Abstract
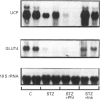
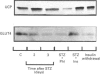
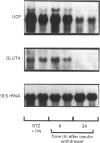
We have studied the time course and relative effects of hypoinsulinaemia and hyperglycaemia on concentrations of uncoupling protein (UCP) and glucose transporter (GLUT4) and their mRNAs in brown adipose tissue (BAT) during the early phase of diabetes induced by streptozotocin. Two days after intravenous injection of streptozotocin, plasma insulin concentration was at its lowest and glycaemia was higher than 22 mmol/l. After 3 days, a 60% decrease in BAT UCP mRNA concentration and a 36% decrease in UCP was observed. Concomitantly, there was an 80% decrease in GLUT4 mRNA and a 44% decrease in GLUT4 levels. When hyperglycaemia was prevented by infusing phlorizin into diabetic rats, BAT UCP mRNA and protein levels were further decreased (respectively 90% and 60% lower than in control rats). In contrast, the marked decreases in GLUT4 mRNA and protein concentrations in BAT were similar in hyperglycaemic and normoglycaemic diabetic rats. Infusion of physiological amounts of insulin restored normoglycaemia in diabetic rats, and BAT UCP and GLUT4 mRNA and protein concentrations were maintained at the level of control rats. When insulin infusion was stopped, a 75% decrease in BAT UCP mRNA level and a 75% decrease in GLUT4 mRNA level were observed after 24 h, but UCP and GLUT4 concentrations did not decrease. This study shows that insulin plays an important role in the regulation of UCP and GLUT4 mRNA and protein concentrations in BAT. Hyperglycaemia partially prevents the rapid decrease in concentration of UCP and its mRNA observed in insulinopenic diabetes whereas it did not affect the decrease in GLUT4 mRNA and protein concentration. It is suggested that UCP is produced by a glucose-dependent gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Katagiri H., Tsukuda K., Lin J. L., Ishihara H., Yazaki Y., Oka Y. Upregulation of GLUT2 mRNA by glucose, mannose, and fructose in isolated rat hepatocytes. Diabetes. 1992 Jan;41(1):22–25. doi: 10.2337/diab.41.1.22. [DOI] [PubMed] [Google Scholar]
- Bouillaud F., Raimbault S., Ricquier D. The gene for rat uncoupling protein: complete sequence, structure of primary transcript and evolutionary relationship between exons. Biochem Biophys Res Commun. 1988 Dec 15;157(2):783–792. doi: 10.1016/s0006-291x(88)80318-8. [DOI] [PubMed] [Google Scholar]
- Bouillaud F., Ricquier D., Thibault J., Weissenbach J. Molecular approach to thermogenesis in brown adipose tissue: cDNA cloning of the mitochondrial uncoupling protein. Proc Natl Acad Sci U S A. 1985 Jan;82(2):445–448. doi: 10.1073/pnas.82.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcelin R., Eddouks M., Kande J., Assan R., Girard J. Evidence that GLUT-2 mRNA and protein concentrations are decreased by hyperinsulinaemia and increased by hyperglycaemia in liver of diabetic rats. Biochem J. 1992 Dec 1;288(Pt 2):675–679. doi: 10.1042/bj2880675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. The biochemistry of an inefficient tissue: brown adipose tissue. Essays Biochem. 1985;20:110–164. [PubMed] [Google Scholar]
- Chan Y. L., Gutell R., Noller H. F., Wool I. G. The nucleotide sequence of a rat 18 S ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18 S ribosomal ribonucleic acid. J Biol Chem. 1984 Jan 10;259(1):224–230. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Decaux J. F., Antoine B., Kahn A. Regulation of the expression of the L-type pyruvate kinase gene in adult rat hepatocytes in primary culture. J Biol Chem. 1989 Jul 15;264(20):11584–11590. [PubMed] [Google Scholar]
- Ferré P., Leturque A., Burnol A. F., Penicaud L., Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J. 1985 May 15;228(1):103–110. doi: 10.1042/bj2280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J. R., Cuendet G. S., Marliss E. B., Kervran A., Rieutort M., Assan R. Fuels, hormones, and liver metabolism at term and during the early postnatal period in the rat. J Clin Invest. 1973 Dec;52(12):3190–3200. doi: 10.1172/JCI107519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Mariash C. N. Cell-specific carbohydrate metabolism regulates S14 gene transcription. Diabetes. 1992 Mar;41(3):339–346. doi: 10.2337/diab.41.3.339. [DOI] [PubMed] [Google Scholar]
- Géloën A., Trayhurn P. Regulation of the level of uncoupling protein in brown adipose tissue by insulin requires the mediation of the sympathetic nervous system. FEBS Lett. 1990 Jul 16;267(2):265–267. doi: 10.1016/0014-5793(90)80941-b. [DOI] [PubMed] [Google Scholar]
- Géloën A., Trayhurn P. Regulation of the level of uncoupling protein in brown adipose tissue by insulin. Am J Physiol. 1990 Feb;258(2 Pt 2):R418–R424. doi: 10.1152/ajpregu.1990.258.2.R418. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J. Brown adipose tissue thermogenesis: interdisciplinary studies. FASEB J. 1990 Aug;4(11):2890–2898. [PubMed] [Google Scholar]
- Jamal Z., Saggerson E. D. Factors influencing the altered thermogenic response of rat brown adipose tissue in streptozotocin-diabetes. Biochem J. 1988 Jan 15;249(2):415–421. doi: 10.1042/bj2490415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Klaus S., Casteilla L., Bouillaud F., Ricquier D. The uncoupling protein UCP: a membraneous mitochondrial ion carrier exclusively expressed in brown adipose tissue. Int J Biochem. 1991;23(9):791–801. doi: 10.1016/0020-711x(91)90062-r. [DOI] [PubMed] [Google Scholar]
- Kozak U. C., Held W., Kreutter D., Kozak L. P. Adrenergic regulation of the mitochondrial uncoupling protein gene in brown fat tumor cells. Mol Endocrinol. 1992 May;6(5):763–772. doi: 10.1210/mend.6.5.1603085. [DOI] [PubMed] [Google Scholar]
- Munnich A., Besmond C., Darquy S., Reach G., Vaulont S., Dreyfus J. C., Kahn A. Dietary and hormonal regulation of aldolase B gene expression. J Clin Invest. 1985 Mar;75(3):1045–1052. doi: 10.1172/JCI111766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G., Locke R. M. Thermogenic mechanisms in brown fat. Physiol Rev. 1984 Jan;64(1):1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Oka Y., Asano T., Shibasaki Y., Lin J. L., Tsukuda K., Akanuma Y., Takaku F. Increased liver glucose-transporter protein and mRNA in streptozocin-induced diabetic rats. Diabetes. 1990 Apr;39(4):441–446. doi: 10.2337/diab.39.4.441. [DOI] [PubMed] [Google Scholar]
- POE R. H., DAVIS T. R. Cold exposure and acclimation in alloxan-diabetic rats. Am J Physiol. 1962 Jun;202:1045–1048. doi: 10.1152/ajplegacy.1962.202.6.1045. [DOI] [PubMed] [Google Scholar]
- Ricquier D., Barlet J. P., Garel J. M., Combes-George M., Dubois M. P. An immunological study of the uncoupling protein of brown adipose tissue mitochondria. Biochem J. 1983 Mar 15;210(3):859–866. doi: 10.1042/bj2100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. Insulin and thermogenesis. Int J Obes. 1988;12(2):93–102. [PubMed] [Google Scholar]
- Seydoux J., Trimble E. R., Bouillaud F., Assimacopoulos-Jeannet F., Bas S., Ricquier D., Giacobino J. P., Girardier L. Modulation of beta-oxidation and proton conductance pathway of brown adipose tissue in hypo- and hyperinsulinemic states. FEBS Lett. 1984 Jan 23;166(1):141–145. doi: 10.1016/0014-5793(84)80060-5. [DOI] [PubMed] [Google Scholar]
- Shih H. M., Towle H. C. Definition of the carbohydrate response element of the rat S14 gene. Evidence for a common factor required for carbohydrate regulation of hepatic genes. J Biol Chem. 1992 Jul 5;267(19):13222–13228. [PubMed] [Google Scholar]
- Thompson K. S., Towle H. C. Localization of the carbohydrate response element of the rat L-type pyruvate kinase gene. J Biol Chem. 1991 May 15;266(14):8679–8682. [PubMed] [Google Scholar]
- Yoshida T., Nishioka H., Nakamura Y., Kondo M. Reduced noradrenaline turnover in streptozotocin-induced diabetic rats. Diabetologia. 1985 Sep;28(9):692–696. doi: 10.1007/BF00291978. [DOI] [PubMed] [Google Scholar]
- Young J. B., Saville E., Rothwell N. J., Stock M. J., Landsberg L. Effect of diet and cold exposure on norepinephrine turnover in brown adipose tissue of the rat. J Clin Invest. 1982 May;69(5):1061–1071. doi: 10.1172/JCI110541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P., Cawthorne M. A., Smith S. A. Brown adipose tissue is a major site of glucose utilisation in C57Bl/6 ob/ob mice treated with a thermogenic beta-adrenoceptor agonist. Biochem Biophys Res Commun. 1985 Jul 16;130(1):241–248. doi: 10.1016/0006-291x(85)90408-5. [DOI] [PubMed] [Google Scholar]