Abstract
The Lassa virus glycoprotein consists of an amino-terminal and a carboxy-terminal cleavage fragment designated GP-1 and GP-2, respectively, that are derived by proteolysis from the precursor GP-C. The membrane-anchored GP-2 obtained from purified virions of the Josiah strain revealed the N-terminal tripeptide GTF262 when analyzed by Edman degradation. Upstream of this site, GP-C contains the tetrapeptide sequence RRLL259, which is conserved in all Lassa virus isolates published to date. Systematic mutational analysis of vector-expressed GP-C revealed that the motif R-X (L/I/V)-L259 (where X stands for L, I, or V) is essential for cleavage of the peptide bond between leucine259 and glycine260. This cleavage motif is homologous to the consensus sequence recognized by a novel class of cellular endoproteases which have so far not been implicated in the processing of viral glycoproteins.
Lassa virus is the causative agent of a hemorrhagic fever endemic in West Africa. In this area, between 100,000 and 500,000 human infections are estimated to occur annually (23), of which approximately 30% result in illness ranging from mild, flu-like symptoms to fulminant hemorrhagic fever with an overall mortality of 10 to 15% (22).
Lassa virus belongs to the family of Arenaviridae, which also includes lymphocytic choriomeningitis virus (LCMV), Mopeia virus, and the new world arenaviruses, such as Junin and Machupo viruses. Virions are composed of a nucleocapsid surrounded by a lipid-containing envelope and viral glycoprotein spikes. The genome consists of two single-stranded RNA molecules, designated L and S, with coding capacities of 7.2 and 3.4 kb, respectively (25). Both RNA strands are arranged in an ambisense coding orientation (1, 32), with the S segment encoding the nucleocapsid protein (NP) in a negative sense orientation at the 3′ end and the viral envelope glycoprotein precursor (GP-C) at the 5′ end in a positive sense orientation.
The Lassa virus glycoprotein is synthesized as a 76-kDa precursor glycoprotein (GP-C) which is posttranslationally cleaved into the amino-terminal subunit GP-1 (44 kDa) and the carboxy-terminal fragment GP-2 (36 kDa) containing the membrane anchor (21, 24). It has been shown for LCMV and Junin virus that glycoprotein cleavage occurs in the Golgi apparatus or a post-Golgi compartment (8, 33). GP-1 and GP-2 of LCMV form homotetrameric spikes on viral particles (33). GP-1 of LCMV and of Lassa virus interacts with a host cell surface receptor that has recently been identified as α-dystroglycan (4, 9), while the ectodomain of GP-2 is believed to contain a fusion peptide (17, 18). After cleavage of the LCMV and Junin virus glycoprotein, the fusion peptide appears to be exposed by a conformational change in a pH-dependent manner to mediate fusion (10, 14, 15).
It has been shown for a wide range of enveloped viruses that endoproteolytic cleavage of the fusogenic glycoprotein is critical for infection and, at least in some cases, also a major determinant of viral tropism and pathogenicity (5, 20). It is therefore reasonable to assume that cleavage of the Lassa virus glycoprotein GP-C is also of high biological significance. Buchmeier and coworkers showed that the cleavage site of LCMV GP-C is limited to a stretch of 9 amino acids by using site-specific antibodies. This stretch contains paired arginine residues, which are highly conserved among the glycoproteins of the arenavirus family, suggesting that this basic sequence likely serves as a recognition site for proteolytic cleavage (6). In the Lassa virus Josiah GP-C, paired arginine residues are present at amino acid positions 256 and 257. To find out if these arginines are located at the cleavage site, we first raised antibodies against the putative carboxy-terminal and amino-terminal ends of GP-1 and GP-2, respectively. For this purpose, peptides corresponding to amino acids 231 to 255 and 259 to 279 (see Fig. 3) were chemically synthesized, conjugated to keyhole limpet hemocyanin (KLH) as a carrier protein, and used for immunization of rabbits. In addition, antibodies were raised against a peptide homologous to the C terminus of GP-2 (amino acids 477 to 491). The resulting antisera were designated anti-GP231, anti-GP259, and anti-GP477, respectively, with anti-GP231 detecting GP-1 and anti-GP259 detecting GP-2 (Fig. 1B). This finding indicates that the cleavage site of the Lassa virus glycoprotein is located within the peptide region comprising amino acids 231 to 279, which agrees with the data obtained for the LCMV glycoprotein (6).
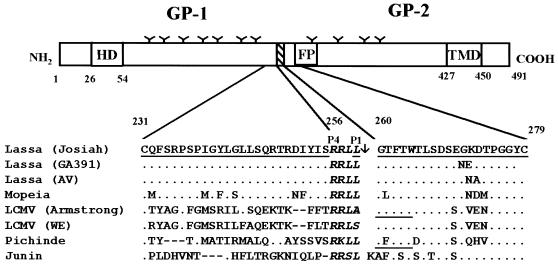
FIG. 3.
Schematic representation of the Lassa virus glycoprotein and alignment of the amino acid sequences around the cleavage regions of different arenaviruses. The Lassa virus glycoprotein precursor contains three hydrophobic amino acid domains, the N-terminal hydrophobic domain (HD), the putative fusion domain (FP), and the transmembrane domain (TMD), and several potential N-glycosylation sites (Y). The precursor glycoprotein GP-C is proteolytically cleaved at a recognition motif (striped box). The sequences of the cleavage region comprising the amino acids from position 231 to 279 are shown for Lassa virus strain Josiah (GenBank accession number P08669) (2), Lassa virus GA391 (GenBank accession number P17332) (12), Lassa virus A.V. (19), Mopeia virus (GenBank accession number P19240) (32), LCMV Armstrong (Genbank accession number P09991) (29), LCMV WE (GenBank accession number P07399) (26), Pichinde virus (GenBank accession number P03540) (1), and Junin virus (GenBank accession number P26313) (16). Only amino acids differing from the Josiah sequence are shown. The cleavage site as determined with the Josiah isolate is marked by an arrow. The N termini of the GP-2s of LCMV and Pichinde virus, as determined by Burns and Buchmeier (7), are underlined. The conserved consensus sequence for protease recognition in African arenaviruses, RRLL, and comparable tetrapeptides for other arenaviruses are shown in boldface italics. The sequences of the peptides used for immunization are underlined for Lassa virus strain Josiah.
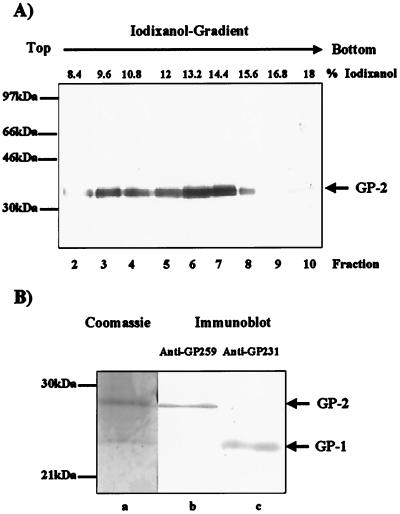
FIG. 1.
Preparation of glycoprotein subunit GP-2 from Lassa virus particles for N-terminal sequencing. (A) Vero-E6 cells were infected with Lassa virus strain Josiah. Virions were purified from the cell culture supernatant by centrifugation through a 20% sucrose cushion followed by iodixanol gradient ultracentrifugation. Fractions of the gradient were SDS treated, subjected to electrophoresis on 12% acrylamide gels, and electrophoretically blotted onto a PVDF membrane. Fractions containing cleaved GP-2 were identified by immunodetection using anti-GP477, horseradish peroxidase-labeled anti-rabbit antibodies from swine (Dako, Glostrup, Denmark), and the Super Signal enhanced chemoluminescence detection kit (Pierce, Rockford, Ill). (B) Proteins of fraction 6 were treated with PNGase F, subjected to SDS-PAGE, and transferred onto a PVDF membrane. Virus glycoproteins GP-1 and GP-2 were detected by immune sera anti-G231 and anti-GP259 (lanes b and c) or were stained with Coomassie blue (lane a). The Coomassie-stained band representing deglycosylated GP-2 was excised and prepared for N-terminal analyses. Molecular mass markers RPN756 used for SDS-PAGE were obtained from Amersham-Pharmacia (Freiburg, Germany).
(O.L. performed this work in partial fulfillment of the requirements for a Ph.D. degree from the Philipps-Universität Marburg.)
To determine the exact cleavage site of GP-C, the Lassa virus strain Josiah was propagated in Vero-E6 cells under biosafety level 4 (BSL-4) biocontainment conditions. On day 5 postinfection (p.i.), virus contained in the cell culture supernatant was centrifuged through a 20% sucrose cushion (at 20,000 rpm in an SW41 rotor [Beckman, Unterschleissheim, Germany] for 2 h) followed by velocity gradient centrifugation (7.2 to 18% iodixanol [Opti-Prep; Sigma, Deisenhofen, Germany] in phosphate-buffered saline [PBS]; 41,000 rpm in an SW28 rotor [Beckman] for 1.5 h). Virus was collected in 1-ml fractions from the top of the iodixanol gradient. Infectious virus was inactivated by boiling with 1% sodium dodecyl sulfate (SDS) for 8 min before further analyses were performed. The viral GP-2 was identified by Western blotting using anti-GP477 after SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Virus peaked in fractions 6 and 7 with 13.2 and 14.4% iodixanol, respectively (Fig. 1A), while the peak around fraction 3 represents GP-2 attached to microsomes (13). In order to get a sufficient amount of purified viral glycoproteins, the proteins of fraction 6 were freed from iodixanol by passage through a NAP5 column (desalting Sephadex G25 columns [Pharmacia, Freiburg, Germany]) and concentrated by speed vacuum centrifugation. To obtain a sharp band stainable with Coomassie blue, the N-linked carbohydrates were removed by treatment with N-glycosidase F (PNGase F) (EC 3.5.1.52). Viral proteins were separated by SDS-PAGE, electrophoretically transferred onto a polyvinylidene difluoride (PVDF) membrane, stained with Coomassie blue, and in parallel identified by immunodetection using the rabbit antiserum specific for GP-2 and GP-1 and an immunoluminescence detection kit (see Fig. 1B). Two distinct bands were found, showing apparent molecular masses of 24 and 27 kDa, respectively. The antiserum against GP-1 detected the 24-kDa protein, while the antiserum against GP-2 clearly stained the 27-kDa protein. The Coomassie blue-stained GP-2 band was excised from the PVDF membrane and submitted for N-terminal amino acid sequencing by automated Edman degradation (Wittmann Institute of Technology and Analysis of Biomolecules, Berlin-Teltow, Germany). Three amino acids were unambiguously identified in the first three cycles of the Edman degradation: glycine (cycle 1), threonine (cycle 2), and phenylalanine (cycle 3). Since GP-C of the Josiah strain contains the tripeptide GTF only in the sequence RRLL259GTFTWTLT, the peptide bond between leucine259 and glycine260 is most likely cleaved.
The observation that GP-C is not cleaved at the carboxy-terminal end of the dibasic sequence RR257 is very interesting, suggesting an uncommon cleavage type for viral glycoproteins. This type of cleavage was already considered as an alternative cleavage for the related arenaviruses LCMV and Pichinde virus by Burns and Buchmeier (7). It was therefore of interest to determine the amino acid consensus sequence recognized by the protease involved in processing of GP-C. Therefore GP-C was subjected to site-directed mutagenesis of the cleavage site. GP-C was cloned into the pTM1 expression plasmid at NcoI and BamHI sites using PCR technology. The glycoprotein was efficiently expressed in Vero-E6, U373, and HeLa cells using the modified vaccinia virus strain Ankara T7 (MVA-T7) system (31). As shown in Fig. 2A, three bands were detected with anti-GP477 when the wild-type Lassa virus glycoprotein was expressed by the MVA-T7 system, while after Lassa virus infection, only two protein bands appeared. The 76- and 36-kDa bands, present in cells used for MVA-T7 expression as well as in virus-infected cells, represent GP-C and GP-2, respectively. The third band, with a molecular mass of about 55 kDa, designated GP-C*, is most likely the unglycosylated GP-C polypeptide that is not correctly translocated into the endoplasmic reticulum as a result of overexpression in the vaccinia virus system (3).
FIG. 2.
Determination of the consensus sequence of the cleavage site of the Lassa virus glycoprotein by mutational analysis. (A) Cleavage of wild-type and mutated Lassa virus glycoprotein expressed in an MVA-T7 polymerase system and in Lassa virus-infected cells. Vero-E6 cells were infected with Lassa virus and lysed 5 days p.i. (lanes a and b). Human astrocytoma U373 cells were infected with MVA-T7 (31) at a multiplicity of infection of 10 PFU per cell and transfected with either pTM1 alone (lane c), pTM1 encoding wild-type GP-C (lane d), or the alanine-scanning mutant A256 or A257 (lanes d and e). Separation of proteins from the lysed cells was performed as described for Fig. 1. Immunoblotting was done on a nitrocellulose membrane using anti-GP477 and immunodetection material as indicated for Fig. 1. (B) Summary of GP-C cleavage of alanine-scanning mutants. Amino acids at positions 248 to 263 (of Josiah GP-C) were individually mutated to alanine. GP-C and its mutants were expressed by the MVA-T7 system. Cleavage is indicated by the GP-2 bands obtained after SDS-PAGE and immunostaining using anti-GP477. (C) Summary of GP-C cleavage of mutants with exchanged amino acids within the consensus sequence. Arginine at position 256 and leucine at positions 258 and 259 were individually mutated to the indicated amino acids, and GP-C was expressed in the MVA-T7 system. Cleavage is indicated by the GP-2 bands obtained after SDS-PAGE and immunostaining using anti-GP477.
First, we analyzed 15 amino acids enclosing the cleavage region in an alanine-scanning experiment. The alanine mutants generated and the corresponding cleavage characteristics are summarized in Fig. 2B. Loss of cleavability was observed when the arginine at position 256 or both arginine residues at positions 256 and 257 and the leucine residues at positions 258 and 259 were individually replaced by alanine. Alanine mutations between positions 248 and 255 and between positions 260 and 262 had no effect on cleavage. To further investigate the requirements for a consensus motif, substitutions with different amino acids were introduced at critical sites (Fig. 2C). Substitution of arginine at position 256 with the basic amino acid lysine rendered GP-C uncleaved. Likewise, when leucine at position 259 was replaced by very closely related amino acids, such as isoleucine or valine, the cleavage motif was no longer recognized by the processing protease, whereas the neighboring amino acid (leucine at position 258) tolerated such substitutions. The amino acid at position 257 seems to be the most variable one of the tetrapeptide motif, because the replacement of the basic arginine by the uncharged, hydrophobic alanine has no effect on GP-C cleavage. The results of the mutational analyses of the cleavage site clearly show that an arginine at position P4, a leucine, isoleucine, or valine at position P2, and a leucine at position P1 are the crucial requirements for cleavage of the Lassa virus GP-C. These findings further support the cleavage of Lassa virus glycoprotein between L259 and G260, since cleavage of GP-C upstream of these residues seems highly unlikely due to the analogous recognition motifs and the cleavage sites of kexin and pyrolysin proteases.
Our data show that the cleavage site of Lassa virus GP-C is located between uncharged amino acids, while glycoproteins of a great number of other enveloped viruses are cleaved C-terminally to a basic amino acid residue, either at a cluster of lysine and arginine residues, at a single arginine, or, in rare cases, at a single lysine (20). The most common enzyme cleaving at clustered basic residues is furin, a member of the family of proprotein convertases, which cleaves at the recognition motif R-X-R/K-R (27, 30). Interestingly, the consensus sequence for cleavage of the Lassa virus glycoprotein also possesses, like furin, an essential arginine residue in position P4 and a variable amino acid in the P3 position. But in contrast to the cleavage motif of furin substrates, the Lassa glycoprotein cleavage motif definitely requires leucine in position P1 and an amino acid with a bulky aliphatic side chain in position P2.
Comparison of the GP-C cleavage sites of different Lassa virus isolates and the Central African Mopeia virus reveals that the tetrapeptide RRLL is highly conserved, indicating that the cleavage site and the motif presented here for the Lassa virus strain Josiah seems to be relevant for African arenaviruses in general (Fig. 3). The glycoproteins of LCMV and the New World arenaviruses have similar tetrapeptides with an arginine residue in position P4 that might also be recognition signals for cleavage. The observation that LCMV strain Armstrong has the tetrapeptide RRLA at the presumptive cleavage site, whereas Lassa virus GP-C did not tolerate an alanine in the P1 position, suggests that the LCMV glycoproteins might be cleaved by a different yet related enzyme. The tetrapeptide located in the cleavage region of the South American arenaviruses Junin and Pichinde complies with the consensus motif identified for Lassa virus strain Josiah (Fig. 3).
The cellular proteases processing the glycoproteins of arenaviruses have not been identified yet. Cleavage of the Lassa virus GP-C by furin can be excluded by the differing consensus sequence and by the lack of effect of the furin-specific inhibitor decanoylated Arg-Val-Lys-Arg chloromethylketone on the cleavage of GP-C (data not shown). The cleavage motif described here for Lassa virus will help to identify these enzymes. It was recently reported that the hamster protease S1p, an essential enzyme in the cholesterol regulation pathway (11), and the human subtilisin kexin isoenzyme 1 (SKI-1) (28) recognize the cleavage site RRLL and other sequences similar to the consensus motif described here. Studies to elucidate the role of SKI-1 for the cleavage of Lassa virus GP-C are already in progress. The elucidation of the cleavage motif of Lassa virus offers the possibility to design substrate analogues that block the cleavage of GP-C. Such protease inhibitors will be efficient tools to throw light on the biological significance of the cleavage of the Lassa virus glycoprotein for virus replication and pathogenesis. They may also have therapeutic potential for treatment of the often devastating haemorrhagic fever caused by Lassa virus and other closely related arenaviruses.
Acknowledgments
We thank S. Becker for help in the BSL-4 facility. MVA-T7 and the pTM1 vector were a generous gift of R. Sutter (Institut für Molekulare Virologie, GSF-Forschungszentrum, Oberschleissheim, Germany). Oligopeptides were synthesized and kindly provided by M. Krause (Institut für Molekularbiologie und Tumorforschung, Philipps-Universität Marburg, Marburg, Germany).
This work was supported by the Deutsche Forschungsgemeinschaft (SFB286), the Fonds der Chemischen Industrie, and the European Community (INCO-grant ERBIC 18 CT9803832).
REFERENCES
- 1.Auperin D D, Romanowski V, Galinski M, Bishop D H. Sequencing studies of Pichinde arenavirus S RNA indicate a novel coding strategy, an ambisense viral S RNA. J Virol. 1984;52:897–904. doi: 10.1128/jvi.52.3.897-904.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auperin D D, Sasso D R, McCormick J B. Nucleotide sequence of the glycoprotein gene and intergenic region of the Lassa virus S genome RNA. Virology. 1986;154:155–167. doi: 10.1016/0042-6822(86)90438-1. [DOI] [PubMed] [Google Scholar]
- 3.Becker S, Klenk H D, Mühlberger E. Intracellular transport and processing of the Marburg virus surface protein in vertebrate and insect cells. Virology. 1996;225:145–155. doi: 10.1006/viro.1996.0582. [DOI] [PubMed] [Google Scholar]
- 4.Borrow P, Oldstone M B. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology. 1994;198:1–9. doi: 10.1006/viro.1994.1001. [DOI] [PubMed] [Google Scholar]
- 5.Bosch F X, Garten W, Klenk H D, Rott R. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of avian influenza viruses. Virology. 1981;113:725–735. doi: 10.1016/0042-6822(81)90201-4. [DOI] [PubMed] [Google Scholar]
- 6.Buchmeier M J, Southern P J, Parekh B S, Wooddell M K, Oldstone M B. Site-specific antibodies define a cleavage site conserved among arenavirus GP-C glycoproteins. J Virol. 1987;61:982–985. doi: 10.1128/jvi.61.4.982-985.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns J W, Buchmeier M J. Glycoproteins of the Arenaviruses. In: Salvato M S, editor. The Arenaviridae. New York, N.Y: Plenum Press; 1993. pp. 17–33. [Google Scholar]
- 8.Candurra N A, Damonte E B. Effect of inhibitors of the intracellular exocytic pathway on glycoprotein processing and maturation of Junin virus. Arch Virol. 1997;142:2179–2193. doi: 10.1007/s007050050234. [DOI] [PubMed] [Google Scholar]
- 9.Cao W, Henry M D, Borrow P, Yamada H, Elder J H, Ravkov E V, Nichol S T, Compans R W, Campbell K P, Oldstone M B. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 10.Castilla V, Mersich S E. Low-pH-induced fusion of Vero cells infected with Junin virus. Arch Virol. 1996;141:1307–1317. doi: 10.1007/BF01718832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng D, Espenshade P J, Slaughter C A, Jaen J C, Brown M S, Goldstein J L. Secreted site-1 protease cleaves peptides corresponding to luminal loop of sterol regulatory element-binding proteins. J Biol Chem. 1999;274:22805–22812. doi: 10.1074/jbc.274.32.22805. [DOI] [PubMed] [Google Scholar]
- 12.Clegg J C, Wilson S M, Oram J D. Nucleotide sequence of the S RNA of Lassa virus (Nigerian strain) and comparative analysis of arenavirus gene products. Virus Res. 1991;18:151–164. doi: 10.1016/0168-1702(91)90015-n. [DOI] [PubMed] [Google Scholar]
- 13.Dettenhofer M, Yu X F. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J Virol. 1999;73:1460–1467. doi: 10.1128/jvi.73.2.1460-1467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Simone C, Buchmeier M J. Kinetics and pH dependence of acid-induced structural changes in the lymphocytic choriomeningitis virus glycoprotein complex. Virology. 1995;209:3–9. doi: 10.1006/viro.1995.1225. [DOI] [PubMed] [Google Scholar]
- 15.Di Simone C, Zandonatti M A, Buchmeier M J. Acidic pH triggers LCMV membrane fusion activity and conformational change in the glycoprotein spike. Virology. 1994;198:455–465. doi: 10.1006/viro.1994.1057. [DOI] [PubMed] [Google Scholar]
- 16.Ghiringhelli P D, Rivera-Pomar R V, Lozano M E, Grau O, Romanowski V. Molecular organization of Junin virus S RNA: complete nucleotide sequence, relationship with other members of the Arenaviridae and unusual secondary structures. J Gen Virol. 1991;72:2129–2141. doi: 10.1099/0022-1317-72-9-2129. [DOI] [PubMed] [Google Scholar]
- 17.Glushakova S E, Lukashevich I S, Baratova L A. Prediction of arenavirus fusion peptides on the basis of computer analysis of envelope protein sequences. FEBS Lett. 1990;269:145–147. doi: 10.1016/0014-5793(90)81140-j. [DOI] [PubMed] [Google Scholar]
- 18.Glushakova S E, Omelyanenko V G, Lukashevitch I S, Bogdanov A A, Jr, Moshnikova A B, Kozytch A T, Torchilin V P. The fusion of artificial lipid membranes induced by the synthetic arenavirus ‘fusion peptide’. Biochim Biophys Acta. 1992;1110:202–208. doi: 10.1016/0005-2736(92)90360-x. [DOI] [PubMed] [Google Scholar]
- 19.Guenther S, Emmerich P, Laue T, Kuehle O, Asper M, Jung A, Grewing T, ter Meulen J, Schmitz H. Imported Lassa fever in Germany: molecular characterization of the virus by full-length amplification and sequencing of the 3.5-kb S-RNA. Emerg Infect Dis. 2000;6:466–476. doi: 10.3201/eid0605.000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klenk H D, Garten W. Activation cleavage of viral spike proteins. In: Wimmer E, editor. Cellular receptors for animal viruses. Monograph 28. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 241–280. [Google Scholar]
- 21.Lukashevich I S, Lemeshko N N, Stelmakh T A, Golubev V P, Stcheslyenok E P. Some biochemical properties of Lassa virus RNA and polypeptides. Med Microbiol Immunol. 1986;175:73–77. doi: 10.1007/BF02122419. [DOI] [PubMed] [Google Scholar]
- 22.McCormick J B, King I J, Webb P A, Johnson K M, O'Sullivan R, Smith E S, Trippel S, Tong T C. A case-control study of the clinical diagnosis and course of Lassa fever. J Infect Dis. 1987;155:445–455. doi: 10.1093/infdis/155.3.445. [DOI] [PubMed] [Google Scholar]
- 23.McCormick J B, Webb P A, Krebs J W, Johnson K M, Smith E S. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis. 1987;155:437–444. doi: 10.1093/infdis/155.3.437. [DOI] [PubMed] [Google Scholar]
- 24.Riviere Y, Ahmed R, Southern P J, Buchmeier M J, Dutko F J, Oldstone M B. The S RNA segment of lymphocytic choriomeningitis virus codes for the nucleoprotein and glycoproteins 1 and 2. J Virol. 1985;53:966–968. doi: 10.1128/jvi.53.3.966-968.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romanowski V, Bishop D H. Conserved sequences and coding of two strains of lymphocytic choriomeningitis virus (WE and ARM) and Pichinde arenavirus. Virus Res. 1985;2:35–51. doi: 10.1016/0168-1702(85)90058-9. [DOI] [PubMed] [Google Scholar]
- 26.Romanowski V, Matsuura Y, Bishop D H. Complete sequence of the S RNA of lymphocytic choriomeningitis virus (WE strain) compared to that of Pichinde arenavirus. Virus Res. 1985;3:101–114. doi: 10.1016/0168-1702(85)90001-2. [DOI] [PubMed] [Google Scholar]
- 27.Seidah N G, Chretien M. Eukaryotic protein processing: endoproteolysis of precursor proteins. Curr Opin Biotechnol. 1997;8:602–607. doi: 10.1016/s0958-1669(97)80036-5. [DOI] [PubMed] [Google Scholar]
- 28.Seidah N G, Mowla S J, Hamelin J, Mamarbachi A M, Benjannet S, Toure B B, Basak A, Munzer J S, Marcinkiewicz J, Zhong M, Barale J C, Lazure C, Murphy R A, Chretien M, Marcinkiewicz M. Mammalian subtilisin/kexin isozyme SKI-1: a widely expressed proprotein convertase with a unique cleavage specificity and cellular localization. Proc Natl Acad Sci USA. 1999;96:1321–1326. doi: 10.1073/pnas.96.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Southern P J, Singh M K, Riviere Y, Jacoby D R, Buchmeier M J, Oldstone M B. Molecular characterization of the genomic S RNA segment from lymphocytic choriomeningitis virus. Virology. 1987;157:145–155. doi: 10.1016/0042-6822(87)90323-0. [DOI] [PubMed] [Google Scholar]
- 30.Steiner D F. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- 31.Sutter G, Ohlmann M, Erfle V. Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett. 1995;371:9–12. doi: 10.1016/0014-5793(95)00843-x. [DOI] [PubMed] [Google Scholar]
- 32.Wilson S M, Clegg J C. Sequence analysis of the S RNA of the African arenavirus Mopeia: an unusual secondary structure feature in the intergenic region. Virology. 1991;180:543–552. doi: 10.1016/0042-6822(91)90068-m. [DOI] [PubMed] [Google Scholar]
- 33.Wright K E, Spiro R C, Burns J W, Buchmeier M J. Post-translational processing of the glycoproteins of lymphocytic choriomeningitis virus. Virology. 1990;177:175–183. doi: 10.1016/0042-6822(90)90471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]