Abstract
The procyclic acidic repetitive protein is the major cell-surface glycoprotein of the insect-dwelling procyclic forms of the Trypanosoma brucei species of African trypanosomes. The glycoprotein contains an acidic Glu-Pro repeat domain, a glycosyl-phosphatidylinositol membrane anchor and a putative asparagine glycosylation site. In this paper we describe a rapid purification scheme for this glycoprotein, using solvent extraction and hydrophobic interaction chromatography, and a partial characterization of the glycosylphosphatidylinositol membrane anchor. The carbohydrate composition of the anchor is extremely unusual; it contains on average nine GlcNAc, nine Gal, and five sialic acid residues. This is the first description of such a heavily substituted and negatively charged anchor. A comparison between the trypanosome procyclic surface and the Leishmania promastigote surface is also presented.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clayton C. E., Mowatt M. R. The procyclic acidic repetitive proteins of Trypanosoma brucei. Purification and post-translational modification. J Biol Chem. 1989 Sep 5;264(25):15088–15093. [PubMed] [Google Scholar]
- Cross G. A. Cellular and genetic aspects of antigenic variation in trypanosomes. Annu Rev Immunol. 1990;8:83–110. doi: 10.1146/annurev.iy.08.040190.000503. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Davies C. R., Cooper A. M., Peacock C., Lane R. P., Blackwell J. M. Expression of LPG and GP63 by different developmental stages of Leishmania major in the sandfly Phlebotomus papatasi. Parasitology. 1990 Dec;101(Pt 3):337–343. doi: 10.1017/s0031182000060522. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A. Colworth Medal Lecture. Glycosyl-phosphatidylinositol membrane anchors: the tale of a tail. Biochem Soc Trans. 1992 May;20(2):243–256. doi: 10.1042/bst0200243. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Homans S. W., Dwek R. A., Rademacher T. W. Glycosyl-phosphatidylinositol moiety that anchors Trypanosoma brucei variant surface glycoprotein to the membrane. Science. 1988 Feb 12;239(4841 Pt 1):753–759. doi: 10.1126/science.3340856. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Low M. G., Cross G. A. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1985 Nov 25;260(27):14547–14555. [PubMed] [Google Scholar]
- Ferguson M. A., Masterson W. J., Homans S. W., McConville M. J. Evolutionary aspects of GPI metabolism in kinetoplastid parasites. Cell Biol Int Rep. 1991 Nov;15(11):991–1005. doi: 10.1016/0309-1651(91)90052-k. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A. Site of palmitoylation of a phospholipase C-resistant glycosylphosphatidylinositol membrane anchor. Biochem J. 1992 Jun 1;284(Pt 2):297–300. doi: 10.1042/bj2840297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Field M. C., Menon A. K., Cross G. A. A glycosylphosphatidylinositol protein anchor from procyclic stage Trypanosoma brucei: lipid structure and biosynthesis. EMBO J. 1991 Oct;10(10):2731–2739. doi: 10.1002/j.1460-2075.1991.tb07821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. C., Menon A. K., Cross G. A. Developmental variation of glycosylphosphatidylinositol membrane anchors in Trypanosoma brucei. In vitro biosynthesis of intermediates in the construction of the GPI anchor of the major procyclic surface glycoprotein. J Biol Chem. 1992 Mar 15;267(8):5324–5329. [PubMed] [Google Scholar]
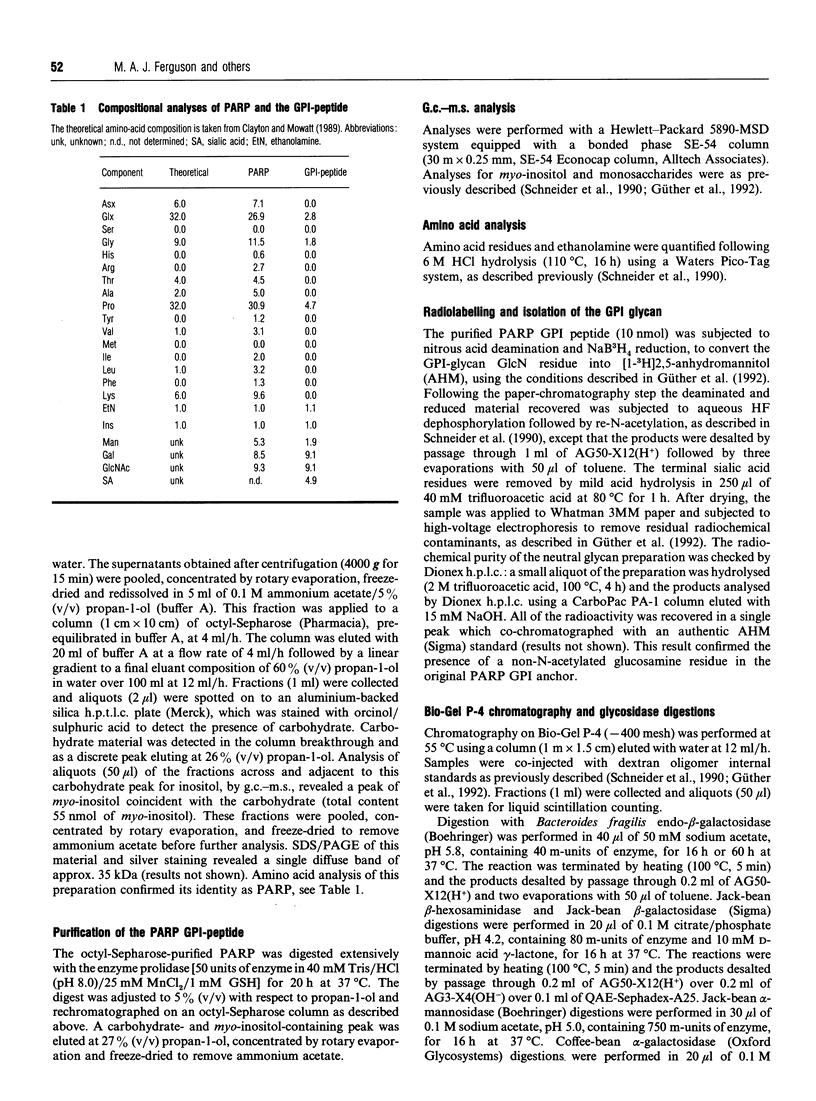
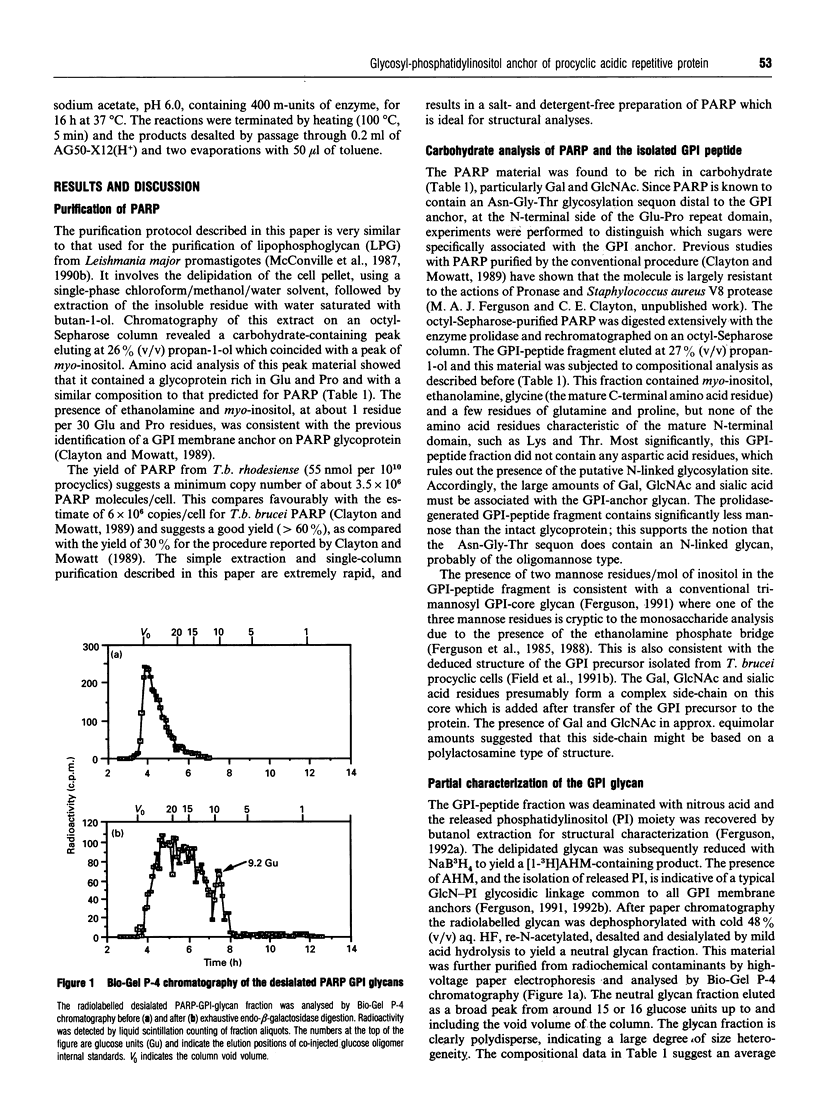
- Güther M. L., de Almeida M. L., Yoshida N., Ferguson M. A. Structural studies on the glycosylphosphatidylinositol membrane anchor of Trypanosoma cruzi 1G7-antigen. The structure of the glycan core. J Biol Chem. 1992 Apr 5;267(10):6820–6828. [PubMed] [Google Scholar]
- Homans S. W., Mehlert A., Turco S. J. Solution structure of the lipophosphoglycan of Leishmania donovani. Biochemistry. 1992 Jan 28;31(3):654–661. doi: 10.1021/bi00118a004. [DOI] [PubMed] [Google Scholar]
- Ilg T., Etges R., Overath P., McConville M. J., Thomas-Oates J., Thomas J., Homans S. W., Ferguson M. A. Structure of Leishmania mexicana lipophosphoglycan. J Biol Chem. 1992 Apr 5;267(10):6834–6840. [PubMed] [Google Scholar]
- Low M. G. The glycosyl-phosphatidylinositol anchor of membrane proteins. Biochim Biophys Acta. 1989 Dec 6;988(3):427–454. doi: 10.1016/0304-4157(89)90014-2. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Bacic A. A family of glycoinositol phospholipids from Leishmania major. Isolation, characterization, and antigenicity. J Biol Chem. 1989 Jan 15;264(2):757–766. [PubMed] [Google Scholar]
- McConville M. J., Bacic A., Mitchell G. F., Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8941–8945. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M. J., Blackwell J. M. Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J Biol Chem. 1991 Aug 15;266(23):15170–15179. [PubMed] [Google Scholar]
- McConville M. J., Homans S. W., Thomas-Oates J. E., Dell A., Bacic A. Structures of the glycoinositolphospholipids from Leishmania major. A family of novel galactofuranose-containing glycolipids. J Biol Chem. 1990 May 5;265(13):7385–7394. [PubMed] [Google Scholar]
- McConville M. J., Thomas-Oates J. E., Ferguson M. A., Homans S. W. Structure of the lipophosphoglycan from Leishmania major. J Biol Chem. 1990 Nov 15;265(32):19611–19623. [PubMed] [Google Scholar]
- McConville M. J., Turco S. J., Ferguson M. A., Sacks D. L. Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage. EMBO J. 1992 Oct;11(10):3593–3600. doi: 10.1002/j.1460-2075.1992.tb05443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowatt M. R., Clayton C. E. Developmental regulation of a novel repetitive protein of Trypanosoma brucei. Mol Cell Biol. 1987 Aug;7(8):2838–2844. doi: 10.1128/mcb.7.8.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi P. A., Jr, Turco S. J. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987 Jul 25;262(21):10384–10391. [PubMed] [Google Scholar]
- Pimenta P. F., Turco S. J., McConville M. J., Lawyer P. G., Perkins P. V., Sacks D. L. Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science. 1992 Jun 26;256(5065):1812–1815. doi: 10.1126/science.1615326. [DOI] [PubMed] [Google Scholar]
- Previato J. O., Andrade A. F., Pessolani M. C., Mendonça-Previato L. Incorporation of sialic acid into Trypanosoma cruzi macromolecules. A proposal for a new metabolic route. Mol Biochem Parasitol. 1985 Jun;16(1):85–96. doi: 10.1016/0166-6851(85)90051-9. [DOI] [PubMed] [Google Scholar]
- Richardson J. P., Beecroft R. P., Tolson D. L., Liu M. K., Pearson T. W. Procyclin: an unusual immunodominant glycoprotein surface antigen from the procyclic stage of African trypanosomes. Mol Biochem Parasitol. 1988 Dec;31(3):203–216. doi: 10.1016/0166-6851(88)90150-8. [DOI] [PubMed] [Google Scholar]
- Roditi I., Carrington M., Turner M. Expression of a polypeptide containing a dipeptide repeat is confined to the insect stage of Trypanosoma brucei. Nature. 1987 Jan 15;325(6101):272–274. doi: 10.1038/325272a0. [DOI] [PubMed] [Google Scholar]
- Roditi I., Pearson T. W. The procyclin coat of African trypanosomes (or the not-so-naked trypanosome). Parasitol Today. 1990 Mar;6(3):79–82. doi: 10.1016/0169-4758(90)90216-q. [DOI] [PubMed] [Google Scholar]
- Roditi I., Schwarz H., Pearson T. W., Beecroft R. P., Liu M. K., Richardson J. P., Bühring H. J., Pleiss J., Bülow R., Williams R. O. Procyclin gene expression and loss of the variant surface glycoprotein during differentiation of Trypanosoma brucei. J Cell Biol. 1989 Feb;108(2):737–746. doi: 10.1083/jcb.108.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman S., Jiang M. S., Hart G. W., Nussenzweig V. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell. 1991 Jun 28;65(7):1117–1125. doi: 10.1016/0092-8674(91)90008-m. [DOI] [PubMed] [Google Scholar]
- Schneider P., Ferguson M. A., McConville M. J., Mehlert A., Homans S. W., Bordier C. Structure of the glycosyl-phosphatidylinositol membrane anchor of the Leishmania major promastigote surface protease. J Biol Chem. 1990 Oct 5;265(28):16955–16964. [PubMed] [Google Scholar]
- Scudder P., Lawson A. M., Hounsell E. F., Carruthers R. A., Childs R. A., Feiz T. Characterisation of oligosaccharides released from human-blood-group O erythrocyte glycopeptides by the endo-beta-galactosidase of Bacteroides fragilis. A study of the enzyme susceptibility of branched poly(N-acetyllactosamine) structures. Eur J Biochem. 1987 Nov 2;168(3):585–593. doi: 10.1111/j.1432-1033.1987.tb13457.x. [DOI] [PubMed] [Google Scholar]
- Stahl N., Baldwin M. A., Hecker R., Pan K. M., Burlingame A. L., Prusiner S. B. Glycosylinositol phospholipid anchors of the scrapie and cellular prion proteins contain sialic acid. Biochemistry. 1992 Jun 2;31(21):5043–5053. doi: 10.1021/bi00136a600. [DOI] [PubMed] [Google Scholar]
- Thomas J. R., Dwek R. A., Rademacher T. W. Structure, biosynthesis, and function of glycosylphosphatidylinositols. Biochemistry. 1990 Jun 12;29(23):5413–5422. doi: 10.1021/bi00475a001. [DOI] [PubMed] [Google Scholar]
- Thomas J. R., McConville M. J., Thomas-Oates J. E., Homans S. W., Ferguson M. A., Gorin P. A., Greis K. D., Turco S. J. Refined structure of the lipophosphoglycan of Leishmania donovani. J Biol Chem. 1992 Apr 5;267(10):6829–6833. [PubMed] [Google Scholar]
- Yamashita K., Mizuochi T., Kobata A. Analysis of oligosaccharides by gel filtration. Methods Enzymol. 1982;83:105–126. doi: 10.1016/0076-6879(82)83008-5. [DOI] [PubMed] [Google Scholar]
- Zamze S. E., Ashford D. A., Wooten E. W., Rademacher T. W., Dwek R. A. Structural characterization of the asparagine-linked oligosaccharides from Trypanosoma brucei type II and type III variant surface glycoproteins. J Biol Chem. 1991 Oct 25;266(30):20244–20261. [PubMed] [Google Scholar]
- Zamze S. E., Wooten E. W., Ashford D. A., Ferguson M. A., Dwek R. A., Rademacher T. W. Characterisation of the asparagine-linked oligosaccharides from Trypanosoma brucei type-I variant surface glycoproteins. Eur J Biochem. 1990 Feb 14;187(3):657–663. doi: 10.1111/j.1432-1033.1990.tb15350.x. [DOI] [PubMed] [Google Scholar]
- Ziegelbauer K., Quinten M., Schwarz H., Pearson T. W., Overath P. Synchronous differentiation of Trypanosoma brucei from bloodstream to procyclic forms in vitro. Eur J Biochem. 1990 Sep 11;192(2):373–378. doi: 10.1111/j.1432-1033.1990.tb19237.x. [DOI] [PubMed] [Google Scholar]
- Zingales B., Carniol C., de Lederkremer R. M., Colli W. Direct sialic acid transfer from a protein donor to glycolipids of trypomastigote forms of Trypanosoma cruzi. Mol Biochem Parasitol. 1987 Nov;26(1-2):135–144. doi: 10.1016/0166-6851(87)90137-x. [DOI] [PubMed] [Google Scholar]


