Abstract
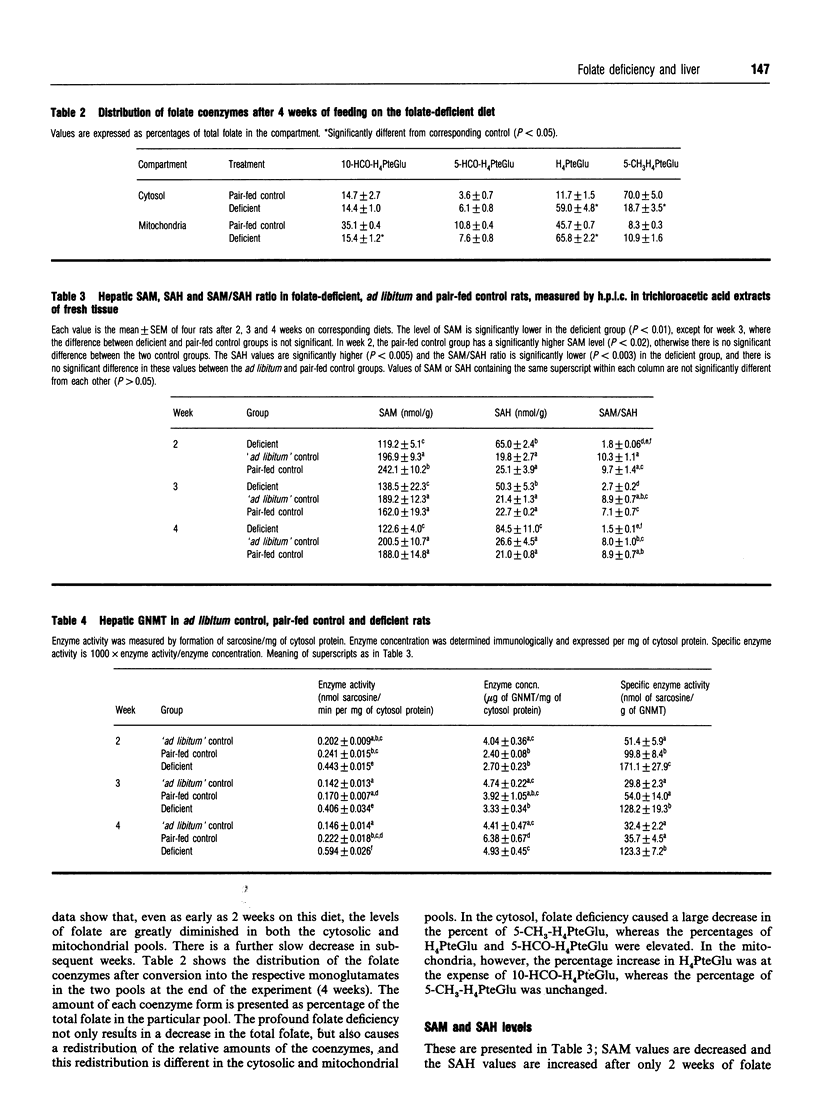
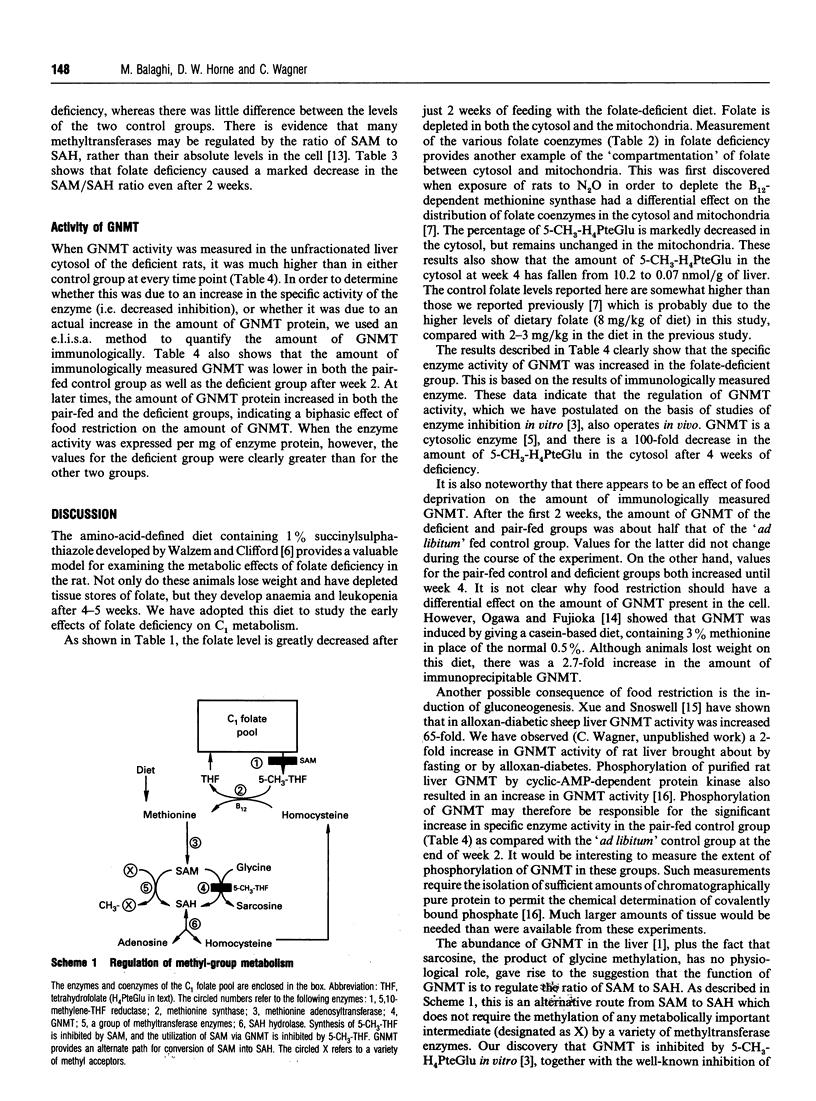
Glycine N-methyltransferase (GNMT) is inhibited by 5-methyltetrahydrofolate polyglutamate in vitro. It is believed to play a regulatory role in the synthesis de novo of methyl groups. We have used the amino-acid-defined diet of Walzem and Clifford [(1988) J. Nutr. 118, 1089-1096] to determine whether folate deficiency in vivo would affect GNMT activity, as predicted by the studies in vitro. Weanling male rats were fed on the folate-deficient diet or a folate-supplemented diet pair-fed to the deficient group. A third group was fed on the folate-supplemented diet ad libitum. Development of folate deficiency rapidly resulted in decreased levels of S-adenosylmethionine (SAM) and elevation of S-adenosylhomocysteine (SAH). The ratios of SAM to SAH were 1.8, 2.7 and 1.5 in the deficient group for weeks 2, 3 and 4 of the experiment, and the values were 9.7, 7.1 and 8.9 for the pair-fed control group and 10.3, 8.8 and 8.0 for the control group ad libitum fed. The activity of GNMT was significantly higher in the deficient group than in either of the two control groups at each time period. This was not due to increased amounts of GNMT protein, but reflected an increase in specific enzyme activity. Levels of folate in both the cytosol and mitochondria were severely lowered after only 2 weeks on the diet. The distribution of folate coenzymes was also affected by the deficiency, which resulted in a marked increase in the percentage of tetrahydrofolate polyglutamates in both cytosol and mitochondria and a very large decrease in cytosolic 5-methyltetrahydrofolate. The increased GNMT activity is therefore consistent with decreased folate levels and decreased inhibition of enzyme activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cook R. J., Blair J. A. The distribution and chemical nature of radioactive folates in rat liver cells and rat liver mitochondria. Biochem J. 1979 Mar 15;178(3):651–659. doi: 10.1042/bj1780651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. J., Horne D. W., Wagner C. Effect of dietary methyl group deficiency on one-carbon metabolism in rats. J Nutr. 1989 Apr;119(4):612–617. doi: 10.1093/jn/119.4.612. [DOI] [PubMed] [Google Scholar]
- Cook R. J., Wagner C. Glycine N-methyltransferase is a folate binding protein of rat liver cytosol. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3631–3634. doi: 10.1073/pnas.81.12.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein J. D. Methionine metabolism in mammals. J Nutr Biochem. 1990 May;1(5):228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- Heady J. E., Kerr S. J. Purification and characterization of glycine N-methyltransferase. J Biol Chem. 1973 Jan 10;248(1):69–72. [PubMed] [Google Scholar]
- Horne D. W., Patterson D., Cook R. J. Effect of nitrous oxide inactivation of vitamin B12-dependent methionine synthetase on the subcellular distribution of folate coenzymes in rat liver. Arch Biochem Biophys. 1989 May 1;270(2):729–733. doi: 10.1016/0003-9861(89)90556-0. [DOI] [PubMed] [Google Scholar]
- Kutzbach C., Stokstad E. L. Feedback inhibition of methylene-tetrahydrofolate reductase in rat liver by S-adenosylmethionine. Biochim Biophys Acta. 1967 May 16;139(1):217–220. doi: 10.1016/0005-2744(67)90140-4. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Fujioka M. Induction of rat liver glycine methyltransferase by high methionine diet. Biochem Biophys Res Commun. 1982 Sep 16;108(1):227–232. doi: 10.1016/0006-291x(82)91855-1. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L., Greenawalt J. W., Reynafarje B., Hullihen J., Decker G. L., Soper J. W., Bustamente E. Preparation and characterization of mitochondria and submitochondrial particles of rat liver and liver-derived tissues. Methods Cell Biol. 1978;20:411–481. doi: 10.1016/s0091-679x(08)62030-0. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Wagner C. Purification and characterization of a folate binding protein from rat liver cytosol. Arch Biochem Biophys. 1980 Jan;199(1):236–248. doi: 10.1016/0003-9861(80)90277-5. [DOI] [PubMed] [Google Scholar]
- Wagner C., Briggs W. T., Cook R. J. Inhibition of glycine N-methyltransferase activity by folate derivatives: implications for regulation of methyl group metabolism. Biochem Biophys Res Commun. 1985 Mar 29;127(3):746–752. doi: 10.1016/s0006-291x(85)80006-1. [DOI] [PubMed] [Google Scholar]
- Wagner C., Decha-Umphai W., Corbin J. Phosphorylation modulates the activity of glycine N-methyltransferase, a folate binding protein. In vitro phosphorylation is inhibited by the natural folate ligand. J Biol Chem. 1989 Jun 5;264(16):9638–9642. [PubMed] [Google Scholar]
- Wagner C. Folate-binding proteins. Nutr Rev. 1985 Oct;43(10):293–299. [PubMed] [Google Scholar]
- Walzem R. L., Clifford A. J. Folate deficiency in rats fed diets containing free amino acids or intact proteins. J Nutr. 1988 Sep;118(9):1089–1096. doi: 10.1093/jn/118.9.1089. [DOI] [PubMed] [Google Scholar]
- Xue G. P., Snoswell A. M. Disturbance of methyl group metabolism in alloxan-diabetic sheep. Biochem Int. 1985 Jun;10(6):897–905. [PubMed] [Google Scholar]


