Abstract
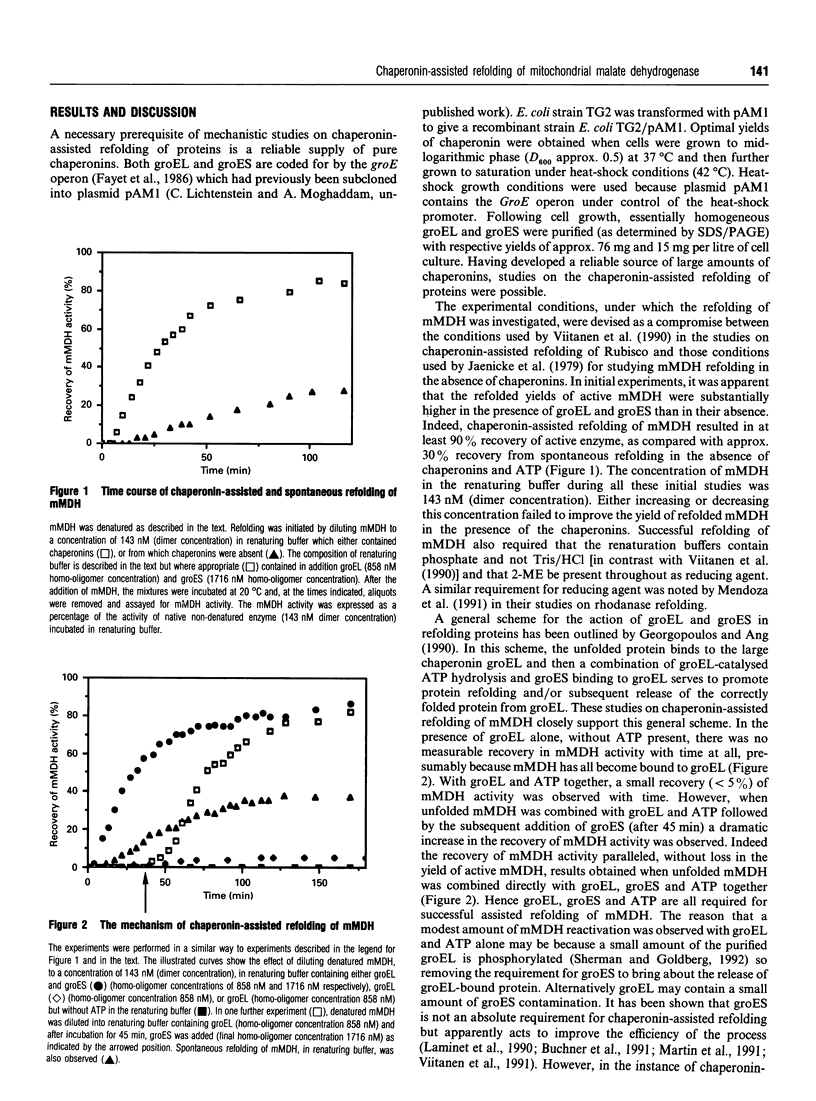
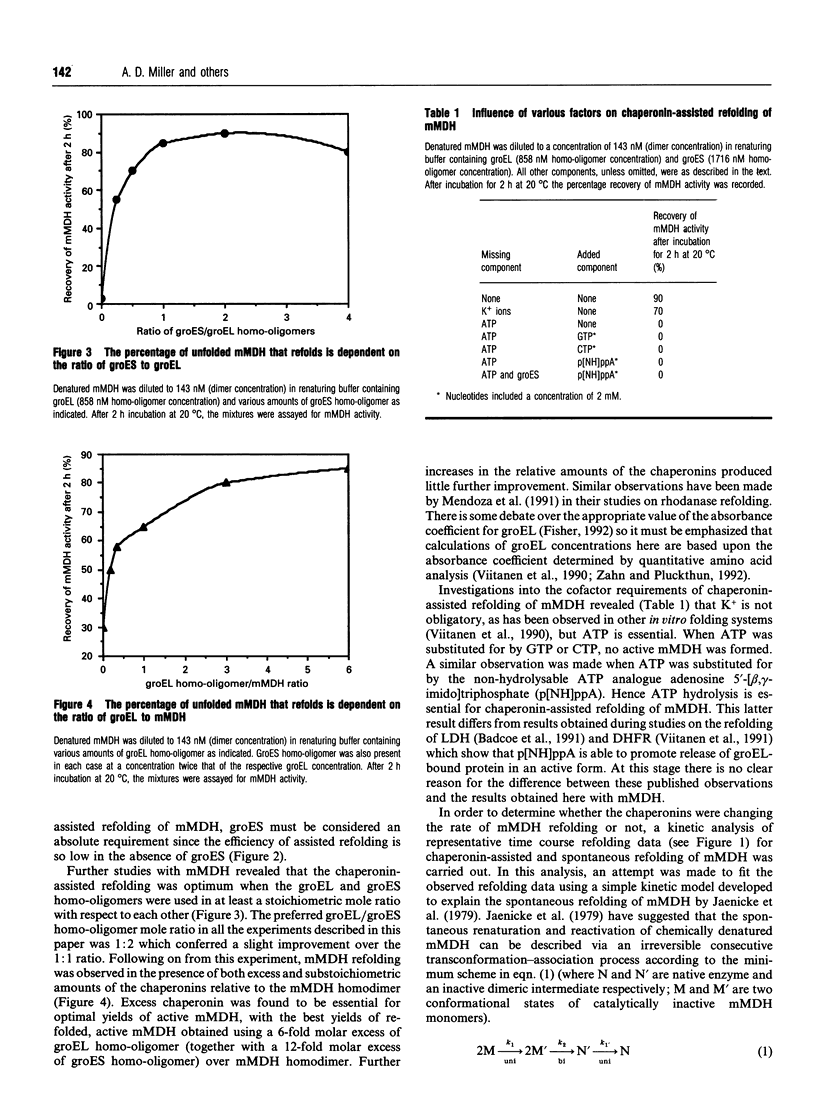
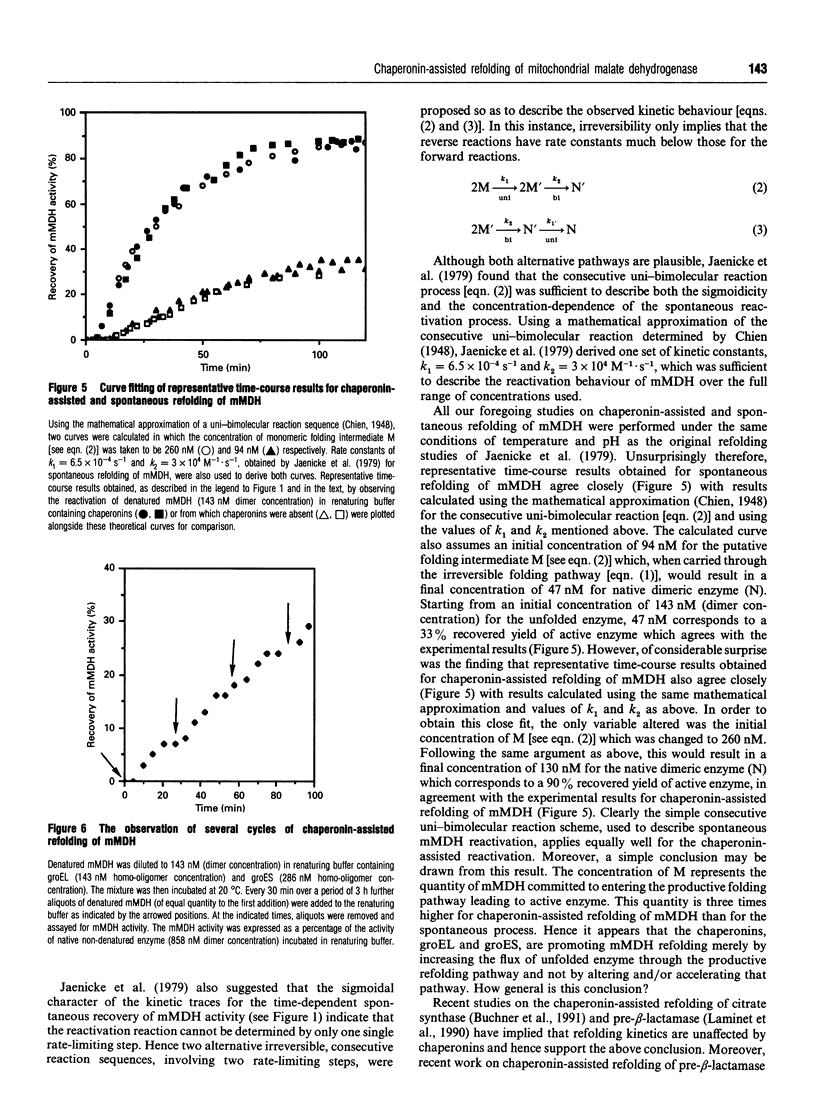
In vitro refolding of pig mitochondrial malate dehydrogenase is investigated in the presence and absence of Escherichia coli chaperonins cpn60 (groEL) and cpn10 (groES). The refolded yields of active malate dehydrogenase are increased almost 3-fold in the presence of groEL, groES, Mg2+/ATP and K+ ions. Chaperonin-assisted refolding of malate dehydrogenase does not have an absolute requirement for K+ ions but Mg2+/ATP is obligatory. When ATP is replaced by other nucleoside triphosphates, or by non-hydrolysable ATP analogues, assisted refolding is prevented. Optimal chaperonin-assisted refolding requires both groEL and groES homo-oligomers in molar excess over malate dehydrogenase. Kinetic analysis shows that the chaperonins do not catalyse the refolding of malate dehydrogenase but increase the flux of unfolded enzyme through the productive refolding pathway without altering and/or accelerating that pathway. Although not acting as refolding catalysts, the chaperonins are able to assist at least six consecutive cycles of malate dehydrogenase refolding.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badcoe I. G., Smith C. J., Wood S., Halsall D. J., Holbrook J. J., Lund P., Clarke A. R. Binding of a chaperonin to the folding intermediates of lactate dehydrogenase. Biochemistry. 1991 Sep 24;30(38):9195–9200. doi: 10.1021/bi00102a010. [DOI] [PubMed] [Google Scholar]
- Buchner J., Schmidt M., Fuchs M., Jaenicke R., Rudolph R., Schmid F. X., Kiefhaber T. GroE facilitates refolding of citrate synthase by suppressing aggregation. Biochemistry. 1991 Feb 12;30(6):1586–1591. doi: 10.1021/bi00220a020. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar G. N., Tilly K., Woolford C., Hendrix R., Georgopoulos C. Purification and properties of the groES morphogenetic protein of Escherichia coli. J Biol Chem. 1986 Sep 15;261(26):12414–12419. [PubMed] [Google Scholar]
- Ellis R. J., van der Vies S. M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Fayet O., Louarn J. M., Georgopoulos C. Suppression of the Escherichia coli dnaA46 mutation by amplification of the groES and groEL genes. Mol Gen Genet. 1986 Mar;202(3):435–445. doi: 10.1007/BF00333274. [DOI] [PubMed] [Google Scholar]
- Fisher M. T. Promotion of the in vitro renaturation of dodecameric glutamine synthetase from Escherichia coli in the presence of GroEL (chaperonin-60) and ATP. Biochemistry. 1992 Apr 28;31(16):3955–3963. doi: 10.1021/bi00131a010. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C., Ang D. The Escherichia coli groE chaperonins. Semin Cell Biol. 1990 Feb;1(1):19–25. [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Christeller J. T., Gatenby A. A., Lorimer G. H. Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfoleded state depends on two chaperonin proteins and Mg-ATP. Nature. 1989 Dec 21;342(6252):884–889. doi: 10.1038/342884a0. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Yost F. J., Jr, Rohrbach M. S., Harrison J. H. Selective chemical modification of malate dehydrogenase. N-ethylmaleimide modification of active center sulfhydryl residues. J Biol Chem. 1971 Sep 10;246(17):5491–5497. [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979 Apr 15;129(3):375–392. doi: 10.1016/0022-2836(79)90502-3. [DOI] [PubMed] [Google Scholar]
- Jaenicke R., Rudolph R., Heider I. Quaternary structure, subunit activity, and in vitro association of porcine mitochondrial malic dehydrogenase. Biochemistry. 1979 Apr 3;18(7):1217–1223. doi: 10.1021/bi00574a016. [DOI] [PubMed] [Google Scholar]
- Laminet A. A., Ziegelhoffer T., Georgopoulos C., Plückthun A. The Escherichia coli heat shock proteins GroEL and GroES modulate the folding of the beta-lactamase precursor. EMBO J. 1990 Jul;9(7):2315–2319. doi: 10.1002/j.1460-2075.1990.tb07403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Martin J., Langer T., Boteva R., Schramel A., Horwich A. L., Hartl F. U. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature. 1991 Jul 4;352(6330):36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- Mendoza J. A., Rogers E., Lorimer G. H., Horowitz P. M. Chaperonins facilitate the in vitro folding of monomeric mitochondrial rhodanese. J Biol Chem. 1991 Jul 15;266(20):13044–13049. [PubMed] [Google Scholar]
- Roderick S. L., Banaszak L. J. The three-dimensional structure of porcine heart mitochondrial malate dehydrogenase at 3.0-A resolution. J Biol Chem. 1986 Jul 15;261(20):9461–9464. [PubMed] [Google Scholar]
- Sherman MYu, Goldberg A. L. Heat shock in Escherichia coli alters the protein-binding properties of the chaperonin groEL by inducing its phosphorylation. Nature. 1992 May 14;357(6374):167–169. doi: 10.1038/357167a0. [DOI] [PubMed] [Google Scholar]
- THORNE C. J., KAPLAN N. O. Physicochemical properties of pig and horse heart mitochondrial malate dehydrogenase. J Biol Chem. 1963 May;238:1861–1868. [PubMed] [Google Scholar]
- Viitanen P. V., Donaldson G. K., Lorimer G. H., Lubben T. H., Gatenby A. A. Complex interactions between the chaperonin 60 molecular chaperone and dihydrofolate reductase. Biochemistry. 1991 Oct 8;30(40):9716–9723. doi: 10.1021/bi00104a021. [DOI] [PubMed] [Google Scholar]
- Viitanen P. V., Lubben T. H., Reed J., Goloubinoff P., O'Keefe D. P., Lorimer G. H. Chaperonin-facilitated refolding of ribulosebisphosphate carboxylase and ATP hydrolysis by chaperonin 60 (groEL) are K+ dependent. Biochemistry. 1990 Jun 19;29(24):5665–5671. doi: 10.1021/bi00476a003. [DOI] [PubMed] [Google Scholar]
- Zahn R., Plückthun A. GroE prevents the accumulation of early folding intermediates of pre-beta-lactamase without changing the folding pathway. Biochemistry. 1992 Mar 31;31(12):3249–3255. doi: 10.1021/bi00127a029. [DOI] [PubMed] [Google Scholar]
- van der Vies S. M., Viitanen P. V., Gatenby A. A., Lorimer G. H., Jaenicke R. Conformational states of ribulosebisphosphate carboxylase and their interaction with chaperonin 60. Biochemistry. 1992 Apr 14;31(14):3635–3644. doi: 10.1021/bi00129a012. [DOI] [PubMed] [Google Scholar]


