Abstract
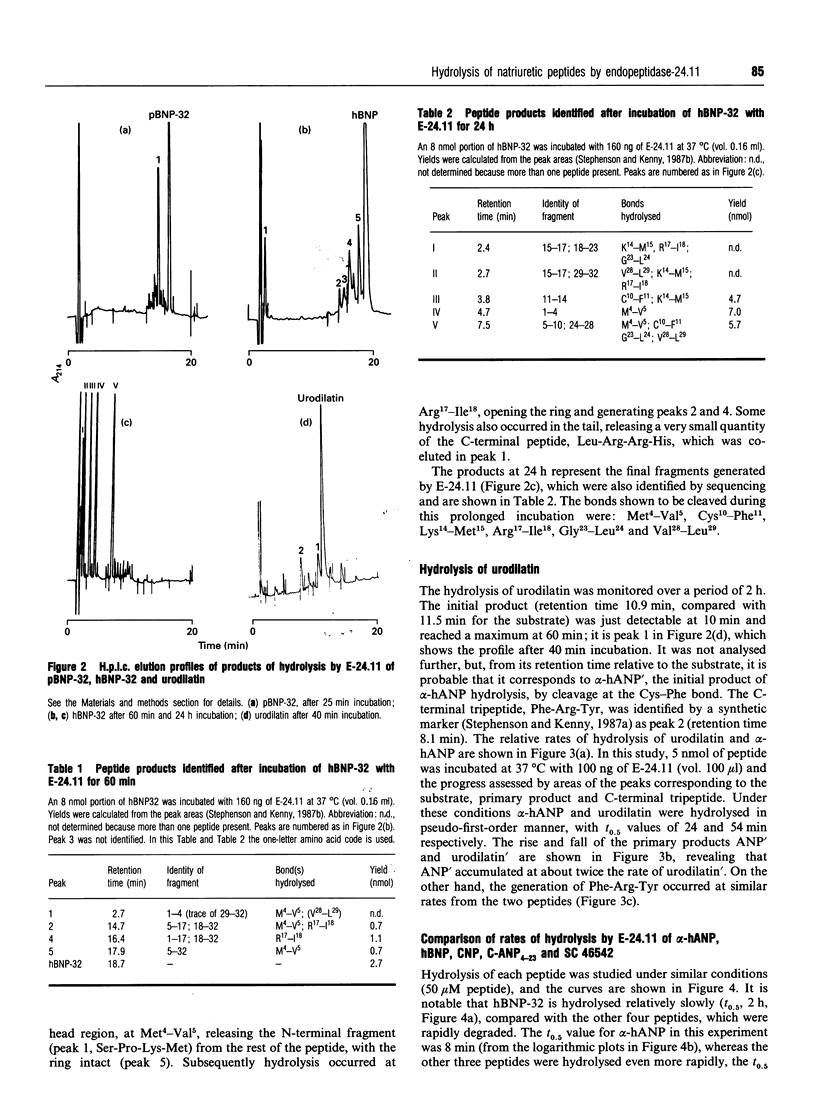
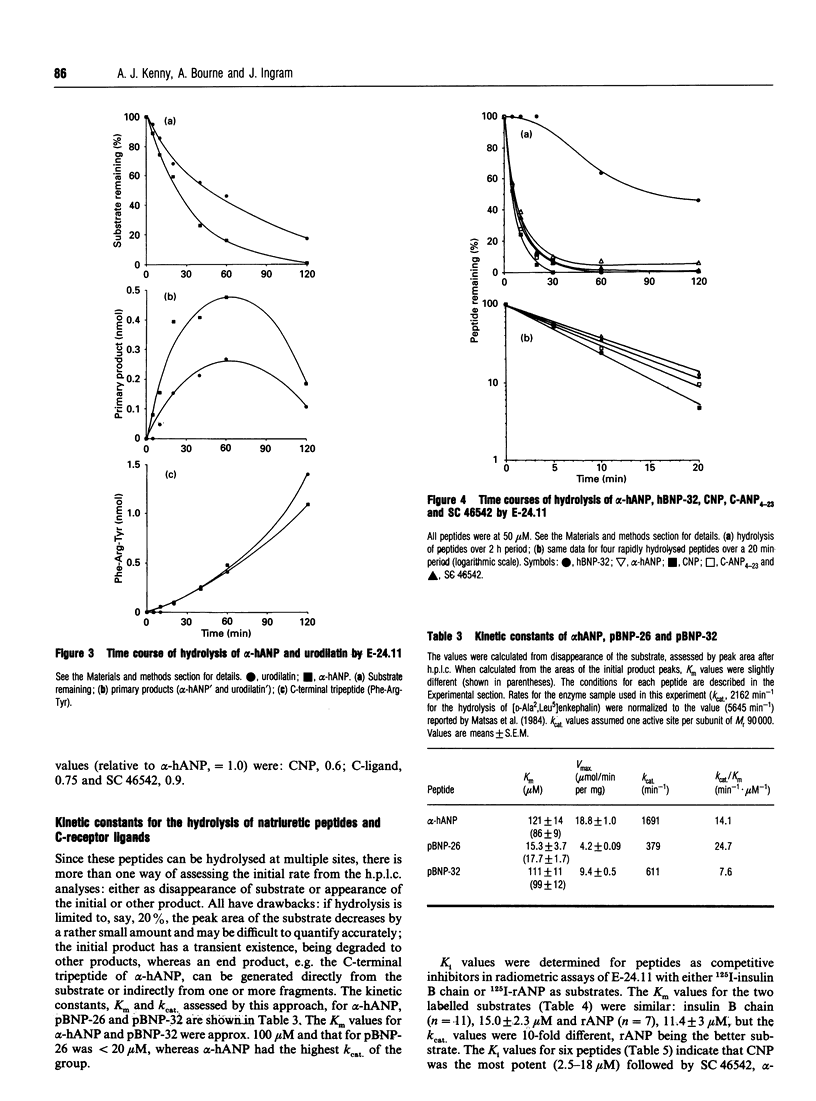
Endopeptidase-24.11 (E-24.11, EC 3.4.24.11) is widely believed to play a physiological role in metabolizing atrial natriuretic peptide (ANP). Since the discovery of ANP, new natriuretic peptides have been isolated and other peptides synthesized as receptor ligands. The hydrolysis in vitro of six related peptides by the endopeptidase has been studied, mainly by h.p.l.c. The initial attack on the 32-residue form of pig brain natriuretic peptide (pBNP-32) was shown to be at the Ser20-Leu21 bond, as had been previously shown for the 26-residue form. In contrast, human brain natriuretic peptide-32 (hBNP-32), which differs in ten residues from pBNP-32, was attacked first at the Met4-Val5 bond, releasing the N-terminal tetrapeptide, and only later at bonds within the ring: at Arg17-Ile18 and subsequently at four other sites. Urodilatin, which has a four-residue extension at the N-terminus compared with alpha-human atrial natriuretic peptide-28 (alpha-hANP), was degraded at about half the rate of the latter, though the C-terminal Phe-Arg-Tyr was released at the same rate. The 22-residue C-type natriuretic peptide was hydrolysed more rapidly than alpha-hANP, as were two C-receptor ligands (peptides with deletions within the ring): C-ANP4-23 (rANP4-23 des-Gln18,Ser19,Gly20,Leu21,Gly22) and SC 46542 (hANP5-28 des-Phe8,Gly9,Ala17,Gln18). Angiotensin-converting enzyme failed to hydrolyse pBNP-32, hBNP-32 or 125I-rat (r) ANP, even after prolonged incubation. Km and kcat values were determined for the hydrolysis of alpha-hANP, porcine BNP-26, porcine BNP-32 and 125I-rANP by E-24.11. Ki values were determined for six peptides, alpha-hANP, urodilatin, hBNP-32, C-type natriuretic peptide (CNP), SC 46542 and C-type natriuretic peptide (C-ANP4-23), in radiometric assays of E-24.11 with either [125I] insulin B chain or [125I] rANP as substrate. The Ki values (2.5-13 microM) for CNP were the lowest of any of the group, whereas those for hBNP-32 (151-172 microM) were the highest. The physiological significance of these results is discussed, especially in regard to the relative resistance of hBNP-32 to attack and the ability of the C-receptor ligands to compete with natriuretic peptides for hydrolysis by E-24.11.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne A., Kenny A. J. The hydrolysis of brain and atrial natriuretic peptides by porcine choroid plexus is attributable to endopeptidase-24.11. Biochem J. 1990 Oct 15;271(2):381–385. doi: 10.1042/bj2710381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L., Chang M. S., Lowe D. G., Chin H. M., Goeddel D. V., Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989 Mar 2;338(6210):78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem J. 1983 Jun 1;211(3):743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller F., Porter J. G., Arfsten A. E., Miller J., Schilling J. W., Scarborough R. M., Lewicki J. A., Schenk D. B. Atrial natriuretic peptide clearance receptor. Complete sequence and functional expression of cDNA clones. J Biol Chem. 1988 Jul 5;263(19):9395–9401. [PubMed] [Google Scholar]
- Gagelmann M., Hock D., Forssmann W. G. Urodilatin (CDD/ANP-95-126) is not biologically inactivated by a peptidase from dog kidney cortex membranes in contrast to atrial natriuretic peptide/cardiodilatin (alpha-hANP/CDD-99-126). FEBS Lett. 1988 Jun 20;233(2):249–254. doi: 10.1016/0014-5793(88)80436-8. [DOI] [PubMed] [Google Scholar]
- Hosoda K., Nakao K., Mukoyama M., Saito Y., Jougasaki M., Shirakami G., Suga S., Ogawa Y., Yasue H., Imura H. Expression of brain natriuretic peptide gene in human heart. Production in the ventricle. Hypertension. 1991 Jun;17(6 Pt 2):1152–1155. doi: 10.1161/01.hyp.17.6.1152. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Arik L., Pitts B. J., Foster C. J. Rapid receptor-mediated catabolism of 125I-atrial natriuretic factor by vascular endothelial cells. Biochem J. 1990 Jun 15;268(3):771–776. doi: 10.1042/bj2680771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangawa K., Matsuo H. Purification and complete amino acid sequence of alpha-human atrial natriuretic polypeptide (alpha-hANP). Biochem Biophys Res Commun. 1984 Jan 13;118(1):131–139. doi: 10.1016/0006-291x(84)91077-5. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., Stephenson S. L. Role of endopeptidase-24.11 in the inactivation of atrial natriuretic peptide. FEBS Lett. 1988 May 9;232(1):1–8. doi: 10.1016/0014-5793(88)80375-2. [DOI] [PubMed] [Google Scholar]
- Komatsu Y., Nakao K., Suga S., Ogawa Y., Mukoyama M., Arai H., Shirakami G., Hosoda K., Nakagawa O., Hama N. C-type natriuretic peptide (CNP) in rats and humans. Endocrinology. 1991 Aug;129(2):1104–1106. doi: 10.1210/endo-129-2-1104. [DOI] [PubMed] [Google Scholar]
- Kuno T., Andresen J. W., Kamisaki Y., Waldman S. A., Chang L. Y., Saheki S., Leitman D. C., Nakane M., Murad F. Co-purification of an atrial natriuretic factor receptor and particulate guanylate cyclase from rat lung. J Biol Chem. 1986 May 5;261(13):5817–5823. [PubMed] [Google Scholar]
- Maack T., Suzuki M., Almeida F. A., Nussenzveig D., Scarborough R. M., McEnroe G. A., Lewicki J. A. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987 Oct 30;238(4827):675–678. doi: 10.1126/science.2823385. [DOI] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino N., Kangawa K., Matsuo H. N-terminally extended form of C-type natriuretic peptide (CNP-53) identified in porcine brain. Biochem Biophys Res Commun. 1990 Jul 31;170(2):973–979. doi: 10.1016/0006-291x(90)92187-5. [DOI] [PubMed] [Google Scholar]
- Mukoyama M., Nakao K., Hosoda K., Suga S., Saito Y., Ogawa Y., Shirakami G., Jougasaki M., Obata K., Yasue H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991 Apr;87(4):1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukoyama M., Nakao K., Obata K., Jougasaki M., Yoshimura M., Morita E., Hosoda K., Suga S., Ogawa Y., Yasue H. Augmented secretion of brain natriuretic peptide in acute myocardial infarction. Biochem Biophys Res Commun. 1991 Oct 15;180(1):431–436. doi: 10.1016/s0006-291x(05)81311-7. [DOI] [PubMed] [Google Scholar]
- Mukoyama M., Nakao K., Saito Y., Ogawa Y., Hosoda K., Suga S., Shirakami G., Jougasaki M., Imura H. Increased human brain natriuretic peptide in congestive heart failure. N Engl J Med. 1990 Sep 13;323(11):757–758. doi: 10.1056/NEJM199009133231114. [DOI] [PubMed] [Google Scholar]
- Norman J. A., Little D., Bolgar M., Di Donato G. Degradation of brain natriuretic peptide by neutral endopeptidase: species specific sites of proteolysis determined by mass spectrometry. Biochem Biophys Res Commun. 1991 Feb 28;175(1):22–30. doi: 10.1016/s0006-291x(05)81194-5. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Nakao K., Mukoyama M., Hosoda K., Shirakami G., Arai H., Saito Y., Suga S., Jougasaki M., Imura H. Natriuretic peptides as cardiac hormones in normotensive and spontaneously hypertensive rats. The ventricle is a major site of synthesis and secretion of brain natriuretic peptide. Circ Res. 1991 Aug;69(2):491–500. doi: 10.1161/01.res.69.2.491. [DOI] [PubMed] [Google Scholar]
- Rathinavelu A., Isom G. E. Differential internalization and processing of atrial-natriuretic-factor B and C receptor in PC12 cells. Biochem J. 1991 Jun 1;276(Pt 2):493–497. doi: 10.1042/bj2760493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough R. M., McEnroe G. A., Arfsten A., Kang L. L., Schwartz K., Lewicki J. A. D-amino acid-substituted atrial natriuretic peptide analogs reveal novel receptor recognition requirements. J Biol Chem. 1988 Nov 15;263(32):16818–16822. [PubMed] [Google Scholar]
- Scarborough R. M., Schenk D. B., McEnroe G. A., Arfsten A., Kang L. L., Schwartz K., Lewicki J. A. Truncated atrial natriuretic peptide analogs. Comparison between receptor binding and stimulation of cyclic GMP accumulation in cultured vascular smooth muscle cells. J Biol Chem. 1986 Oct 5;261(28):12960–12964. [PubMed] [Google Scholar]
- Schulz-Knappe P., Forssmann K., Herbst F., Hock D., Pipkorn R., Forssmann W. G. Isolation and structural analysis of "urodilatin", a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klin Wochenschr. 1988 Sep 1;66(17):752–759. doi: 10.1007/BF01726570. [DOI] [PubMed] [Google Scholar]
- Seymour A. A., Norman J. A., Asaad M. M., Fennell S. A., Abboa-Offei B., Little D. K., Kratunis V. J., Delaney N. G., Hunt J. T., Di Donato G. Possible regulation of atrial natriuretic factor by neutral endopeptidase 24.11 and clearance receptors. J Pharmacol Exp Ther. 1991 Mar;256(3):1002–1009. [PubMed] [Google Scholar]
- Stephenson S. L., Kenny A. J. Metabolism of neuropeptides. Hydrolysis of the angiotensins, bradykinin, substance P and oxytocin by pig kidney microvillar membranes. Biochem J. 1987 Jan 1;241(1):237–247. doi: 10.1042/bj2410237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson S. L., Kenny A. J. The hydrolysis of alpha-human atrial natriuretic peptide by pig kidney microvillar membranes is initiated by endopeptidase-24.11. Biochem J. 1987 Apr 1;243(1):183–187. doi: 10.1042/bj2430183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudoh T., Kangawa K., Minamino N., Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988 Mar 3;332(6159):78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- Sudoh T., Maekawa K., Kojima M., Minamino N., Kangawa K., Matsuo H. Cloning and sequence analysis of cDNA encoding a precursor for human brain natriuretic peptide. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1427–1434. doi: 10.1016/0006-291x(89)92269-9. [DOI] [PubMed] [Google Scholar]
- Sudoh T., Minamino N., Kangawa K., Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990 Apr 30;168(2):863–870. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- Ueda S., Minamino N., Aburaya M., Kangawa K., Matsukura S., Matsuo H. Distribution and characterization of immunoreactive porcine C-type natriuretic peptide. Biochem Biophys Res Commun. 1991 Mar 29;175(3):759–767. doi: 10.1016/0006-291x(91)91631-l. [DOI] [PubMed] [Google Scholar]
- Vanneste Y., Michel A., Dimaline R., Najdovski T., Deschodt-Lanckman M. Hydrolysis of alpha-human atrial natriuretic peptide in vitro by human kidney membranes and purified endopeptidase-24.11. Evidence for a novel cleavage site. Biochem J. 1988 Sep 1;254(2):531–537. doi: 10.1042/bj2540531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste Y., Pauwels S., Lambotte L., Deschodt-Lanckman M. In vivo metabolism of brain natriuretic peptide in the rat involves endopeptidase 24.11 and angiotensin converting enzyme. Biochem Biophys Res Commun. 1990 Nov 30;173(1):265–271. doi: 10.1016/s0006-291x(05)81051-4. [DOI] [PubMed] [Google Scholar]
- Vogt-Schaden M., Gagelmann M., Hock D., Herbst F., Forssmann W. G. Degradation of porcine brain natriuretic peptide (pBNP-26) by endoprotease-24.11 from kidney cortical membranes. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1177–1183. doi: 10.1016/0006-291x(89)91366-1. [DOI] [PubMed] [Google Scholar]


