Abstract
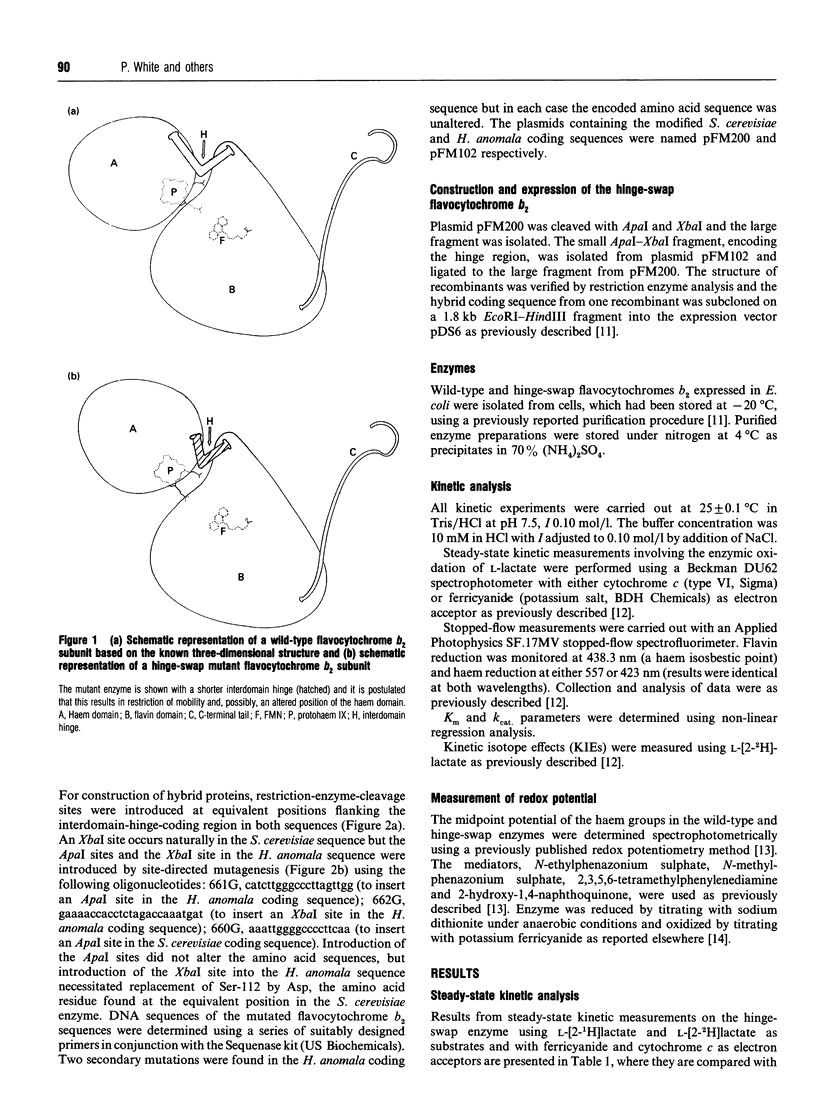
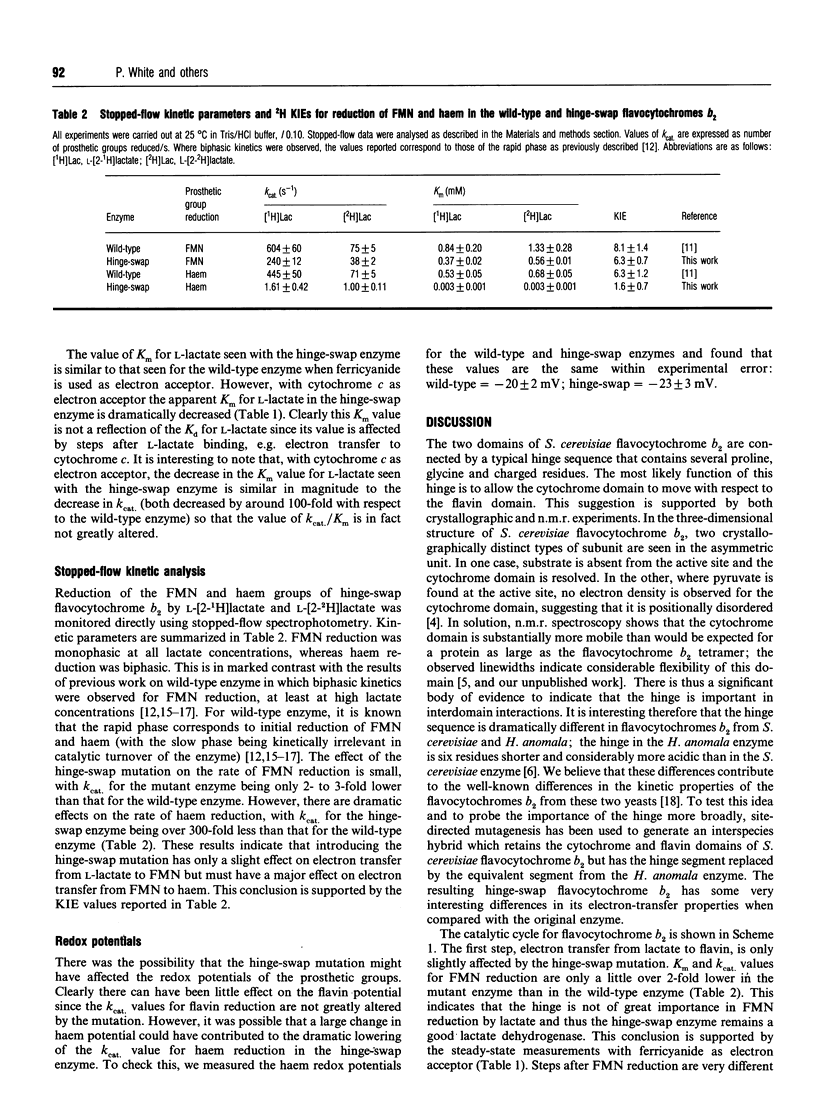
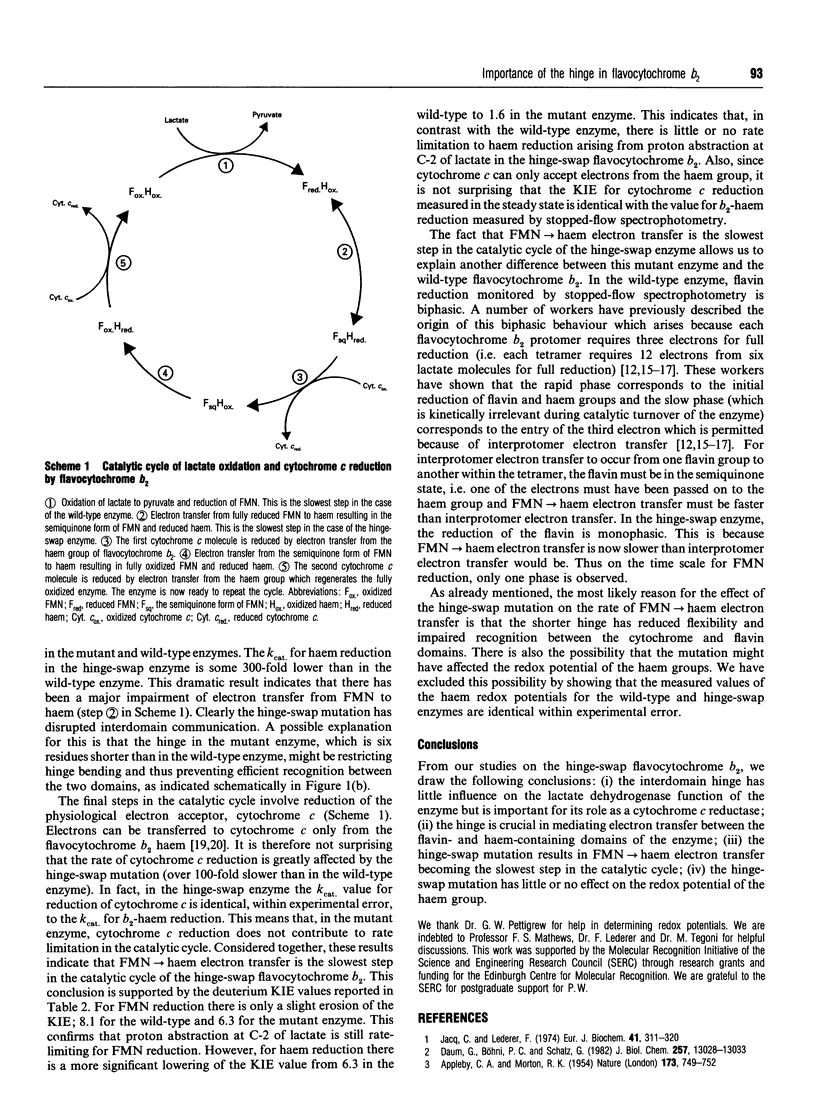
The two distinct domains of flavocytochrome b2 (L-lactate:cytochrome c oxidoreductase) are connected by a typical hinge peptide. The amino acid sequence of this interdomain hinge is dramatically different in flavocytochromes b2 from Saccharomyces cerevisiae and Hansenula anomala. This difference in the hinge is believed to contribute to the difference in kinetic properties between the two enzymes. To probe the importance of the hinge, an interspecies hybrid enzyme has been constructed comprising the bulk of the S. cerevisiae enzyme but containing the H. anomala flavocytochrome b2 hinge. The kinetic properties of this 'hinge-swap' enzyme have been investigated by steady-state and stopped-flow methods. The hinge-swap enzyme remains a good lactate dehydrogenase as is evident from steady-state experiments with ferricyanide as acceptor (only 3-fold less active than wild-type enzyme) and stopped-flow experiments monitoring flavin reduction (2.5-fold slower than in wild-type enzyme). The major effect of the hinge-swap mutation is to lower dramatically the enzyme's effectiveness as a cytochrome c reductase; kcat. for cytochrome c reduction falls by more than 100-fold, from 207 +/- 10 s-1 (25 degrees C, pH 7.5) in the wild-type enzyme to 1.62 +/- 0.41 s-1 in the mutant enzyme. This fall in cytochrome c reductase activity results from poor interdomain electron transfer between the FMN and haem groups. This can be demonstrated by the fact that the kcat. for haem reduction in the hinge-swap enzyme (measured by the stopped-flow method) has a value of 1.61 +/- 0.42 s-1, identical with the value for cytochrome c reduction and some 300-fold lower than the value for the wild-type enzyme. From these and other kinetic parameters, including kinetic isotope effects with [2-2H]lactate, we conclude that the hinge plays a crucial role in allowing efficient electron transfer between the two domains of flavocytochrome b2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEBY C. A., MORTON R. K. Crystalline cytochrome b2 and lactic dehydrogenase of yeast. Nature. 1954 Apr 24;173(4408):749–752. doi: 10.1038/173749a0. [DOI] [PubMed] [Google Scholar]
- Black M. T., Gunn F. J., Chapman S. K., Reid G. A. Structural basis for the kinetic differences between flavocytochromes b2 from the yeasts Hansenula anomala and Saccharomyces cerevisiae. Biochem J. 1989 Nov 1;263(3):973–976. doi: 10.1042/bj2630973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M. T., White S. A., Reid G. A., Chapman S. K. High-level expression of fully active yeast flavocytochrome b2 in Escherichia coli. Biochem J. 1989 Feb 15;258(1):255–259. doi: 10.1042/bj2580255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt C. E., Cox M. C., Thurgood A. G., Moore G. R., Reid G. A., Chapman S. K. Isolation and characterization of the cytochrome domain of flavocytochrome b2 expressed independently in Escherichia coli. Biochem J. 1992 Apr 1;283(Pt 1):87–90. doi: 10.1042/bj2830087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capeillère-Blandin C. Electron-transfer steps involved in the reactivity of Hansenula anomala flavocytochrome b2 as deduced from deuterium isotope effects and simulation studies. Biochem J. 1991 Feb 15;274(Pt 1):207–217. doi: 10.1042/bj2740207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Dutton P. L. Redox potentiometry: determination of midpoint potentials of oxidation-reduction components of biological electron-transfer systems. Methods Enzymol. 1978;54:411–435. doi: 10.1016/s0076-6879(78)54026-3. [DOI] [PubMed] [Google Scholar]
- Jacq C., Lederer F. Cytochrome b2 from bakers' yeast (L-lactate dehydrogenase). A double-headed enzyme. Eur J Biochem. 1974 Jan 16;41(2):311–320. doi: 10.1111/j.1432-1033.1974.tb03271.x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeyrie F., Baudras A., Lederer F. Flavocytochrome b 2 or L-lactate cytochrome c reductase from yeast. Methods Enzymol. 1978;53:238–256. doi: 10.1016/s0076-6879(78)53030-9. [DOI] [PubMed] [Google Scholar]
- Labeyrie F., Beloeil J. C., Thomas M. A. Evidence by NMR for mobility of the cytochrome domain within flavocytochrome b2. Biochim Biophys Acta. 1988 Mar 23;953(2):134–141. doi: 10.1016/0167-4838(88)90018-0. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Miles C. S., Rouvière-Fourmy N., Lederer F., Mathews F. S., Reid G. A., Black M. T., Chapman S. K. Tyr-143 facilitates interdomain electron transfer in flavocytochrome b2. Biochem J. 1992 Jul 1;285(Pt 1):187–192. doi: 10.1042/bj2850187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y., Nakamura T. Kinetic studies on the oxidation and reduction of the protoheme moiety of yeast L(+)-lactate dehydrogenases. J Biochem. 1966 Jul;60(1):77–86. doi: 10.1093/oxfordjournals.jbchem.a128402. [DOI] [PubMed] [Google Scholar]
- Pompon D. Flavocytochrome b2 from baker's yeast. Computer-simulation studies of a new scheme for intramolecular electron transfer. Eur J Biochem. 1980 May;106(1):151–159. [PubMed] [Google Scholar]
- Pompon D., Iwatsubo M., Lederer F. Flavocytochrome b2 (Baker's yeast). Deuterium isotope effect studied by rapid-kinetic methods as a probe for the mechanism of electron transfer. Eur J Biochem. 1980 Mar;104(2):479–488. doi: 10.1111/j.1432-1033.1980.tb04450.x. [DOI] [PubMed] [Google Scholar]
- Reid G. A., White S., Black M. T., Lederer F., Mathews F. S., Chapman S. K. Probing the active site of flavocytochrome b2 by site-directed mutagenesis. Eur J Biochem. 1988 Dec 15;178(2):329–333. doi: 10.1111/j.1432-1033.1988.tb14454.x. [DOI] [PubMed] [Google Scholar]
- Xia Z. X., Mathews F. S. Molecular structure of flavocytochrome b2 at 2.4 A resolution. J Mol Biol. 1990 Apr 20;212(4):837–863. doi: 10.1016/0022-2836(90)90240-M. [DOI] [PubMed] [Google Scholar]


