ABSTRACT
Background
The importance of albuminuria as opposed to proteinuria in predicting kidney outcomes in primary immunoglobulin A nephropathy (IgAN) is not well established.
Methods
From 2010 to 2012, 421 patients with biopsy-proven IgAN have been enrolled into the German Chronic Kidney Disease (GCKD) cohort, a prospective observational cohort study (N = 5217). Adjudicated endpoints include a composite kidney endpoint (CKE) consisting of eGFR decline >40%, eGFR <15 ml/min/1.73 m2 and initiation of kidney replacement therapy; the individual components of the CKE; and combined major adverse cardiac events (MACE), including non-fatal myocardial infarction, non-fatal stroke and all-cause mortality. The associations between the incidence of CKE and baseline factors, including demographics, laboratory values and comorbidities were analysed using the Cox proportional hazards regression model.
Results
The mean age of IgAN patients at baseline was 51.6 years (± 13.6) and 67% were male. The patient-reported duration of disease at baseline was 5.9 ± 8.1 years. Baseline median urine albumin:creatinine ratio (UACR) was 0.4 g/g [interquartile range (IQR) 0.1–0.8] and mean eGFR was 52.5 ± 22.4 ml/min/1.73 m2. Over a follow-up of 6.5 years, 64 (15.2%) patients experienced a >40% eGFR decline, 3 (0.7%) reached eGFR <15 ml/min/1.73 m2, 53 (12.6%) initiated kidney replacement therapy and 28% of the patients experienced the CKE. Albuminuria, with reference to <0.1 g/g, was most associated with CKE. Hazard ratios (HRs) at UACRs of 0.1–0.6 g/g, 0.6–1.4 g/g, 1.4–2.2 g/g and >2.2 g/g were 2.03 [95% confidence interval (CI) 1.02–4.05], 3.8 (95% CI 1.92–7.5), 5.64 (95% CI 2.58–12.33) and 5.02 (95% CI 2.29–11-03), respectively. Regarding MACE, the presence of diabetes [HR 2.53 (95% CI 1.11–5.78)] was the most strongly associated factor, whereas UACR and eGFR did not show significant associations.
Conclusion
In the GCKD IgAN subcohort, more than every fourth patient experienced a CKE event within 6.5 years. Our findings support the use of albuminuria as a surrogate to assess the risk of poor kidney outcomes.
Keywords: albuminuria, CKD, glomerulonephritis, IgA nephropathy, proteinuria
KEY LEARNING POINTS.
What was known:
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis worldwide and poses a significant risk of kidney failure over a patient's lifetime.
Low-grade proteinuria (<1 g/day) has been recognized as prognostically relevant, with no benign course of IgAN in the presence of proteinuria.
The urine albumin:creatinine ratio (UACR) offers higher specificity and sensitivity to changes in glomerular permeability compared with traditional proteinuria measurements.
This study adds:
In the German Chronic Kidney Disease cohort, more than a quarter of patients with IgAN experienced a composite kidney endpoint event.
Albuminuria was the strongest predictor of poor kidney outcomes.
A substantial risk of kidney failure is already seen in patients with a UACR of 0.1–0.6 g/g.
Relapse after partial or complete remission was relatively uncommon, occurring in only 3.1% of the cohort.
Potential impact:
These findings support the use of albuminuria as a surrogate marker for assessing the risk of poor kidney outcomes in IgAN patients.
The study emphasizes the importance of targeting albuminuria reduction in the clinical management of IgAN to improve long-term kidney outcomes.
INTRODUCTION
Immunoglobulin A nephropathy (IgAN), the most common primary glomerulonephritis globally, presents a significant risk of kidney failure over a patient's lifetime [1]. The disease exhibits a remarkably heterogeneous course, ranging from mild forms with potential spontaneous remission to rapidly progressive forms [2, 3]. Overall, life expectancy is modestly reduced for patients with IgAN but is highly dependent on whether the disease progresses to kidney failure [4].
Prognostic assessment of IgAN currently mostly relies on the extent of proteinuria, estimated glomerular filtration rate (eGFR) and blood pressure (BP) control [5–10]. Increasing proteinuria is associated with an increased risk of both kidney failure and cardiovascular risk [11, 12]. There is now increasing evidence that even low-grade proteinuria (<1 g/day) is prognostically relevant and that there is no ‘benign course’ of IgAN as long as there is proteinuria [8, 13]. Recently, regulatory authorities have acknowledged the reduction in proteinuria as a surrogate endpoint for IgAN [14]. Traditionally, 24-h urine protein excretion (UPE) has been the standard for assessing proteinuria in randomized controlled trials (RCTs), but it is not without limitations [15]. Compared with proteinuria [either UPE or urine protein:creatinine ratio (UPCR)], the urine albumin:creatinine ratio (UACR) offers higher specificity and increased sensitivity to changes in glomerular permeability [16–19]. In two Chinese cohorts of patients with IgAN, UACR was found to better predict kidney failure compared with UPE and UPCR [20, 21].
In the present study, we analysed clinical data from patients with IgAN enrolled into the German Chronic Kidney Disease (GCKD) cohort, a national non-interventional cohort study with highly granular data and adjudicated endpoints. We sought to investigate their characteristics and outcomes and the association between albuminuria and the risk of cardiovascular and kidney outcomes.
MATERIALS AND METHODS
Study design and population
Between 2010 and 2012, the GCKD study enrolled 5217 participants of European ancestry ages 18–74 years under nephrological care with an eGFR of 30–60 ml/min/1.73 m2 (corresponding to CKD stages G3, A1–3) or an eGFR ≥60 ml/min/1.73 m2 in the presence of increased albuminuria in a spot urine sample (i.e. UACR >300 mg/g creatinine; corresponding to CKD stages G1–2, A3). The main exclusion criteria were non-European ancestry, active malignancy in the previous 2 years, previous transplantation, or heart failure (New York Heart Association class IV). All 421 participants with biopsy-proven IgAN as the leading cause of CKD were included in the analysis. It should be noted that the general inclusion criteria and methodology of the GCKD study were applicable to this specific IgAN cohort. The baseline date was defined as the first study visit. The median follow-up time after baseline was 6.5 years.
Every participant in the study gave written informed consent. The ethics committees of all nine German regional centres participating in the study approved the study. The study was carried out in accordance with approved guidelines and the Declaration of Helsinki. The study was registered in the German Clinical Trials Register (www.drks.de; DRKS00003971). The reporting guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology were followed [22].
Details of the study enrolment and follow-up procedures have been described previously [23]. At baseline and follow-up study visits, trained and certified personnel used standardized questionnaires to obtain information about each patient's medical history, sociodemographic and lifestyle factors and medication intake. Further information about medical history and additional medical records were obtained from the treating nephrologists. All clinical measurements were performed according to predefined standard operating procedures.
Hypertension was defined as either systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg or use of antihypertensive medication. Diabetes mellitus was defined as haemoglobin A1c ≥6.5% or the use of antidiabetic medication. Complete and partial remission were defined as UACR <0.1 g/g and UACR ≥0.1–<0.6 g/g, respectively. Relapse was defined as a single albuminuria value ≥0.6 g/g after any remission (complete or partial). Ever-smoker was defined as currently smoking or having smoked in the past. At baseline and follow-up visits, biomaterials including serum, plasma and urine were collected and transported frozen to a central biobank for future analyses following standard operating procedures. Blood and urine specimens were analysed in a central certified laboratory. Serum creatinine was analysed using an isotope dilution mass spectrometry traceable methodology (Creatinine Plus, Roche Diagnostics, Rotkreuz, Switzerland). Measures of kidney function included eGFR calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula and UACR.
Outcomes
Endpoints were continuously recorded based on hospital discharge letters and death certificates and were centrally adjudicated by experienced physicians. For this project, we included endpoints occurring over the first 6.5 years of follow-up. Adjudicated endpoints included a composite kidney endpoint (CKE) consisting of eGFR decline >40%, eGFR <15 ml/min/1.73 m2 and initiation of kidney replacement therapy, the individual components of the CKE and combined major adverse cardiac events (MACE), including non-fatal myocardial infarction, non-fatal stroke and all-cause mortality. All events until February 2022 (data export) were taken into account for the analysis.
Statistical analysis
Continuous variables were expressed as means and standard deviations (SDs) in case of normally distributed variables or medians with interquartile ranges (IQRs) for non-normally distributed variables. Categorical variables were expressed as numbers with percentages. The eGFR slopes were evaluated in linear mixed effects models. Repeated measurements of a participant were included by random intercept and random slope term (i.e. random effects). The random intercept allows a participant-specific eGFR at baseline and the random slope reflects a participant-specific change in eGFR over time. All data were collected and managed using Askimed as a cloud-based web platform (https://www.askimed.com). Data extraction from Askimed was performed in February 2022. To identify determinants of eGFR levels, we used linear regression modelling, including the following baseline covariates: age, gender, body mass index (BMI), diabetes, systolic BP and UACR in categories as time dependent variables and presented the estimate (β) with 95% CIs.
Time-to-event outcomes were defined as follows: If patients failed to complete the 6-year follow-up period, censoring was performed at the time of the last follow-up, e.g. when participants were lost to follow-up or refused to further participate in the study. The Cox regression analyses for the specific outcomes (CKE and MACE) were conducted with a competing risks approach using cause-specific Cox regression. Confounding risk factors at baseline included age, sex, BMI, diabetes, systolic BP, eGFR (using the CKD-EPI 2009 equation) and as time-dependent UACR in categories. Estimates are presented as HRs with 95% CIs. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Characteristics of the study population
The mean age at baseline was 51.6 ± 13.6 years and 67% (n = 282) were men (Table 1). The mean patient reported duration of the disease before enrolment was 5.9 ± 8.1 years. At baseline, the median UACR was 0.4 ± 0.1 g/g and the mean eGFR was 52.5 ± 22.4 ml/min/1.73 m2. Approximately 28% of the patients were in CKD stage 1 or 2 at baseline, 61.9% in CKD stage 3 and 10% in CKD stage 4 or 5. Regarding albuminuria, only 26.1% of patients had a UACR <0.1 g/g, while 60% had a UACR of 0.1–1.4 g/g and ≈13% had a UACR >1.4 g/g. Among the patients, 13.5% had diabetes at baseline and nearly all had hypertension (98%). At baseline, 92% of the patients were on therapy with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker and 15.6% were on dual treatment. A total of 15% received corticosteroids, while 6% were receiving immunosuppressive treatment other than steroids, with azathioprine being the most common. Systolic BP was 134 ± 17.3 mmHg and diastolic BP was 81.3 ± 11.2 mmHg.
Table 1:
Demographics and clinical parameters at baseline.
| UACR categories (baseline) | |||||
|---|---|---|---|---|---|
| Variable | Total (N = 421) |
≥0–<0.1 g/g [n = 110 (26.1%)] | ≥0.1–<0.6 g/g [n = 158 (37.5%)] | ≥0.6–<1.4 g/g [n = 99 (23.5%)] | ≥1.4 g/g [n = 54 (12.9%)] |
| Sex, n (%) | |||||
| Male | 282 (67) | 67 (61) | 118 (75) | 61 (62) | 36 67) |
| Female | 139 (33) | 43 (39) | 40 (25) | 38 (38) | 18 (33) |
| Age (years), mean ± SD | 51.6 ± 13.6 | 57.0 ± 11.9 | 51.8 ± 13.2 | 48.2 ± 14.4 | 45.9 ± 12.9 |
| Duration of onset (years), mean ± SD | 5.9 ± 8.1 | 8.8 ± 10.7 | 5.4 ± 7.0 | 4.7 ± 6.3 | 7.7 ± 2.3 |
| BMI (kg/m2), mean ± SD | 28 ± 5.2 | 28.9 ± 5.8 | 27.4 ± 4.5 | 27.9 ± 5.3 | 27.9 ± 5.6 |
| Systolic BP (mmHg), mean ± SD | 134.4 ± 17.3 | 131.2 ± 17.3 | 133.4 ± 16.4 | 135.2 ± 17.3 | 142.3 ± 17.6 |
| Diastolic BP (mmHg), mean ± SD | 81.3 ± 11.2 | 77.8 ± 10.9 | 80.6 ± 11.1 | 83.2 ± 10.7 | 87.2 ± 10.0 |
| Smoking, n (%) | |||||
| Non-smoker | 177 (42.2) | 46 (42.2) | 73 (46.2) | 41 (41.4) | 17 (32.1) |
| Former smoker | 156 (37.2) | 46 (42.2) | 54 (34.2) | 36 (36.4) | 20 (37.7) |
| Current smoker | 86 (20.5) | 17 (15.6) | 31 (19.6) | 22 (22.2) | 16 (30.2) |
| eGFR (CKD-EPI) (ml/min/1.73 m2), mean ± SD | 52.5 ± 22.4 | 48.1 ± 14.8 | 53.5 ± 22.9 | 56.8 ± 28.2 | 50.4 ± 20.1 |
| eGFR category, n (%) | |||||
| G1: CKD-EPI ≥90 | 38 (9) | 1 (0.9) | 17 (10.8) | 17 (17.2) | 3 (5.6) |
| G2: CKD-EPI ≥60–<90 | 80 (19) | 26 (23.6) | 28 (17.8) | 14 (14.1) | 12 (22.2) |
| G3a: CKD-EPI ≥45–<60 | 103 (24.5) | 26 (23.6) | 40 (25.5) | 22 (22.2) | 15 (27.8) |
| G3b: CKD-EPI ≥30–<45 | 157 (37.4) | 49 (44.5) | 55(35.0) | 36 (36.4) | 17 (31.5) |
| G4: CKD-EPI ≥15–<30 | 41 (9.8) | 8 (7.3) | 17 (10.8) | 9 (9.1) | 7 (13.0) |
| G5: CKD-EPI <15 | 1 (0.2) | 0 | 0 | 1 (1.0) | 0 |
| Diabetes mellitus, n (%) | 57 (13.5) | 18 (31.6) | 23 (40.3) | 9 (15.8) | 7 (12.4) |
| Hypertension, n (%) | 413 (98.1) | 106 (25.7) | 157 (38.0) | 97 (23.5) | 53 (12.8) |
| CHD, n (%) | 29 (6.9) | 9 (31.0) | 12 (41.4) | 4 (13.8) | 4 (13.8) |
| Antihypertensive therapy, n (%) | 402 (96.4) | 104 (25.9) | 155 (38.6) | 91 (22.5) | 52 (12.9) |
| ACEi, n (%) | 246 (59) | 63 (25.6) | 91 (37.0) | 56 (22.8) | 36 (14.6) |
| ARB, n (%) | 205 (49.2) | 47 (22.9) | 88 (42.9) | 47 (22.9) | 23 (11.2) |
| Dihydropyridines (nifedipine-type), n (%) | 149 (35.7) | 44 (29.5) | 50 (33.6) | 29 (19.5) | 26 (17.4) |
| Diuretics, n (%) | 203 (48.7) | 57 (28.1) | 82 (40.4) | 44 (21.7) | 20 (9.6) |
| Thiazides, n (%) | 103 (24.7) | 24 (23.3) | 45 (43.7) | 26 (25.2) | 8 (7.8) |
| Loop diuretics, n (%) | 96 (23) | 33 (34.4) | 33 (34.4) | 19 (19.8) | 11 (11.4) |
| Aldosterone antagonists, n (%) | 14 (3.4) | 5 (35.7) | 6 (42.9) | 1 (7.1) | 2 (14.3) |
| Statins, n (%) | 186 (44.6) | 43 (23.1) | 74 (39.8) | 39 (21.0) | 30 (16.1) |
| Immunosuppressives, n (%) | 25 (6) | 7 (28.0) | 9 (36.1) | 5 (20.0) | 4 (16.0) |
| Glucocorticoids, n (%) | 63 (15.1) | 18 (28.6) | 14 (22.2) | 18 (28.6) | 13 (20.6) |
| Proton pump inhibitors, n (%) | 87 (20.9) | 30 (34.5) | 24 (27.6) | 21 (24.1) | 12 (13.8) |
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker; CHD: coronary heart disease.
Kidney outcomes
Over a follow-up of 6.5 years, 64 (15.2%) patients experienced a >40% eGFR decline, 3 (0.7%) reached an eGFR <15 ml/min, 53 (12.6%) initiated kidney replacement therapy and 28% experienced a CKE (Supplementary Table S1). Relapse after partial or complete remission was relatively uncommon, occurring in only 3.1% of the cohort.
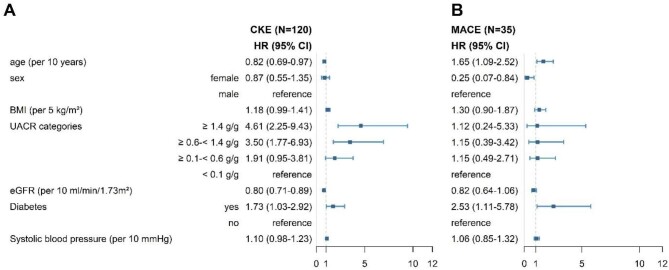
Albuminuria, with reference to <0.1 g/g was most strongly associated with CKE. HRs at a UACR ≥0.1–0.6 g/g, ≥0.6–1.4 g/g, ≥1.4–2.2 g/g and ≥2.2 g/g were 2.03 (95% CI 1.02–4.05), 3.8 (95% CI 1.92–7.5), 5.64 (95% CI 2.58–12.33) and 5.02 (95% CI 2.29–11-03), respectively (Fig. 1A and Table 2). Each 10 ml/min higher eGFR at baseline was found to be protective against CKE [HR 0.80 (95% CI 0.71–0.89)]. A history of diabetes was found to be associated with an increased HR of 1.73 (95% CI 1.03–2.92), while each 10 years older age was associated with a decreased risk of CKE [HR 0.82 (95% CI 0.69–0.97)].
Figure 1:
Forest plots of (A) CKE and (B) MACE showing HRs and 95% CIs.
Table 2:
Multivariate Cox model for predictors of CKE and MACE.
| Variables | CKE (n = 120/421), HR (95% CI) |
MACE (n = 35/421), HR (95% CI) |
|---|---|---|
| Age per 10-year increase (baseline) | 0.82 (0.69–0.97) | 1.65 (1.09–2.52) |
| Sex | ||
| Female | 0.87 (0.55–1.35) | 0.25 (0.07–0.84) |
| Male | Reference | Reference |
| BMI per 5 kg/m2 (baseline) increase | 1.18 (0.99–1.41) | 1.30 (0.90–1.87) |
| UACR categories, g/g | ||
| ≥1.4 | 4.61 (2.25–9.43) | 1.12 (0.24–5.33) |
| >0.6–<1.4 | 3.50 (1.77–6.93) | 1.15 (0.39–3.42) |
| ≥0.1–<0.6 | 1.91 (0.95–3.81) | 1.15 (0.49–2.71) |
| ≥0–<0.1 | Reference | Reference |
| eGFR per 10 ml/min/1.73 m2 (baseline) increase | 0.80 (0.71–0.89) | 0.82 (0.64–1.06) |
| Diabetes mellitus | ||
| Yes | 1.73 (1.03–2.92) | 2.53 (1.11–5.78) |
| No | Reference | Reference |
| Systolic BP (per 10 mmHg) increase | 1.10 (0.98–1.23) | 1.06 (0.85–1.32) |
Relationship between albuminuria, eGFR decline and kidney failure
The rate of eGFR decline was greater among patients with albuminuria >1.4 g/g, with an average decrease of 3.16 ml/min/1.73 m2/year (Fig. 2 and Supplementary Table S2). Conversely, the lowest rate of decline was observed in patients with a UACR <0.1 g/g, at 0.90 ml/min/1.73 m2/year, indicating rather stable kidney function over time. Intermediate rates of eGFR decline were noted in the UACR categories of 0.6–<1.4 g/g and 0.1–<0.6 g/g, with slopes of −2.34 and −1.49 ml/min/1.73 m2/year, respectively.
Figure 2:
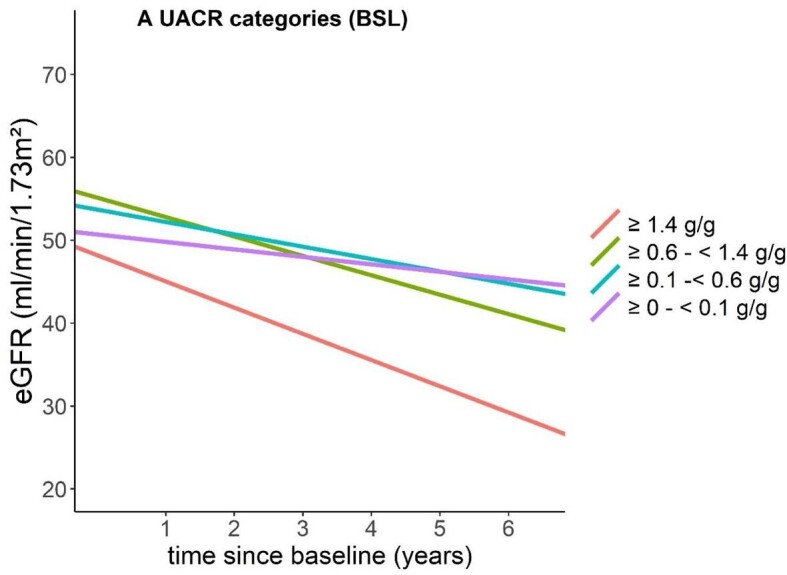
eGFR slopes according to UACR levels. Patients with higher baseline UACR levels (≥1.4 g/g) show a significantly steeper decline in eGFR over the 6-year follow-up period, indicating a greater risk of kidney function deterioration compared with those with lower UACR levels.
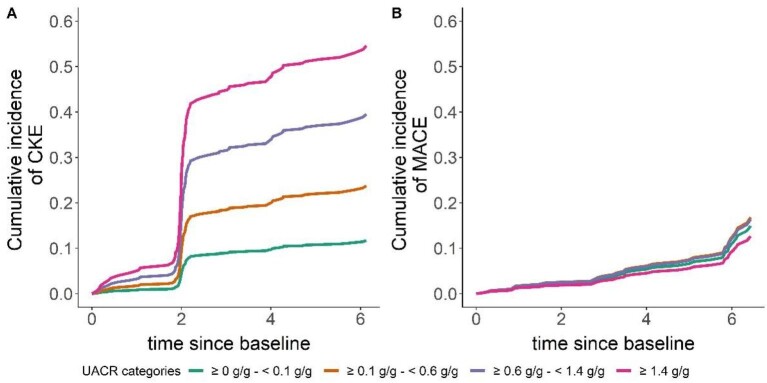
Albuminuria from baseline over the total follow-up was analysed as a categorical variable, with higher albuminuria associated with worse kidney survival. Nearly 40% of individuals with a UACR of 0.6–1.4 g/g experienced CKE over the specified period, while notably 15% of individuals with a UACR of 0.1–0.6 experienced CKE (Fig. 3, Table 3).
Figure 3:
Cumulative incidence of (A) CKE and (B) MACE according to baseline UACR levels. The sudden increases in CKE incidence at 2 years (and subsequent smaller steps) are likely due to the fact that changes in eGFR were assessed for the first time at 2 years and then subsequently at 4 and 6 years.
Table 3:
Cumulative incidence of CKE and MACE according to UACR categories.
| CKE, % | MACE, % | |||||||
|---|---|---|---|---|---|---|---|---|
| UACR categories BSL | ≥0–<0.1 g/g | ≥0.1–<0.6 g/g | ≥0.6–<1.4 g/g | ≥1.4 g/g | ≥0–<0.1 g/g | ≥0.1–<0.6 g/g | ≥0.6–<1.4 g/g | ≥1.4 g/g |
| FU2 | 4.77 | 10.10 | 17.93 | 26.64 | 2.12 | 2.42 | 2.35 | 1.78 |
| FU4 | 9.65 | 19.83 | 33.66 | 47.44 | 5.04 | 5.72 | 5.57 | 4.23 |
| FU6 | 11.25 | 22.89 | 38.29 | 53.07 | 5.04 | 5.72 | 5.57 | 4.23 |
BSL: baseline; FU2: follow-up at 2 years; FU4: follow-up at 4 years; FU6: follow-up at 6 years.
The percentages indicate the proportion of patients within each UACR category who experienced CKE or MACE during the specified follow-up periods.
Cardiovascular outcomes
During the follow-up period, 35 individuals experienced a first MACE (n = 19 patients with non-fatal myocardial infraction or stroke, n = 16 with all-cause mortality) (Supplementary Table S3). A statistically significant association between age and MACE was found, where a 10-year increase in age was associated with a 1.65-fold higher hazard (95% CI 1.09–2.52) (Table 2, Fig. 1B). Additionally, the presence of diabetes was significantly associated with a 2.53-fold higher hazard of MACE (95% CI 1.11–5.78). Female gender was associated with a significantly lower hazard of MACE [HR 0.25 (95% CI 0.07–0.84)]. Other variables such as BMI, UACR, BP and eGFR did not show statistically significant associations with MACE in the analysis. Notably, ≈5% of individuals with a UACR of 0.6–1.4 g/g experienced MACE during the study period (Fig. 3, Table 3).
DISCUSSION
The present study explored the clinical characteristics and outcomes of patients diagnosed with IgAN within the GCKD cohort. Over a 6.5-year follow-up period, 28% of patients with IgAN experienced a CKE. Relapse after partial or complete remission was relatively uncommon, occurring in only 3.1% of the cohort, suggesting that once remission is achieved, the risk of a significant increase in albuminuria remains low. Albuminuria was the strongest predictor of kidney failure, with higher levels correlated with increased risk.
Regarding relative risks, our findings indicate that compared with a UACR <0.1 g/g, any higher UACR level was independently associated with a greater rate of CKE. Specifically, we observed that patients with a UACR of 0.1–0.6 g/g had a 2.03-fold increased risk of experiencing a CKE. For those with a UACR of 0.6–1.4 g/g, the risk was 3.8 times higher, highlighting a significant linear association between elevated UACR levels and worse kidney outcomes. These findings are aligned with findings in 2 Chinese cohorts and a recently published Swedish cohort [8, 13, 24]. The recent Swedish study found a strong and incremental association between UACR and the risk of adverse kidney events. Conversely, as expected, higher baseline eGFR was protective. An eGFR decline was observed across all albuminuria categories, yet patients with <0.1 g/g of albuminuria demonstrated a more stable disease course, with a ‘physiological’ decline of <0.9 ml/min/1.73 m2/year.
Current phase 2 and 3 RCTs for IgAN use proteinuria as a surrogate endpoint, usually enrolling patients with baseline proteinuria >1 g/day (approximately equivalent to a UPCR of 0.88 g/g or a UACR of 0.6 g/g). Recently it was shown that ≈20% of patients with a time-averaged UPCR <0.44 g/g and 30% with a time-averaged UPCR of 0.44–<0.88 g/g progressed to kidney failure within 10 years of diagnosis [8]. Correspondingly, in our study, 15% of individuals with a UACR in the range of 0.1–0.6 g/g experienced CKE within <10 years of follow-up.
Regarding cardiovascular events and mortality, our analysis did not find any association between elevated UACR or lower eGFR. Research on the connection between proteinuria and cardiovascular events in IgAN is limited. A Canadian study reported a 10-year risk of 7.4%, with both proteinuria and lower eGFR linked to a higher incidence of cardiovascular events, though not specific to IgAN [25]. A recent US cohort study also found elevated proteinuria associated with higher cardiovascular disease risk and mortality [26]. One explanation could be our study's small sample size and the relatively short follow-up period of 6.5 years.
Currently, numerous new RCTs are investigating how to halt the progression of IgAN. This surge of RCTs is largely based on the recognition that a reduction of proteinuria in so-called high-risk patients is associated with improved outcomes and a slower rate of kidney function loss [14]. New drugs such as targeted release budesonide, sparsentan and potentially inhibitors targeting the APRIL (a proliferation-inducing ligand)/BAFF (B cell activating factor from the TNF family) signalling pathway, as well as complement activation, are undergoing evaluation, particularly in patients with high proteinuria [3, 27, 28]. However, there is a notable absence of IgAN patients with lower proteinuria levels in current RCTs. Based on our data, which suggest that renal risk continuously decreases with decreasing UACR, it will be important to study whether drugs that reduce high levels of proteinuria are also effective at reducing lower levels and if these effects translate to better kidney outcomes.
A limitation of our study is that patients were recruited into the study during their natural disease course and that data between recruitment into the cohort and disease diagnosis were not available. Second, despite the large size of the GCKD cohort, our sample size was relatively small, which may influence the statistical power of our results. Our analysis also did not include other clinical or histological parameters known to influence the progression of IgAN, such as the MEST-C score or the presence/absence of haematuria [29–31]. Additionally, given the fact that the pathophysiology of IgAN seems to be different between Asian and Caucasian populations, the fact that our study was limited to people with European ancestry may affect the generalizability of our findings. The participants were enrolled in Germany on the basis of prevalent non-dialysis-dependent CKD, thus there is a selection bias of participants owing to this defined study cohort. Further larger-scale studies with longer follow-up periods are warranted to validate our findings and further elucidate the factors influencing the prognosis of patients with IgAN. Third, we did not measure urine protein concentrations at baseline, which precludes a comparison with UACR.
The strengths of our analysis include that this cohort consists exclusively of patients treated by nephrologists, ensuring relatively standardized care. This minimizes potential cofounding variables arising from variations in medical management and highlights the clinical relevance of our results. Additionally, the GCKD cohort provides uniquely granular data related to patient demographics, medical history, treatment regimens and outcomes. Systematic endpoint adjudication further enhances outcome data accuracy and reduces bias. The use of standardized questionnaires to evaluate participants’ characteristics, in-person study visits conducted by trained study nurses, along with continuous evaluation of outcomes by experienced physicians following predefined criteria further enhances the reliability of our findings.
In summary, our study further supports the significant disease burden of IgAN. We confirm in a purely Caucasian population the critical importance of albuminuria as a modifiable risk factor in the management of IgAN. The need for targeted intervention in patients with elevated albuminuria is clear, as is the potential benefit of aggressive treatment strategies to maintain or improve renal function.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all the participants of the GCKD study. The enormous effort of the study personnel at the regional centres is highly appreciated. We also thank the large number of nephrologists for their support of the GCKD study (the list of nephrologists currently collaborating with the GCKD study is available at http://www.gckd.org).
Current GCKD Investigators and Collaborators with the GCKD Study: Kai-Uwe Eckardt, Heike Meiselbach, Markus P. Schneider, Mario Schiffer, Hans-Ulrich Prokosch, Barbara Bärthlein, Andreas Beck, André Reis, Arif B. Ekici, Susanne Becker, Ulrike Alberth-Schmidt, Sabine Marschall and Anke Weigel (University of Erlangen-Nürnberg); Gerd Walz, Anna Köttgen, Ulla T. Schultheiß, Fruzsina Kotsis, Simone Meder, Erna Mitsch and Ursula Reinhard (University of Freiburg); Jürgen Floege and Turgay Saritas (RWTH Aachen University); Elke Schaeffner, Seema Baid-Agrawal and Kerstin Theisen (Charité – Universitätsmedizin Berlin); Kai Schmidt-Ott (Hannover Medical School); Martin Zeier, Claudia Sommerer and Mehtap Aykac (University Hospital, Renal Center, Heidelberg); Gunter Wolf, Martin Busch and Andi Steiner (University Hospital Jena); Thomas Sitter (Ludwig-Maximilians University of München); Christoph Wanner, Vera Krane and Britta Bauer (University of Würzburg); Florian Kronenberg, Julia Raschenberger, Barbara Kollerits, Lukas Forer, Sebastian Schönherr and Hansi Weissensteiner (Medical University of Innsbruck, Division of Genetic Epidemiology); Peter Oefner and Wolfram Gronwald (University of Regensburg, Institute of Functional Genomics); Matthias Schmid and Jennifer Nadal (Department of Medical Biometry, Informatics and Epidemiology, University Hospital of Bonn).
Contributor Information
Eleni Stamellou, Division of Nephrology and Clinical Immunology, RWTH Aachen University Hospital, Aachen, Germany; Department of Nephrology, School of Medicine, University of Ioannina, Ioannina, Greece.
Jennifer Nadal, Department of Medical Biometry, Informatics and Epidemiology, Faculty of Medicine, University Hospital Bonn, Bonn, Germany.
Bruce Hendry, Travere Therapeutics, San Diego, CA, USA.
Alex Mercer, JAMCO Pharma Consulting, Stockholm, Sweden.
Claudia Seikrit, Division of Nephrology and Clinical Immunology, RWTH Aachen University Hospital, Aachen, Germany.
Wibke Bechtel-Walz, Department of Medicine IV, University Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany; Berta‐Ottenstein Program, Faculty of Medicine, University of Freiburg, Freiburg, Germany.
Matthias Schmid, Department of Medical Biometry, Informatics and Epidemiology, Faculty of Medicine, University Hospital Bonn, Bonn, Germany.
Marcus J Moeller, Division of Nephrology and Clinical Immunology, RWTH Aachen University Hospital, Aachen, Germany.
Mario Schiffer, Department of Nephrology and Hypertension, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany.
Kai-Uwe Eckardt, Department of Nephrology and Hypertension, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany; Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
Rafael Kramann, Division of Nephrology and Clinical Immunology, RWTH Aachen University Hospital, Aachen, Germany.
Jürgen Floege, Division of Nephrology and Clinical Immunology, RWTH Aachen University Hospital, Aachen, Germany; Department of Cardiology, RWTH Aachen University Hospital, Aachen, Germany.
the GCKD study investigators:
Kai-Uwe Eckardt, Heike Meiselbach, Markus P Schneider, Mario Schiffer, Hans-Ulrich Prokosch, Barbara Bärthlein, Andreas Beck, André Reis, Arif B Ekici, Susanne Becker, Ulrike Alberth-Schmidt, Sabine Marschall, Anke Weigel, Gerd Walz, Anna Köttgen, Ulla T Schultheiß, Fruzsina Kotsis, Simone Meder, Erna Mitsch, Ursula Reinhard, Jürgen Floege, Turgay Saritas, Elke Schaeffner, Seema Baid-Agrawal, Kerstin Theisen, Kai Schmidt-Ott, Martin Zeier, Claudia Sommerer, Mehtap Aykac, Gunter Wolf, Martin Busch, Andi Steiner, Thomas Sitter, Christoph Wanner, Vera Krane, Britta Bauer, Florian Kronenberg, Julia Raschenberger, Barbara Kollerits, Lukas Forer, Sebastian Schönherr, Hansi Weissensteiner, Peter Oefner, Wolfram Gronwald, Matthias Schmid, and Jennifer Nadal
FUNDING
The GCKD study is supported by the German Ministry of Education and Research (Bundesministerium für Bildung und Forschung; FKZ 01ER 0804, 01ER 0818, 01ER 0819, 01ER 0820 and 01ER 0821), KfH Foundation for Preventive Medicine, Innovative Medicines Initiative 2 Joint Undertaking (BEAt-DKD; grant 115974) and corporate sponsors (www.gckd.org). J.F., E.S. and C.S. are funded by the Deutsche Forschungsgemeinschaft (German Research Foundation; KFO 5011, project 445703531) and are members of ERK-NET. E.S. is supported by a START grant (19/21) and by a clinician scientist program of the Faculty of Medicine of RWTH Aachen University; by the STOP-FSGS consortium of the German Ministry for Science and Education (STOP-FSGS-01GM2202C) and has received a research grant from the German Society of Nephrology. The analysis received funding from Travere Therapeutics.
AUTHORS’ CONTRIBUTIONS
J.F., B.H. and A.M. were responsible for conceptualization. W.B.-W., M.S. and K.-U.E. were responsible for data curation/formal analysis of the GCKD cohort. E.S., J.N., C.S., J.F., M.S., A.M., B.H. and M.J.M. were responsible for the investigation. J.N., J.F., M.S., A.M., B.H. and M.J.M. were responsible for the methodology. J.F. was responsible for supervision. E.S. and J.N. wrote the original draft. J.F., A.M., B.H., M.J.M., M.S., W.B.-W., M.S., K.-U.E. and R.K. were responsible for writing, review and editing of the manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its online supplementary material.
CONFLICT OF INTEREST STATEMENT
J.F. is the Editor-in-Chief of CKJ.
REFERENCES
- 1. McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 2011;26:414–30. 10.1093/ndt/gfq665 [DOI] [PubMed] [Google Scholar]
- 2. Berthoux F, Mohey H, Laurent B et al. Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 2011;22:752–61. 10.1681/ASN.2010040355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stamellou E, Seikrit C, Tang SCW et al. IgA nephropathy. Nat Rev Dis Primers 2023;9:67. 10.1038/s41572-023-00476-9 [DOI] [PubMed] [Google Scholar]
- 4. Jarrick S, Lundberg S, Welander A et al. Mortality in IgA nephropathy: a nationwide population-based cohort study. J Am Soc Nephrol 2019;30:866–76. 10.1681/ASN.2018101017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inker LA, Mondal H, Greene T et al. Early change in urine protein as a surrogate end point in studies of IgA nephropathy: an individual-patient meta-analysis. Am J Kidney Dis 2016;68:392–401. 10.1053/j.ajkd.2016.02.042 [DOI] [PubMed] [Google Scholar]
- 6. Reich HN, Troyanov S, Scholey JW et al. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 2007;18:3177–83. 10.1681/ASN.2007050526 [DOI] [PubMed] [Google Scholar]
- 7. Le W, Liang S, Hu Y et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant 2012;27:1479–85. 10.1093/ndt/gfr527 [DOI] [PubMed] [Google Scholar]
- 8. Pitcher D, Braddon F, Hendry B et al. Long-term outcomes in IgA nephropathy. Clin J Am Soc Nephrol 2023;18:727–38. 10.2215/CJN.0000000000000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bartosik LP, Lajoie G, Sugar L et al. Predicting progression in IgA nephropathy. Am J Kidney Dis 2001;38:728–35. 10.1053/ajkd.2001.27689 [DOI] [PubMed] [Google Scholar]
- 10. Goto M, Wakai K, Kawamura T et al. A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospective cohort study. Nephrol Dial Transplant 2009;24:3068–74. 10.1093/ndt/gfp273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wagner DK, Harris T, Madans JH. Proteinuria as a biomarker: risk of subsequent morbidity and mortality. Environ Res 1994;66:160–72. 10.1006/enrs.1994.1052 [DOI] [PubMed] [Google Scholar]
- 12. Kannel WB, Stampfer MJ, Castelli WP et al. The prognostic significance of proteinuria: the Framingham study. Am Heart J 1984;108:1347–52. 10.1016/0002-8703(84)90763-4 [DOI] [PubMed] [Google Scholar]
- 13. Knoop T, Vikse BE, Mwakimonga A et al. Long-term outcome in 145 patients with assumed benign immunoglobulin A nephropathy. Nephrol Dial Transplant 2017;32:1841–50. 10.1093/ndt/gfx242 [DOI] [PubMed] [Google Scholar]
- 14. Thompson A, Carroll K, Inker LA et al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol 2019;14:469–81. 10.2215/CJN.08600718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harrison TG, Tonelli M. Measuring albuminuria or proteinuria: does one answer fit all? Kidney Int 2023;104:904–9. 10.1016/j.kint.2023.08.008 [DOI] [PubMed] [Google Scholar]
- 16. Levin A, Stevens PE. Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 2014;85:49–61. 10.1038/ki.2013.444 [DOI] [PubMed] [Google Scholar]
- 17. Ballantyne FC, Gibbons J, O'Reilly DS. Urine albumin should replace total protein for the assessment of glomerular proteinuria. Ann Clin Biochem 1993;30:101–3. 10.1177/000456329303000119 [DOI] [PubMed] [Google Scholar]
- 18. Newman DJ, Thakkar H, Medcalf EA et al. Use of urine albumin measurement as a replacement for total protein. Clin Nephrol 1995;43:104–9. [PubMed] [Google Scholar]
- 19. Shihabi ZK, Konen JC, O'Connor ML. Albuminuria vs urinary total protein for detecting chronic renal disorders. Clin Chem 1991;37:621–4. 10.1093/clinchem/37.5.621 [DOI] [PubMed] [Google Scholar]
- 20. Yu G, Cheng J, Li H et al. Comparison of 24-h urine protein, urine albumin-to-creatinine ratio, and protein-to-creatinine ratio in IgA nephropathy. Front Med (Lausanne) 2022;9:809245. 10.3389/fmed.2022.809245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao YF, Zhu L, Liu LJ et al. Measures of urinary protein and albumin in the prediction of progression of IgA nephropathy. Clin J Am Soc Nephrol 2016;11:947–55. 10.2215/CJN.10150915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. von Elm E, Altman DG, Egger M et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eckardt KU, Barthlein B, Baid-Agrawal S et al. The German Chronic Kidney Disease (GCKD) study: design and methods. Nephrol Dial Transplant 2012;27:1454–60. 10.1093/ndt/gfr456 [DOI] [PubMed] [Google Scholar]
- 24. Faucon AL, Lundberg S, Lando S et al. Albuminuria predicts kidney events in IgA nephropathy. Nephrol Dial Transplant 2024;gfae085. 10.1093/ndt/gfae085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Canney M, Gunning HM, Zheng Y et al. The risk of cardiovascular events in individuals with primary glomerular diseases. Am J Kidney Dis 2022;80:740–50. 10.1053/j.ajkd.2022.04.005 [DOI] [PubMed] [Google Scholar]
- 26. Lerma EV, Thakker KM, Bensink ME et al. Kidney failure events, cardiovascular disease events, and all-cause mortality in patients with IgA nephropathy in a real-world database. Kidney360 2024;5:427–36. 10.34067/KID.0000000000000379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barratt J, Lafayette R, Kristensen J et al. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int 2023;103:391–402. 10.1016/j.kint.2022.09.017 [DOI] [PubMed] [Google Scholar]
- 28. Rovin BH, Barratt J, Heerspink HJL et al. Efficacy and safety of sparsentan versus irbesartan in patients with IgA nephropathy (PROTECT): 2-year results from a randomised, active-controlled, phase 3 trial. Lancet 2023;402:2077–90. 10.1016/S0140-6736(23)02302-4 [DOI] [PubMed] [Google Scholar]
- 29. Barbour SJ, Espino-Hernandez G, Reich HN et al. The MEST score provides earlier risk prediction in IgA nephropathy. Kidney Int 2016;89:167–75. 10.1038/ki.2015.322 [DOI] [PubMed] [Google Scholar]
- 30. Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis 1997;29:829–42. 10.1016/S0272-6386(97)90456-X [DOI] [PubMed] [Google Scholar]
- 31. Nozawa R, Suzuki J, Takahashi A et al. Clinicopathological features and the prognosis of IgA nephropathy in Japanese children on long-term observation. Clin Nephrol 2005;64:171–9. 10.5414/CNP64171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.