Abstract
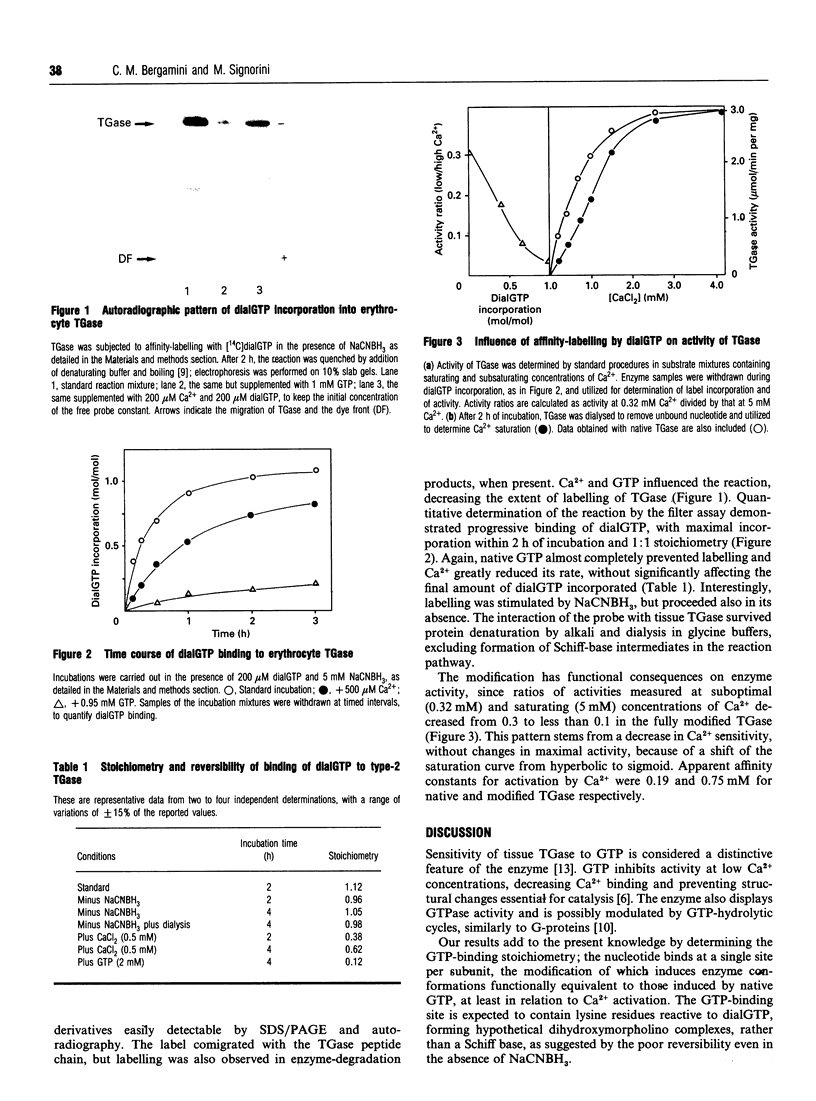
Ca2+ and GTP are the main modulators of type-2 transglutaminases. To study the interaction of the enzyme with GTP, we have employed periodate-oxidized GTP as an affinity-label probe. Dialdehyde GTP bound irreversibly to type-2 transglutaminase in a time-dependent way with 1:1 stoichiometry at complete modification. The reaction took place in the absence, but was more rapid in the presence, of cyanoborohydride. Native GTP prevented incorporation of dialdehyde GTP, and Ca2+ significantly slowed down the reaction rate. The modified enzyme displayed decreased sensitivity to Ca2+, with a sigmoid saturation curve. We conclude that type-2 transglutaminase has a single GTP-binding site, the modification of which by dialdehyde GTP mimics nucleotide binding to the enzyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achyuthan K. E., Greenberg C. S. Identification of a guanosine triphosphate-binding site on guinea pig liver transglutaminase. Role of GTP and calcium ions in modulating activity. J Biol Chem. 1987 Feb 5;262(4):1901–1906. [PubMed] [Google Scholar]
- Bergamini C. M. GTP modulates calcium binding and cation-induced conformational changes in erythrocyte transglutaminase. FEBS Lett. 1988 Nov 7;239(2):255–258. doi: 10.1016/0014-5793(88)80928-1. [DOI] [PubMed] [Google Scholar]
- Bergamini C. M., Signorini M., Poltronieri L. Inhibition of erythrocyte transglutaminase by GTP. Biochim Biophys Acta. 1987 Nov 5;916(1):149–151. doi: 10.1016/0167-4838(87)90222-6. [DOI] [PubMed] [Google Scholar]
- Colman R. F. Affinity labeling of purine nucleotide sites in proteins. Annu Rev Biochem. 1983;52:67–91. doi: 10.1146/annurev.bi.52.070183.000435. [DOI] [PubMed] [Google Scholar]
- Fesus L., Tarcsa E., Kedei N., Autuori F., Piacentini M. Degradation of cells dying by apoptosis leads to accumulation of epsilon(gamma-glutamyl)lysine isodipeptide in culture fluid and blood. FEBS Lett. 1991 Jun 17;284(1):109–112. doi: 10.1016/0014-5793(91)80773-v. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Chung S. I. Molecular and catalytic properties of transglutaminases. Adv Enzymol Relat Areas Mol Biol. 1973;38:109–191. doi: 10.1002/9780470122839.ch3. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Finlayson J. S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- Gentile V., Saydak M., Chiocca E. A., Akande O., Birckbichler P. J., Lee K. N., Stein J. P., Davies P. J. Isolation and characterization of cDNA clones to mouse macrophage and human endothelial cell tissue transglutaminases. J Biol Chem. 1991 Jan 5;266(1):478–483. [PubMed] [Google Scholar]
- Greenberg C. S., Birckbichler P. J., Rice R. H. Transglutaminases: multifunctional cross-linking enzymes that stabilize tissues. FASEB J. 1991 Dec;5(15):3071–3077. doi: 10.1096/fasebj.5.15.1683845. [DOI] [PubMed] [Google Scholar]
- Ikura K., Nasu T., Yokota H., Tsuchiya Y., Sasaki R., Chiba H. Amino acid sequence of guinea pig liver transglutaminase from its cDNA sequence. Biochemistry. 1988 Apr 19;27(8):2898–2905. doi: 10.1021/bi00408a035. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. N., Birckbichler P. J., Patterson M. K., Jr GTP hydrolysis by guinea pig liver transglutaminase. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1370–1375. doi: 10.1016/0006-291x(89)90825-5. [DOI] [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Nakanishi K., Nara K., Hagiwara H., Aoyama Y., Ueno H., Hirose S. Cloning and sequence analysis of cDNA clones for bovine aortic-endothelial-cell transglutaminase. Eur J Biochem. 1991 Nov 15;202(1):15–21. doi: 10.1111/j.1432-1033.1991.tb16338.x. [DOI] [PubMed] [Google Scholar]
- Signorini M., Bortolotti F., Poltronieri L., Bergamini C. M. Human erythrocyte transglutaminase: purification and preliminary characterisation. Biol Chem Hoppe Seyler. 1988 Apr;369(4):275–281. doi: 10.1515/bchm3.1988.369.1.275. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Birckbichler P. J., Patterson M. K., Jr, Lee K. N. Putative nucleotide binding sites of guinea pig liver transglutaminase. FEBS Lett. 1992 Jul 28;307(2):177–180. doi: 10.1016/0014-5793(92)80762-6. [DOI] [PubMed] [Google Scholar]
- Tsai P. K., Hogenkamp H. P. Affinity labeling of ribonucleotide reductase by the 2',3'-dialdehyde derivatives of ribonucleotides. Arch Biochem Biophys. 1983 Oct 1;226(1):276–284. doi: 10.1016/0003-9861(83)90294-1. [DOI] [PubMed] [Google Scholar]