Abstract
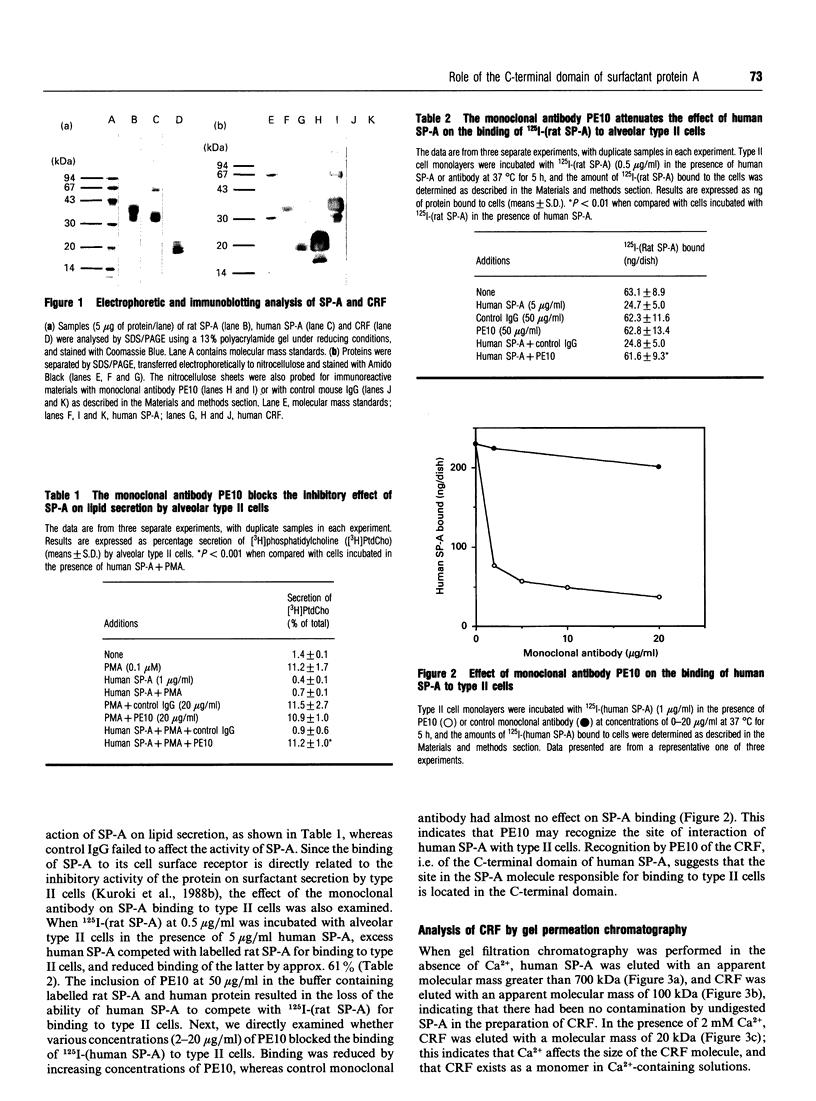
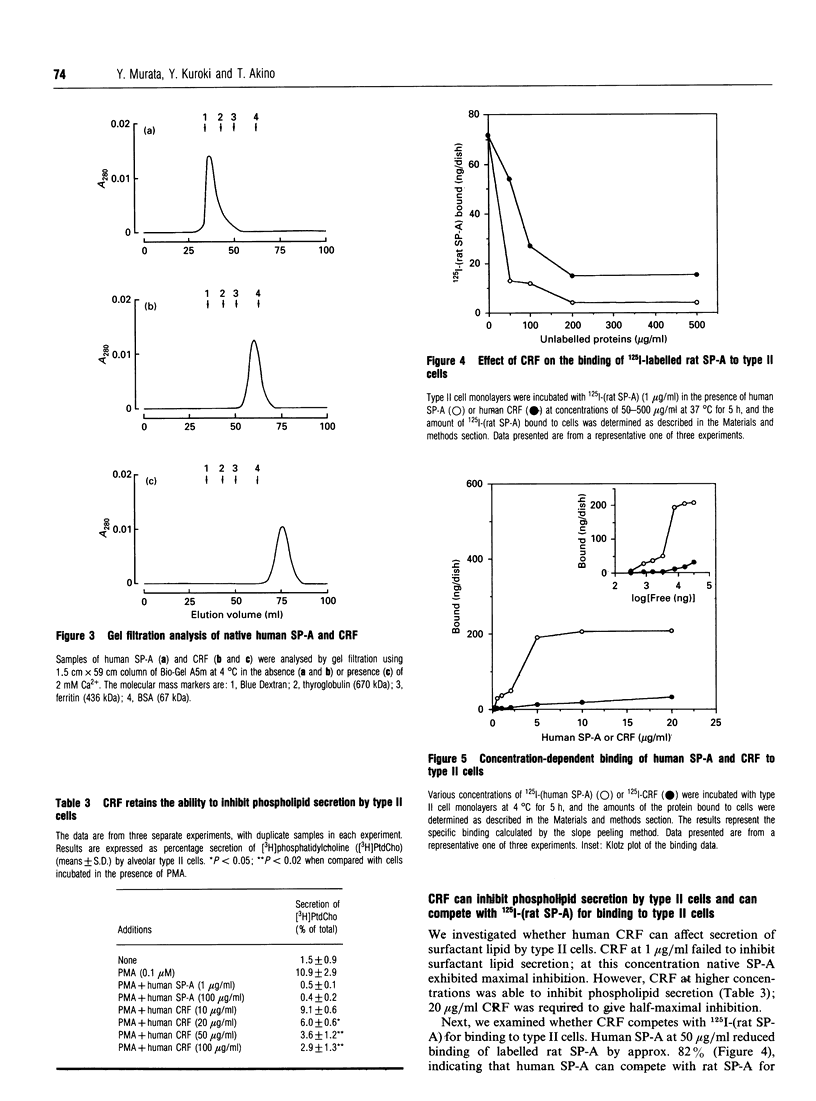
Surfactant protein A (SP-A), with a reduced denatured molecular mass of 26-38 kDa, is characterized by a collagen-like sequence in the N-terminal half of the protein. This protein forms an oligomeric structure which is dependent upon this collagenous domain. SP-A has been demonstrated to function as an inhibitor of phospholipid secretion by primary cultures of alveolar type II cells via a cell surface receptor for the protein. However, the receptor-binding domain of SP-A has not been identified. The purpose of the present study was to investigate the role of the C-terminal domain of SP-A in binding to type II cells and regulation of phospholipid secretion. A monoclonal antibody to human SP-A, whose epitope was localized at the C-terminal domain of the protein, abolished the inhibitory activity of human SP-A on lipid secretion by type II cells, and attenuated the ability of human SP-A to compete with 125I-(rat SP-A) for receptor binding. SP-A was then digested with collagenase and the collagenase-resistant fragment (CRF), which is the C-terminal domain of SP-A (thus lacking the N-terminal domain), was isolated. Gel filtration chromatography revealed that CRF exists as a monomer in solution containing Ca2+. CRF had the ability to inhibit phospholipid secretion, although at a higher concentration than for SP-A, and was also able to compete with 125I-(rat SP-A) for binding to type II cells. A direct binding study showed that CRF bound to type II cells in a concentration-dependent manner. The present study demonstrates that the non-collagenous, C-terminal, domain of SP-A is responsible for the protein's inhibitory effect on lipid secretion and its binding to type II cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson B., Hawgood S., Schilling J., Clements J., Damm D., Cordell B., White R. T. Structure of canine pulmonary surfactant apoprotein: cDNA and complete amino acid sequence. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6379–6383. doi: 10.1073/pnas.82.19.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggaram V., Qing K., Mendelson C. R. The major apoprotein of rabbit pulmonary surfactant. Elucidation of primary sequence and cyclic AMP and developmental regulation. J Biol Chem. 1988 Feb 25;263(6):2939–2947. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs R. A., Wright J. R., Ross G. F., Yuen C. T., Lawson A. M., Chai W., Drickamer K., Feizi T. Specificity of lung surfactant protein SP-A for both the carbohydrate and the lipid moieties of certain neutral glycolipids. J Biol Chem. 1992 May 15;267(14):9972–9979. [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Brody A. R., Hook G. E. Induction of intra- and extra-cellular phospholipids in the lungs of rats exposed to silica. Biochem J. 1986 Jan 1;233(1):111–118. doi: 10.1042/bj2330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs L. G., Mason R. J. Pulmonary alveolar type II cells isolated from rats. Release of phosphatidylcholine in response to beta-adrenergic stimulation. J Clin Invest. 1979 Mar;63(3):378–387. doi: 10.1172/JCI109313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs L. G., Wright J. R., Hawgood S., Gonzalez R., Venstrom K., Nellenbogen J. Pulmonary surfactant and its components inhibit secretion of phosphatidylcholine from cultured rat alveolar type II cells. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1010–1014. doi: 10.1073/pnas.84.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanestil D. D., Barrows C. H., Jr Aging in the rotifer. J Gerontol. 1965 Oct;20(4):462–469. [PubMed] [Google Scholar]
- Floros J., Phelps D. S., Taeusch H. W. Biosynthesis and in vitro translation of the major surfactant-associated protein from human lung. J Biol Chem. 1985 Jan 10;260(1):495–500. [PubMed] [Google Scholar]
- Fornstedt N., Porath J. Characterization studies on a new lectin found in seeds of Vicia ervilia. FEBS Lett. 1975 Sep 15;57(2):187–191. doi: 10.1016/0014-5793(75)80713-7. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Haagsman H. P., Hawgood S., Sargeant T., Buckley D., White R. T., Drickamer K., Benson B. J. The major lung surfactant protein, SP 28-36, is a calcium-dependent, carbohydrate-binding protein. J Biol Chem. 1987 Oct 15;262(29):13877–13880. [PubMed] [Google Scholar]
- Haagsman H. P., Sargeant T., Hauschka P. V., Benson B. J., Hawgood S. Binding of calcium to SP-A, a surfactant-associated protein. Biochemistry. 1990 Sep 25;29(38):8894–8900. doi: 10.1021/bi00490a003. [DOI] [PubMed] [Google Scholar]
- Haas C., Voss T., Engel J. Assembly and disulfide rearrangement of recombinant surfactant protein A in vitro. Eur J Biochem. 1991 May 8;197(3):799–803. doi: 10.1111/j.1432-1033.1991.tb15974.x. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Hamilton R. L., Jr Effects of a surfactant-associated protein and calcium ions on the structure and surface activity of lung surfactant lipids. Biochemistry. 1985 Jan 1;24(1):184–190. doi: 10.1021/bi00322a026. [DOI] [PubMed] [Google Scholar]
- King R. J., Clements J. A. Surface active materials from dog lung. I. Method of isolation. Am J Physiol. 1972 Sep;223(3):707–714. doi: 10.1152/ajplegacy.1972.223.3.707. [DOI] [PubMed] [Google Scholar]
- King R. J., Klass D. J., Gikas E. G., Clements J. A. Isolation of apoproteins from canine surface active material. Am J Physiol. 1973 Apr;224(4):788–795. doi: 10.1152/ajplegacy.1973.224.4.788. [DOI] [PubMed] [Google Scholar]
- Klotz I. M. Numbers of receptor sites from Scatchard graphs: facts and fantasies. Science. 1982 Sep 24;217(4566):1247–1249. doi: 10.1126/science.6287580. [DOI] [PubMed] [Google Scholar]
- Kuroki Y., Akino T. Pulmonary surfactant protein A (SP-A) specifically binds dipalmitoylphosphatidylcholine. J Biol Chem. 1991 Feb 15;266(5):3068–3073. [PubMed] [Google Scholar]
- Kuroki Y., Fukada Y., Takahashi H., Akino T. Monoclonal antibodies against human pulmonary surfactant apoproteins: specificity and application in immunoassay. Biochim Biophys Acta. 1985 Sep 11;836(2):201–209. [PubMed] [Google Scholar]
- Kuroki Y., Gasa S., Ogasawara Y., Makita A., Akino T. Binding of pulmonary surfactant protein A to galactosylceramide and asialo-GM2. Arch Biochem Biophys. 1992 Dec;299(2):261–267. doi: 10.1016/0003-9861(92)90273-y. [DOI] [PubMed] [Google Scholar]
- Kuroki Y., Mason R. J., Voelker D. R. Alveolar type II cells express a high-affinity receptor for pulmonary surfactant protein A. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5566–5570. doi: 10.1073/pnas.85.15.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki Y., Mason R. J., Voelker D. R. Chemical modification of surfactant protein A alters high affinity binding to rat alveolar type II cells and regulation of phospholipid secretion. J Biol Chem. 1988 Nov 25;263(33):17596–17602. [PubMed] [Google Scholar]
- Kuroki Y., Mason R. J., Voelker D. R. Pulmonary surfactant apoprotein A structure and modulation of surfactant secretion by rat alveolar type II cells. J Biol Chem. 1988 Mar 5;263(7):3388–3394. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rice W. R., Ross G. F., Singleton F. M., Dingle S., Whitsett J. A. Surfactant-associated protein inhibits phospholipid secretion from type II cells. J Appl Physiol (1985) 1987 Aug;63(2):692–698. doi: 10.1152/jappl.1987.63.2.692. [DOI] [PubMed] [Google Scholar]
- Rice W. R., Singleton F. M. Regulation of surfactant phospholipid secretion from isolated rat alveolar type II cells by lectins. Biochim Biophys Acta. 1988 Feb 4;958(2):205–210. doi: 10.1016/0005-2760(88)90178-6. [DOI] [PubMed] [Google Scholar]
- Ross G. F., Notter R. H., Meuth J., Whitsett J. A. Phospholipid binding and biophysical activity of pulmonary surfactant-associated protein (SAP)-35 and its non-collagenous COOH-terminal domains. J Biol Chem. 1986 Oct 25;261(30):14283–14291. [PubMed] [Google Scholar]
- Sano K., Fisher J., Mason R. J., Kuroki Y., Schilling J., Benson B., Voelker D. Isolation and sequence of a cDNA clone for the rat pulmonary surfactant-associated protein (PSP-A). Biochem Biophys Res Commun. 1987 Apr 14;144(1):367–374. doi: 10.1016/s0006-291x(87)80519-3. [DOI] [PubMed] [Google Scholar]
- Thorkelsson T., Ciraolo G. M., Ross G. F., Whitsett J. A., Morris R. E. Lectin activity of the major surfactant protein (SP-A) may participate in, but is not required for, binding to rat type II cells. J Histochem Cytochem. 1992 May;40(5):643–649. doi: 10.1177/40.5.1573247. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss T., Eistetter H., Schäfer K. P., Engel J. Macromolecular organization of natural and recombinant lung surfactant protein SP 28-36. Structural homology with the complement factor C1q. J Mol Biol. 1988 May 5;201(1):219–227. doi: 10.1016/0022-2836(88)90448-2. [DOI] [PubMed] [Google Scholar]
- White R. T., Damm D., Miller J., Spratt K., Schilling J., Hawgood S., Benson B., Cordell B. Isolation and characterization of the human pulmonary surfactant apoprotein gene. 1985 Sep 26-Oct 2Nature. 317(6035):361–363. doi: 10.1038/317361a0. [DOI] [PubMed] [Google Scholar]
- Wright J. R., Borchelt J. D., Hawgood S. Lung surfactant apoprotein SP-A (26-36 kDa) binds with high affinity to isolated alveolar type II cells. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5410–5414. doi: 10.1073/pnas.86.14.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. R., Wager R. E., Hawgood S., Dobbs L., Clements J. A. Surfactant apoprotein Mr = 26,000-36,000 enhances uptake of liposomes by type II cells. J Biol Chem. 1987 Feb 25;262(6):2888–2894. [PubMed] [Google Scholar]



