Abstract
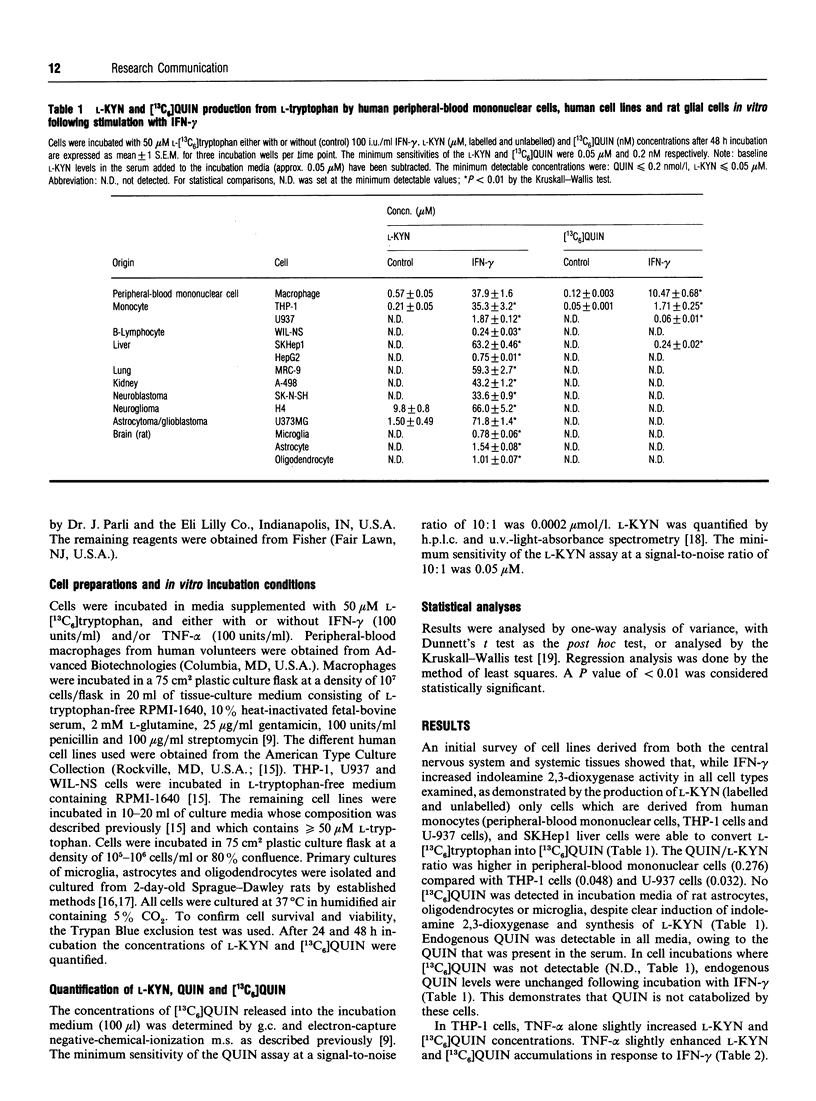
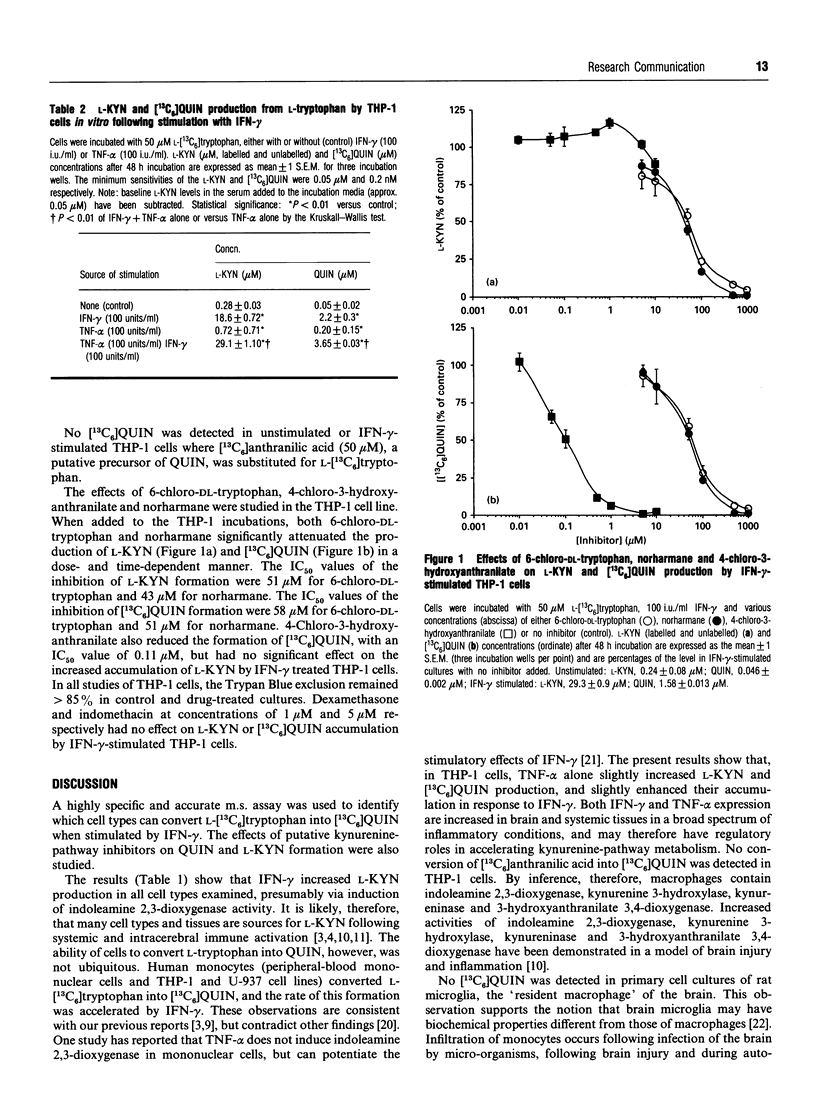
Accumulation of quinolinic acid and L-kynurenine occurs in the brain and/or blood following immune activation, and may derive from L-tryptophan following induction of indoleamine 2,3-dioxygenase and other kynurenine-pathway enzymes. In the present study a survey of various cell lines derived from either brain or systemic tissues showed that, while all cells examined responded to interferon-gamma by increased conversion of L-[13C6]tryptophan into L-kynurenine (human: B-lymphocytes, neuroblastoma, glioblastoma, lung, liver, kidney; rat brain: microglia, astrocytes and oligodendrocytes), only macrophage-derived cells (peripheral-blood mononuclear cells; THP-1, U-937) and certain liver cells (SKHep1) synthesized [13C6]quinolinic acid. Tumour necrosis factor-alpha enhanced the effects of interferon-gamma in THP-1 cells. Norharmane, 6-chloro-DL-tryptophan and 4-chloro-3-hydroxyanthranilate attenuated quinolinic acid formation by THP-1 cells with IC50 values of 51 microM, 58 microM and 0.11 microM respectively. Norharmane and 6-chloro-DL-tryptophan attenuated L-kynurenine formation with IC50 values of 43 microM and 51 microM respectively, whereas 4-chloro-3-hydroxyanthranilate had no effect on L-kynurenine accumulation. The reductions in L-kynurenine and quinolinic acid formation are consistent with the reports that norharmane is an inhibitor of indoleamine 2,3-dioxygenase, 6-chloro-DL-tryptophan is metabolized through the kynurenine pathway, and 4-chloro-3-hydroxyanthranilate is an inhibitor of 3-hydroxyanthranilate 3,4-dioxygenase. These results suggest that many tissues may contribute to the production of L-kynurenine following indoleamine 2,3-dioxygenase induction and immune activation. Quinolinic acid may be directly synthesized from L-tryptophan in both macrophages and certain types of liver cells, although uptake of quinolinic acid precursors from blood may contribute to quinolinic acid synthesis in cells that cannot convert L-kynurenine into quinolinic acid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baran H., Schwarcz R. Presence of 3-hydroxyanthranilic acid in rat tissues and evidence for its production from anthranilic acid in the brain. J Neurochem. 1990 Sep;55(3):738–744. doi: 10.1111/j.1471-4159.1990.tb04553.x. [DOI] [PubMed] [Google Scholar]
- Bender D. A. Biochemistry of tryptophan in health and disease. Mol Aspects Med. 1983;6(2):101–197. doi: 10.1016/0098-2997(83)90005-5. [DOI] [PubMed] [Google Scholar]
- Eguchi N., Watanabe Y., Kawanishi K., Hashimoto Y., Hayaishi O. Inhibition of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase by beta-carboline and indole derivatives. Arch Biochem Biophys. 1984 Aug 1;232(2):602–609. doi: 10.1016/0003-9861(84)90579-4. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Brew B. J., Martin A., Price R. W., Salazar A. M., Sidtis J. J., Yergey J. A., Mouradian M. M., Sadler A. E., Keilp J. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991 Feb;29(2):202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Brew B. J., Saito K., Quearry B. J., Price R. W., Lee K., Bhalla R. B., Der M., Markey S. P. Inter-relationships between quinolinic acid, neuroactive kynurenines, neopterin and beta 2-microglobulin in cerebrospinal fluid and serum of HIV-1-infected patients. J Neuroimmunol. 1992 Sep;40(1):71–80. doi: 10.1016/0165-5728(92)90214-6. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Saito K., Crowley J. S., Davis L. E., Demitrack M. A., Der M., Dilling L. A., Elia J., Kruesi M. J., Lackner A. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992 Oct;115(Pt 5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Saito K., Jacobowitz D., Markey S. P., Takikawa O., Vickers J. H. Poliovirus induces indoleamine-2,3-dioxygenase and quinolinic acid synthesis in macaque brain. FASEB J. 1992 Aug;6(11):2977–2989. doi: 10.1096/fasebj.6.11.1322853. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Saito K., Markey S. P. Human macrophages convert L-tryptophan into the neurotoxin quinolinic acid. Biochem J. 1992 May 1;283(Pt 3):633–635. doi: 10.1042/bj2830633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes M. P., Swartz K. J., Markey S. P., Beal M. F. Regional brain and cerebrospinal fluid quinolinic acid concentrations in Huntington's disease. Neurosci Lett. 1991 Jan 28;122(2):265–269. doi: 10.1016/0304-3940(91)90874-s. [DOI] [PubMed] [Google Scholar]
- Holmes E. W. Determination of serum kynurenine and hepatic tryptophan dioxygenase activity by high-performance liquid chromatography. Anal Biochem. 1988 Aug 1;172(2):518–525. doi: 10.1016/0003-2697(88)90478-2. [DOI] [PubMed] [Google Scholar]
- Martin A., Heyes M. P., Salazar A. M., Kampen D. L., Williams J., Law W. A., Coats M. E., Markey S. P. Progressive slowing of reaction time and increasing cerebrospinal fluid concentrations of quinolinic acid in HIV-infected individuals. J Neuropsychiatry Clin Neurosci. 1992 Summer;4(3):270–279. doi: 10.1176/jnp.4.3.270. [DOI] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan C. E., Cunningham T. J., Levitt P. Differential immunochemical markers reveal the normal distribution of brain macrophages and microglia in the developing rat brain. J Comp Neurol. 1991 Dec 1;314(1):125–135. doi: 10.1002/cne.903140112. [DOI] [PubMed] [Google Scholar]
- Parli C. J., Krieter P., Schmidt B. Metabolism of 6-chlorotryptophan to 4-chloro-3-hydroxyanthranilic acid: a potent inhibitor of 3-hydroxyanthranilic acid oxidase. Arch Biochem Biophys. 1980 Aug;203(1):161–166. doi: 10.1016/0003-9861(80)90164-2. [DOI] [PubMed] [Google Scholar]
- Saito K., Lackner A., Markey S. P., Heyes M. P. Cerebral cortex and lung indoleamine-2,3-dioxygenase activity is increased in type-D retrovirus infected macaques. Brain Res. 1991 Feb 1;540(1-2):353–356. doi: 10.1016/0006-8993(91)90536-5. [DOI] [PubMed] [Google Scholar]
- Saito K., Markey S. P., Heyes M. P. Chronic effects of gamma-interferon on quinolinic acid and indoleamine-2,3-dioxygenase in brain of C57BL6 mice. Brain Res. 1991 Apr 12;546(1):151–154. doi: 10.1016/0006-8993(91)91171-v. [DOI] [PubMed] [Google Scholar]
- Saito K., Markey S. P., Heyes M. P. Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience. 1992 Nov;51(1):25–39. doi: 10.1016/0306-4522(92)90467-g. [DOI] [PubMed] [Google Scholar]
- Saito K., Nowak T. S., Jr, Markey S. P., Heyes M. P. Mechanism of delayed increases in kynurenine pathway metabolism in damaged brain regions following transient cerebral ischemia. J Neurochem. 1993 Jan;60(1):180–192. doi: 10.1111/j.1471-4159.1993.tb05836.x. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Okuno E., White R. J., Bird E. D., Whetsell W. O., Jr 3-Hydroxyanthranilate oxygenase activity is increased in the brains of Huntington disease victims. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4079–4081. doi: 10.1073/pnas.85.11.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W., Connick J. H. Quinolinic acid and other kynurenines in the central nervous system. Neuroscience. 1985 Jul;15(3):597–617. doi: 10.1016/0306-4522(85)90063-6. [DOI] [PubMed] [Google Scholar]
- Suzumura A., Mezitis S. G., Gonatas N. K., Silberberg D. H. MHC antigen expression on bulk isolated macrophage-microglia from newborn mouse brain: induction of Ia antigen expression by gamma-interferon. J Neuroimmunol. 1987 Jul-Aug;15(3):263–278. doi: 10.1016/0165-5728(87)90121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Felmayer G., Werner E. R., Fuchs D., Hausen A., Reibnegger G., Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim Biophys Acta. 1989 Jul 11;1012(2):140–147. doi: 10.1016/0167-4889(89)90087-6. [DOI] [PubMed] [Google Scholar]
- Werner-Felmayer G., Werner E. R., Fuchs D., Hausen A., Reibnegger G., Wachter H. Tumour necrosis factor-alpha and lipopolysaccharide enhance interferon-induced tryptophan degradation and pteridine synthesis in human cells. Biol Chem Hoppe Seyler. 1989 Sep;370(9):1063–1069. doi: 10.1515/bchm3.1989.370.2.1063. [DOI] [PubMed] [Google Scholar]


