12.8 |
| Self-reported pre-pregnancy health | ||
| Excellent | 14 | 35.9 |
| Very good | 13 | 33.3 |
| Good | 5 | 12.8 |
| Fair | 1 | 2.6 |
| Missing data | 6 | 15.4 |
| Existing medical conditions at onset of pregnancy | ||
| None | 14 | 35.9 |
| At least one condition | 14 | 35.9 |
| Asthma | 6 | 15.4 |
| Crohn’s disease | 1 | 2.6 |
| Hyperthyroidism | 1 | 2.6 |
| Hypothyroidism | 2 | 5.1 |
| Low blood pressure | 1 | 2.6 |
| Polycystic ovary syndrome | 6 | 15.4 |
| Other | 2 | 5.1 |
| Missing data | 11 | 28.2 |
COVID-19 coronavirus disease 2019, COVID-PR COVID-19 International Drug Pregnancy Registry
None of the treated COVID-19 episodes was classified as severe. Four were classified as moderate-severe, 21 as moderate, 13 as mild-moderate, and one as mild.
Maternal/Neonatal Outcomes
As of 30 November 2023, 26 participants had given birth, with nine deliveries conducted via cesarean section. The status of one participant was undetermined (due date has passed but no information on the birth is available), and 12 participants had been lost to follow-up (Fig. 1). The birthweights of the 26 infants ranged from 2381 to 4762 g, with a mean birthweight of 3439.91 g. Among the infants, 25 were delivered at ≥ 37 weeks, and one was born at < 37 weeks.
Fig. 1.
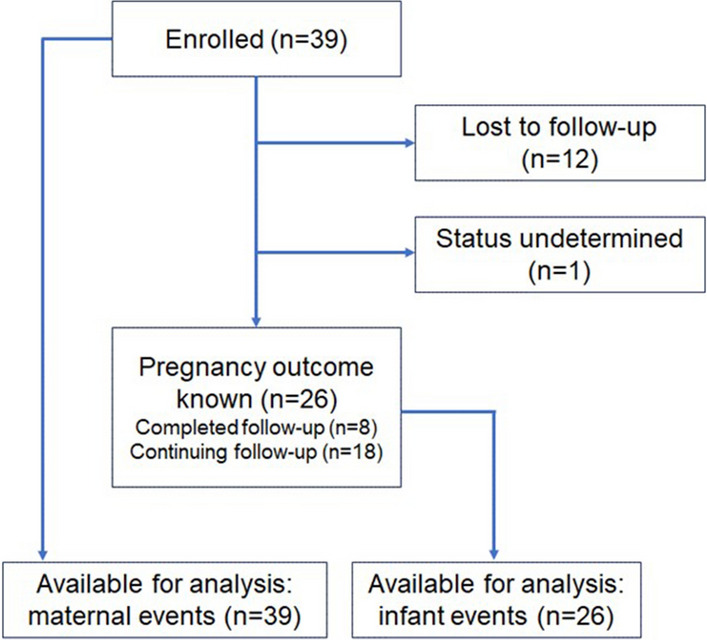
CONSORT diagram for the sotrovimab arm of the COVID-PR study, as of 30 November 2023. CONSORT Consolidated Standards of Reporting Trials. COVID-PR COVID-19 International Drug Pregnancy Registry
Maternal Adverse Events (AEs)
A total of 31 maternal AEs were reported by 12 participants. The most frequently reported AE was gestational hypertension, occurring in three participants. COVID-19 re-infection, fatigue, gestational diabetes, headache, and morning sickness were each reported in two participants. Among the non-SAEs (n = 23 events), 10 were deemed related to sotrovimab treatment after assessment by the COVID-PR safety physician (back pain, pyrexia, pain, hypotension, dizziness, diarrhea, chills, fatigue, headache [n = 2 events]) and one was not assessable (uterine malposition) as insufficient information was provided to evaluate the case.
Eight AEs (in five participants) were categorized as serious (Tables 3, 4), including four AEs (prolonged labor, pre-eclampsia, polyhydramnios, premature labor) in three participants that affected pregnancy (Table 3). Seven of the eight SAEs were found to be unrelated to sotrovimab, with one event (urinary retention) not assessable. The urinary retention event occurred 27 days after receipt of sotrovimab, with available data suggesting it was likely due to uterine incarceration rather than a direct effect of sotrovimab. Notably, details regarding potential uterine incarceration risk factors (such as post-surgical adhesions, pelvic inflammatory disease, fibroids, or tissue laxity) were not documented. Although the relationship between this event and sotrovimab treatment could not be assessed, the likelihood of it being treatment-related was considered to be low.
Table 3.
Summary of pregnancy outcomes
| Maternal age group, years | Serious adverse event |
Time since sotrovimab treatment |
Potential considerations (confounding factors and timing) |
|---|---|---|---|
| Maternal events | |||
| 26–30 | Prolonged labor | –a | Obesity, nulliparity |
| 31–35 | – | – | – |
| 36–40 | Pre-eclampsia | 163 days | Unspecified disability, overweight, tobacco use, temporal relationship |
| Polyhydramnios | 60 days | – | |
| Premature labor | 105 days | PCOS, obesity, tobacco use | |
| Infant/fetal events | |||
| 26–30 | Fetal cardiac disorder | 90 days | Obesity, recreational drug use; relationship to treatment not assessable due to insufficient data |
| Congenital ankyloglossia | 73 days | Temporal relationship not suggestive of treatment causality | |
| 31–35 | Persistent right umbilical vein, congenital ankyloglossia | 59 days | Temporal relationship not suggestive of treatment causality |
| Congenital hydronephrosis | 185 days | Temporal relationship not suggestive of treatment causality; lack of plausible mechanism | |
PCOS polycystic ovary syndrome
aNo information available on the timing of sotrovimab administration
Table 4.
Summary of serious adverse events
| Maternal age group, years | Serious adverse event |
Time since sotrovimab treatment |
Potential confounding factors |
|---|---|---|---|
| Maternal events | |||
| 31–35 | Gestational diabetes | 163 days | PCOS, obesity |
| 36–40 | Urinary retention | 27 days | – |
| Hypothyroidism | 187 days | – | |
| Gestational diabetes | 29 days | PCOS, obesity, tobacco use | |
| Infant/fetal events | |||
| 26–30 | Neonatal jaundice | 109 days | – |
| Seizure | 194 days | – | |
| Breech birth | – | – | |
| 31–35 | Bronchiolitis | 391 days | – |
| 36–40 | Neonatal dyspnea | 82 days | Hypertension, recreational drug use |
| Decreased blood glucose | 82 days | Hypertension, recreational drug use | |
| Infectious croup | 264 days | Hypertension, recreational drug use | |
| Decreased blood glucose | 105 days | Maternal gestational diabetes, hypertension | |
| Neonatal dyspnea | 106 days | Maternal gestational diabetes, hypertension | |
| Respiratory syncytial virus infection | 251 days | – | |
| Infectious croup | 333 days | – | |
| Neonatal dyspnea | 212 days | – | |
PCOS polycystic ovary syndrome
Confounding factors (i.e., factors that may explain, at least in part, the presence of the AE) were identified for five of the eight SAEs (Tables 3, 4) and were used to exclude a relationship to sotrovimab treatment for three of the assessable events (gestational diabetes, polyhydramnios, and premature labor). For the remaining assessable SAEs, causality of sotrovimab treatment was ruled out based on lack of a temporal relationship (hypothyroidism, gestational diabetes) or absence of a plausible mechanism (pre-eclampsia, prolonged labor).
Infant/Fetal AEs
A total of 44 AEs were reported in 19 delivered infants or in utero fetuses. The most common were COVID-19 (n = 6 events), ear infection (n = 5 events), neonatal dyspnea (n = 3 events), and respiratory syncytial virus infection (n = 3 events). Congenital ankyloglossia, decreased blood glucose, and infectious croup were also recurrent (n = 2 events for each). None of the assessable AEs reported in infants/fetuses were considered to be related to sotrovimab treatment; one SAE (fetal cardiac disorder) was not assessable as insufficient information was provided to assess the case.
Sixteen AEs (in 11 infants/fetuses) were classified as serious (Tables 3, 4); these included four AEs (fetal cardiac disorder, congenital ankyloglossia, persistent right umbilical vein, congenital hydronephrosis) that were infant/fetal pregnancy outcome events (Table 3). One of these (congenital hydronephrosis) was considered a major congenital malformation [24]. Confounding factors were identified for 6 of the 16 events. For the assessable SAEs, causality of sotrovimab treatment was ruled out based on lack of a temporal relationship alone (persistent right umbilical vein, congenital ankyloglossia, neonatal jaundice) or in combination with absence of a plausible mechanism (congenital hydronephrosis, breech birth, neonatal dyspnea, decreased blood glucose, infectious croup, bronchiolitis, respiratory syncytial virus infection, seizure).
Discussion
Pregnancy introduces a heightened vulnerability to severe COVID-19, yet the dearth of data on COVID-19 treatment effects in pregnant women stems from their exclusion from clinical trials [13, 14]. Such data are typically provided by postmarketing studies. The COVID-PR was initiated to accumulate real-world data concerning mAb and antiviral drug utilization in pregnancy for managing mild, moderate, or severe COVID-19. To 13 July 2023, 39 participants were treated with sotrovimab and were followed up for up to 1 year. Many had pre-existing medical conditions linked to the risk of severe COVID-19, such as obesity (29%) and asthma (6%). A total of 75 AEs were recorded, encompassing 31 in study participants and 44 in their offspring. Among these events, 8 in mothers and 16 in infants were classified as serious, but none of these SAEs seemed attributable to sotrovimab treatment. Ten of the maternal non-SAEs were considered attributable to sotrovimab treatment, although most of these were consistent with infusion-site reactions, as highlighted in the sotrovimab prescribing information [17].
A number of studies have provided details on the characteristics of patients who received sotrovimab in a real-world setting. A retrospective analysis of de-identified patients diagnosed with COVID-19 in the US FAIR Health National Private Insurance database included more than 15,000 patients treated with sotrovimab between September 2021 and April 2022 [25]. Among this group, 27.73% had obesity and 4.54% had asthma; other reported comorbidities included chronic kidney disease (10.05%), diabetes (26.11%), and immunocompromising conditions/immunosuppressive therapy (41.74%). A report from the DISCOVER-NOW study (currently published as a preprint), included 696 patients treated with sotrovimab in the UK between December 2021 and May 2022 [26]. In this group, 25.6% had long-term respiratory conditions and 2.3% had obesity.
Research on other mAbs, intravenous immunoglobulin (Ig) G, and plasma validates their risk–benefit profile when used in pregnancy [4, 27–31]. Although pregnant patients were excluded from clinical trials during the development of sotrovimab, other studies of sotrovimab have included pregnant patients. For example, the US Fair Health National Private Insurance database included 1203 pregnant participants, who were analyzed separately. The risk of 30-day all-cause hospitalization or mortality was significantly reduced in pregnant participants who received sotrovimab compared with those who received no mAb [25]. A case series in Japan reported on 60 patients, including three pregnant individuals, treated with sotrovimab during the Omicron era. Although data were not reported separately for the pregnant patients, the overall outcomes were positive; only two patients experienced severe or critical disease progression, no patient required mechanical ventilation or intensive care unit admission, and no deaths were reported [32]. AEs were reported in four patients (6.7%), including infusion-related reaction, liver dysfunction, and post-dose fever in two patients.
An observational study conducted in Qatar assessed the real-world effectiveness of sotrovimab for preventing severe COVID-19 outcomes primarily caused by the Omicron BA.2 variant [33]. The study included 94 pregnant women in the treated group of 340 patients, along with 213 pregnant women among the 1043 untreated controls. Although results for the pregnant participants were not presented separately, an analysis focused on a subgroup at higher risk of severe COVID-19 (which included pregnant participants) revealed a reduction in the risk of disease progression, although statistical significance was not reached. No AE data were included in the report.
A US cohort study in pregnant patients (identified between 30 April 2021 and 21 January 2022) with mild-to-moderate COVID-19 investigated the use of therapeutic COVID-19 mAbs, with sotrovimab being the most commonly administered treatment (n = 382, 69.2%). The study reported mild drug-related AEs in eight (1.4%) mAb-treated patients. There were no differences in obstetric-associated safety outcomes between mAb treatment and no treatment among those who delivered. Additionally, there were no significant differences in 28-day COVID-19-related outcomes or non-COVID-19-related hospital admissions between the mAb treatment and no mAb treatment groups in a propensity score-matched cohort [29].
Tuan et al. [4] reviewed electronic medical records of 22 pregnant patients treated with sotrovimab at the Yale New Haven Health Hospital System and reported that no abortions, fetal losses, or other birth/neurodevelopmental defects were noted.
Three single-case reports from Canada, the United Arab Emirates, and the US described successful outcomes of pregnant women treated with sotrovimab, with resolution of symptoms, no need for emergency care or hospitalization, and no reported fetal or pregnancy-related complications [27, 34, 35].
There are sparse data from this study on pregnancy outcomes. Given the sample size, rare congenital abnormalities are extremely unlikely to be detected, since thousands of exposures would likely be required. Although there was low sample size, there was no indication or pattern of birth defects associated with sotrovimab. Most of the exposures occurred outside of the window of development and confounding factors were present; therefore the outcomes observed were not likely related to sotrovimab administration.
This study has several limitations. First, it was based on a small sample size, and therefore low-frequency AEs are less likely to be detected. As the majority of participants received sotrovimab after the first trimester of pregnancy (the period during which congenital abnormalities are most likely to arise), this may also limit the study’s ability to detect these SAEs. Second, most of the participants came from the US and were treated during a time period when the Omicron variant was dominant [36]. It is therefore unclear how the results of this study relate to other regions, time periods, or COVID-19 variants.
The potential for selection bias and lack of representativeness is another limitation. The enrollment process may introduce biases as participants may self-select to participate based on their experiences or outcomes. This may impact the generalizability of the findings to the wider pregnant population. Additionally, the study faces challenges in recruiting a representative sample due to various factors such as limited access to healthcare and reluctance to participate. Reluctance to receive sotrovimab treatment, particularly due to a perception of the lower severity of current COVID-19 variants, may also limit the number of potential participants available.
Another limitation is the high rate of losses to follow-up, which is a well-recognized challenge for pregnancy registry studies [37, 38]; participants who are lost to follow-up may differ from those who remain in the study, potentially leading to biases in the observed outcomes, which may impact the validity of the results. There is also a high rate of missing information in this study, which may affect the accuracy of the data and limit the ability to draw robust conclusions. Missing demographic variables will restrict the capacity to carry out adjustment in future analyses. Furthermore, many of the AEs are self-reported and not confirmed by a healthcare professional. There is a potential for underreporting, although this is probably more likely for AEs that the participant considers to be less serious. Furthermore, some participants did not provide sufficient information to allow for a comprehensive assessment of the causality of reported AEs. Participants are encouraged to provide medical records, where applicable, and are sent reminders, therefore it is possible that more information may become available in the future.
Finally, there is currently no comparison group for this case series of pregnant patients exposed to sotrovimab. The COVID-PR protocol allows for the recruitment of two comparison groups: an active comparator group who received any medication other than sotrovimab specifically indicated for COVID-19 infection and an unexposed group of hospitalized women who did not receive any specifically indicated treatment. It is hoped they will be available for future analyses.
Conclusion
A sizable proportion of the sotrovimab-treated participants in the COVID-PR had underlying medical conditions associated with an increased risk of severe COVID-19. Although definitive conclusions regarding the relationship between sotrovimab exposure and adverse pregnancy outcomes cannot be drawn at this stage, none of the assessable SAEs were considered to be related to sotrovimab treatment. The COVID-PR study continues to monitor the safety outcomes of pregnant women treated with sotrovimab and other COVID-19 treatments during the 12 months following delivery for obstetric, neonatal, and infant outcomes. As data accrue and additional analyses are conducted, these findings will contribute to a more comprehensive understanding of sotrovimab's impact on pregnant patients, informing clinical decision making and healthcare strategies for managing COVID-19 during pregnancy.
Acknowledgements
Editorial assistance for an earlier version of this manuscript was provided by Alison Thornton, a freelance medical writer funded by Pregistry. Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables, grammatical editing, and referencing) was provided by Tony Reardon of OPEN Health Communications, and funded by GSK and Vir Biotechnology, Inc., in accordance with Good Publication Practice (GPP) guidelines (www.ismpp.org/gpp-2022).
Declarations
Funding
The COVID-PR receives funding from Gilead Sciences, GSK/Vir Biotechnology, Inc., MSD, and Roche. This study (216978) was funded by GSK and Vir Biotechnology, Inc. The open access fee for this paper was funded by GSK and Vir Biotechnology, Inc.
Conflicts of interest
Diego F. Wyszynski, Cheryl Renz, and Dragutin Rafailovic are employees of Pregistry, a company contracted by GSK and others (see the Funding statement above) to conduct the COVID-PR. Lydia Demetriou, Shirin Aliabadi, Myriam Drysdale, and Keele E. Wurst are employees of GSK and hold shares in the company. Lee P. Shulman has no conflicts of interest to declare.
Ethics approval
The study protocol has been approved in the United States by the WCG IRB (20214420), in Japan by the Medical Corporation TOUKEIKAI Kitamachi Clinic ERB (RVG0947720230315), and in Australia by the Bellberry Human Research Ethics Committee (2022-04-403). Clearance that approval is not required to conduct the study has been given by Ethics Committees in the following countries: Bulgaria, Canada, Croatia, Czech Republic, Denmark, Germany, Greece, Hong Kong, Italy, Malaysia, New Zealand, Poland, Romania, Singapore, South Africa, South Korea, and the United Kingdom. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for participation
Eligible subjects signed an electronic consent form prior to enrolling in this study.
Consent for publication
Participants provided informed written consent before taking part in the study.
Availability of data and material
This manuscript has no associated data or the data will not be deposited. The COVID-PR study protocol may be made available by the corresponding author on reasonable request, following applicable regulations.
Code availability
Not applicable.
Author contributions
The original study protocol was designed by Diego F. Wyszynski, who planned the study, designed the study methodology, supervised data collection and data verification, and drafted the first version of the article. Lydia Demetriou, Cheryl Renz, Shirin Aliabadi, Dragutin Rafailovic, Lee P. Shulman, Myriam Drysdale, and Keele E. Wurst provided advice on the study methodology and edited the first and final versions of the article. All authors have read and approved the final version of the manuscript.
References
- 1.Pham-Huy A, Top KA, Constantinescu C, Seow CH, El-Chaâr D. The use and impact of monoclonal antibody biologics during pregnancy. CMAJ. 2021;193:E1129–36. 10.1503/cmaj.202391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirshberg JS, Cooke E, Oakes MC, Odibo AO, Raghuraman N, Kelly JC. Monoclonal antibody treatment of symptomatic COVID-19 in pregnancy: initial report. Am J Obstet Gynecol. 2021;225:688–9. 10.1016/j.ajog.2021.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciobanu AM, Dumitru AE, Gica N, Botezatu R, Peltecu G, Panaitescu AM. Benefits and risks of IgG transplacental transfer. Diagnostics (Basel). 2020;10:583. 10.3390/diagnostics10080583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuan JJ, Sharma M, Kayani J, Davis MW, McManus D, Topal JE, et al. Outcomes of pregnant women exposed to sotrovimab for the treatment of COVID-19 in the BA.1 Omicron predominant era (PRESTO). BMC Infect Dis. 2023;23:258. 10.1186/s12879-023-08198-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cathcart AL, Havenar-Daughton C, Lempp FA, Ma D, Schmid MA, Agostini ML, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv. 2022. 10.1101/2021.03.09.434607. 10.1101/2021.03.09.434607 [DOI] [Google Scholar]
- 6.Gaudinski MR, Coates EE, Houser KV, Chen GL, Yamshchikov G, Saunders JG, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med. 2018;15: e1002493. 10.1371/journal.pmed.1002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–5. 10.1038/nature13612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto D, Park YJ, Beltramello M, Walls AC, Tortorici MA, Bianchi S, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–5. 10.1038/s41586-020-2349-y [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–50. 10.1056/NEJMoa2107934 [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Rodrigues Falci D, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327:1236–46. 10.1001/jama.2022.2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen SA, Jamieson DJ. COVID-19 and pregnancy. Infect Dis Clin North Am. 2022;36:423–33. 10.1016/j.idc.2022.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol. 2022;226:177–86. 10.1016/j.ajog.2021.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370: m3320. 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–7. 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heo YA. Sotrovimab: first approval. Drugs. 2022;82:477–84. 10.1007/s40265-022-01690-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyszynski DF, Papageorghiou AT, Renz C, Metz TD, Hernández-Díaz S. The COVID-19 International Drug Pregnancy Registry (COVID-PR): protocol considerations. Drug Saf. 2024;47(3):195–204. 10.1007/s40264-023-01377-2. 10.1007/s40264-023-01377-2 [DOI] [PubMed] [Google Scholar]
- 17.European Medicines Agency. Xevudy summary of product characteristics. 2023. https://www.ema.europa.eu/en/documents/product-information/xevudy-epar-product-information_en.pdf. Accessed 2 Nov 2023.
- 18.US FDA. Guidance for industry: establishing pregnancy exposure registries. 2002. https://www.fda.gov/media/75607/download. Accessed 10 Oct 2023.
- 19.European Medicines Agency. Guideline on the exposure to medicinal products during pregnancy: need for post-authorisation data. 2005. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-exposure-medicinal-products-during-pregnancy-need-post-authorisation-data_en.pdf. Accessed 2 Nov 2023.
- 20.Andrews EA, Avorn J, Bortnichak EA, Chen R, Dai WS, Dieck GS, et al. Guidelines for Good Epidemiology Practices for drug, device, and vaccine research in the United States. Pharmacoepidemiol Drug Saf. 1996;5:333–8. [DOI] [PubMed] [Google Scholar]
- 21.European Medicines Agency. Good pharmacovigilance practices. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/good-pharmacovigilance-practices. Accessed 20 Oct 2023.
- 22.US FDA. Postapproval pregnancy safety studies: guidance for industry. 2019. https://www.fda.gov/media/124746/download. Accessed 10 Oct 2023.
- 23.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Metropolitan Atlanta Congenital Defects Program. https://www.cdc.gov/ncbddd/birthdefects/macdp.html. Accessed 9 Apr 2024.
- 25.Cheng MM, Reyes C, Satram S, Birch H, Gibbons DC, Drysdale M, et al. Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the USA. Infect Dis Ther. 2023;12:607–21. 10.1007/s40121-022-00755-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel V, Yarwood MJ, Levick B, Gibbons DC, Drysdale M, Kerr W, et al. Characteristics and outcomes of patients with COVID-19 at high-risk of disease progression receiving sotrovimab, oral antivirals or no treatment in England. medRxiv. 2022. 10.1101/2022.11.28.22282808. 10.1101/2022.11.28.22282808 [DOI] [PubMed] [Google Scholar]
- 27.Aberumand B, Kamal R, McKinney B, Betschel S. Monoclonal antibody treatment of COVID-19 in a pregnant woman with common variable immunodeficiency. Allergy Asthma Clin Immunol. 2022;18:91. 10.1186/s13223-022-00730-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandhi RT, Malani PN, Del Rio C. COVID-19 therapeutics for nonhospitalized patients. JAMA. 2022;327:617–8. 10.1001/jama.2022.0335 [DOI] [PubMed] [Google Scholar]
- 29.McCreary EK, Lemon L, Megli C, Oakes A, Seymour CW, UPMC Magee Monoclonal Antibody Treatment Group. Monoclonal antibodies for treatment of SARS-CoV-2 infection during pregnancy: a cohort study. Ann Intern Med. 2022;175:1707–15. 10.7326/M22-1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richley M, Rao RR, Afshar Y, Mei J, Mok T, Vijayan T, et al. Neutralizing monoclonal antibodies for coronavirus disease 2019 (COVID-19) in pregnancy: a case series. Obstet Gynecol. 2022;139:368–72. 10.1097/AOG.0000000000004689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thilagar BP, Ghosh AK, Nguyen J, Theiler RN, Wick MJ, Hurt RT, et al. Anti-spike monoclonal antibody therapy in pregnant women with mild-to-moderate coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2022;139:616–8. 10.1097/AOG.0000000000004700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izumo T, Awano N, Kuse N, Sakamoto K, Takada K, Muto Y, et al. Efficacy and safety of sotrovimab for vaccinated or unvaccinated patients with mild-to-moderate COVID-19 in the omicron era. Drug Discov Ther. 2022;16:124–7. 10.5582/ddt.2022.01036 [DOI] [PubMed] [Google Scholar]
- 33.Zaqout A, Almaslamani MA, Chemaitelly H, Hashim SA, Ittaman A, Alimam A, et al. Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild-to-moderate SARS-CoV-2 in Qatar. Int J Infect Dis. 2022;124:96–103. 10.1016/j.ijid.2022.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AlKindi F, Chaaban A, Al Hakim M, Boobes Y. Sotrovimab use for COVID-19 infection in pregnant kidney transplant recipient. Transplantation. 2022;106:e277–8. 10.1097/TP.0000000000004083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta I, Arguello Perez ES. Use of sotrovimab in a pregnant patient with COVID-19 infection. Cureus. 2022;14: e22658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Genomic surveillance for SARS-CoV-2 variants: circulation of Omicron lineages—United States, January 2022–May 2023. 2023. https://www.cdc.gov/mmwr/volumes/72/wr/mm7224a2.htm#:~:text=Omicron%20remained%20predominant%20during%20January,%2C%202022%2C%20followed%20by%20BA. Accessed 10 Oct 2023.
- 37.Favre G, Maisonneuve E, Pomar L, Winterfeld U, Daire C, Martinez de Tejada B, et al. COVID-19 mRNA vaccine in pregnancy: results of the Swiss COVI-PREG registry, an observational prospective cohort study. Lancet Reg Health Eur. 2022;18:100410. 10.1016/j.lanepe.2022.100410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernández-Diáz S, Smith LH, Wyszynski DF, Rasmussen SA. First trimester COVID-19 and the risk of major congenital malformations-International Registry of Coronavirus Exposure in Pregnancy. Birth Defects Res. 2022;114:906–14. 10.1002/bdr2.2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonhoeffer J, Kochhar S, Hirschfeld S, Heath PT, Jones CE, Bauwens J, et al. Global alignment of immunization safety assessment in pregnancy—the GAIA project. Vaccine. 2016;34:5993–7. 10.1016/j.vaccine.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 40.McCoy DC, Sudfeld CR, Bellinger DC, Muhihi A, Ashery G, Weary TE, et al. Development and validation of an early childhood development scale for use in low-resourced settings. Popul Health Metr. 2017;15:3. 10.1186/s12963-017-0122-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


