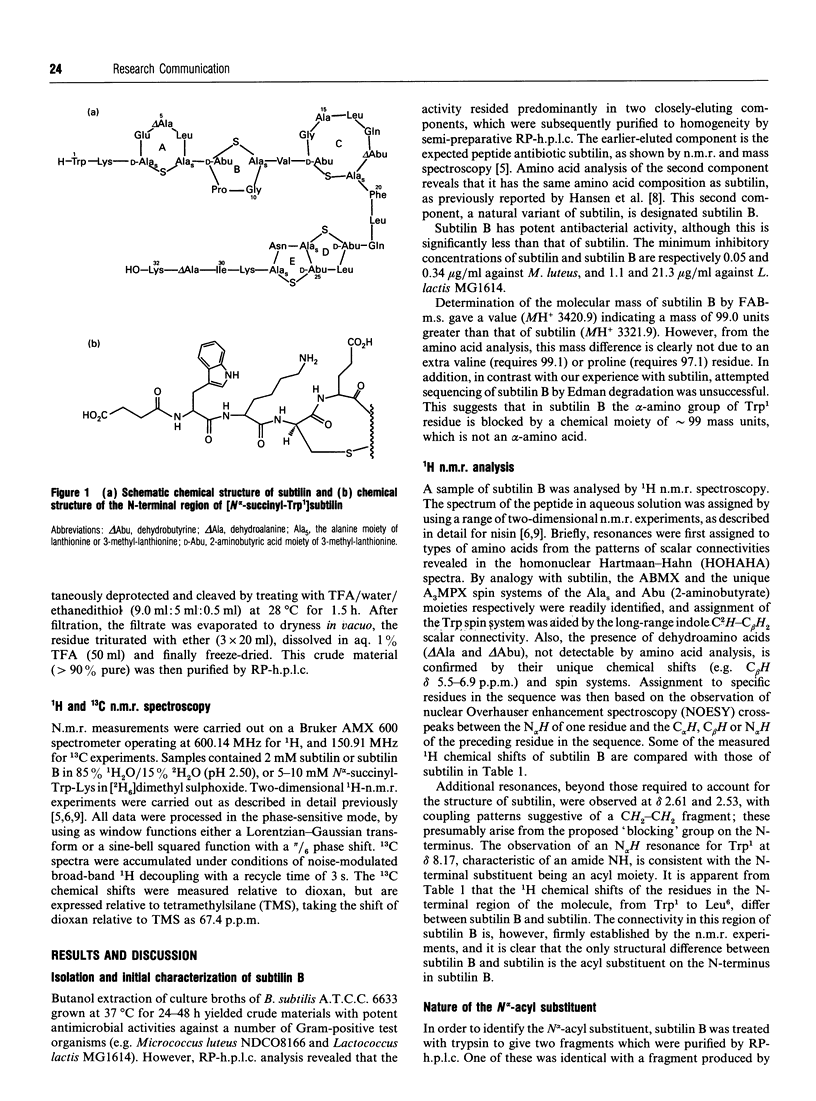
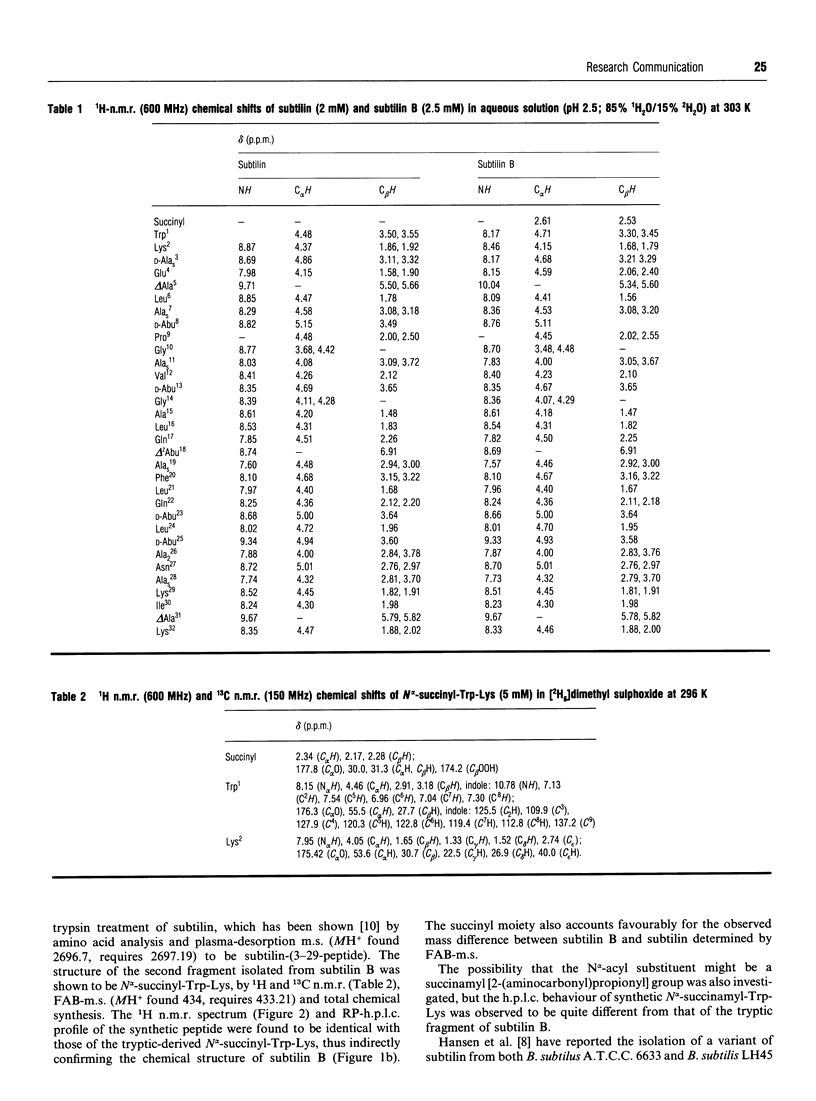
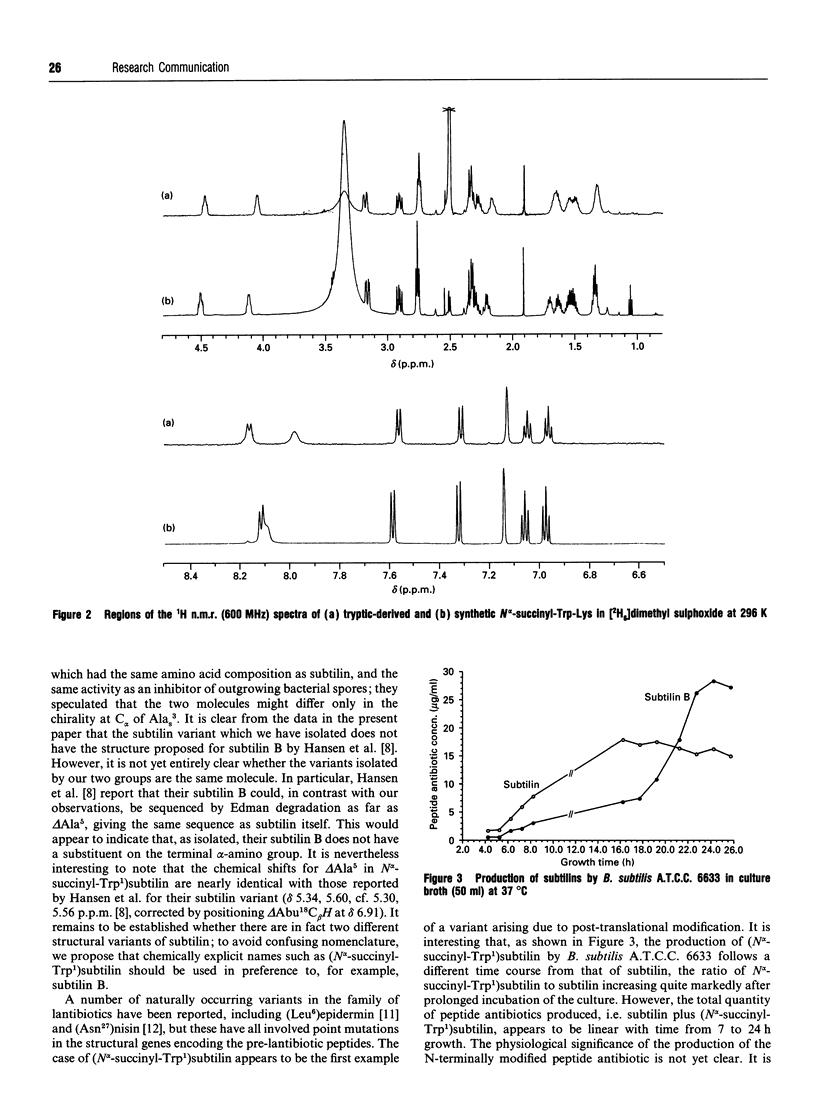
Abstract
A variant of the peptide antibiotic subtilin has been isolated from Bacillus subtilis A.T.C.C. 6633, and its structure has been shown to be [N alpha-succinyl-Trp1]subtilin. The chemical structure of a fragment derived by tryptic hydrolysis of the variant is shown to be N alpha-succinyl-Trp-Lys by 1H and 13C n.m.r., fast-atom-bombardment m.s. and total chemical synthesis [N alpha-Succinyl-Trp1]-subtilin is produced later in the growth of the bacterium than is subtilin; reverse-phase h.p.l.c. analysis shows that after 24 h growth the ratio subtilin/[N alpha-succinyl-Trp1]subtilin is approx. 1:2. Although [N alpha-succinyl-Trp1]subtilin retains significant antibacterial activity, it is 10-20 times less active than subtilin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S., Hansen J. N. Structure and expression of a gene encoding the precursor of subtilin, a small protein antibiotic. J Biol Chem. 1988 Jul 5;263(19):9508–9514. [PubMed] [Google Scholar]
- Chan W. C., Bycroft B. W., Leyland M. L., Lian L. Y., Yang J. C., Roberts G. C. Sequence-specific resonance assignment and conformational analysis of subtilin by 2D NMR. FEBS Lett. 1992 Mar 23;300(1):56–62. doi: 10.1016/0014-5793(92)80163-b. [DOI] [PubMed] [Google Scholar]
- Gross E., Kiltz H. H., Nebelin E. Subtilin, VI. Die Struktur des Subtilins. Hoppe Seylers Z Physiol Chem. 1973 Jul;354(7):810–812. [PubMed] [Google Scholar]
- Kellner R., Jung G., Hörner T., Zähner H., Schnell N., Entian K. D., Götz F. Gallidermin: a new lanthionine-containing polypeptide antibiotic. Eur J Biochem. 1988 Oct 15;177(1):53–59. doi: 10.1111/j.1432-1033.1988.tb14344.x. [DOI] [PubMed] [Google Scholar]
- Kordel M., Benz R., Sahl H. G. Mode of action of the staphylococcinlike peptide Pep 5: voltage-dependent depolarization of bacterial and artificial membranes. J Bacteriol. 1988 Jan;170(1):84–88. doi: 10.1128/jb.170.1.84-88.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian L. Y., Chan W. C., Morley S. D., Roberts G. C., Bycroft B. W., Jackson D. Solution structures of nisin A and its two major degradation products determined by n.m.r. Biochem J. 1992 Apr 15;283(Pt 2):413–420. doi: 10.1042/bj2830413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders J. W., Boerrigter I. J., Rollema H. S., Siezen R. J., de Vos W. M. Identification and characterization of the lantibiotic nisin Z, a natural nisin variant. Eur J Biochem. 1991 Nov 1;201(3):581–584. doi: 10.1111/j.1432-1033.1991.tb16317.x. [DOI] [PubMed] [Google Scholar]
- Smyth D. G., Massey D. E., Zakarian S., Finnie M. D. Endorphins are stored in biologically active and inactive forms: isolation of alpha-N-acetyl peptides. Nature. 1979 May 17;279(5710):252–254. doi: 10.1038/279252a0. [DOI] [PubMed] [Google Scholar]


