Abstract
The overuse of antibiotics has caused the emergence of antibiotic-resistant strains, such as multidrug-resistant, extensively drug-resistant, and pandrug-resistant bacteria. The treatment of infections caused by such strains has become a formidable challenge. In the post-antibiotic era, phage therapy is an attractive solution for this problem and some successful phase 1 and 2 studies have demonstrated the efficacy and safety of phage therapy over the last decade. It is a form of evolutionary medicine, phages exhibit immunomodulatory and anti-inflammatory properties. However, phage therapy is limited by factors, such as the narrow spectrum of host strains, the special pharmacokinetics and pharmacodynamics in vivo, immune responses, and the development of phage resistance. The aim of this minireview was to compare the potencies of lytic phages and chemical antibiotics to treat bacterial infections. The advantages of phage therapy has fewer side effects, self-replication, evolution, bacterial biofilms eradication, immunomodulatory and anti-inflammatory properties compared with chemical antibiotics. Meanwhile, the disadvantages of phage therapy include the narrow spectrum of available host strains, the special pharmacokinetics and pharmacodynamics in vivo, immune responses, and phage resistance hurdles. Recently, some researchers continue to make efforts to overcome these limitations of phage therapy. Phage therapy will be a welcome addition to the gamut of options available for treating antibiotic-resistant bacterial infections. We focus on the advantages and limitations of phage therapy with the intention of exploiting the advantages and overcoming the limitations.
Keywords: Bacteria, Antimicrobial resistance, Phage, Lytic phage, Phage therapy, Antibiotic
1. Introduction
One of the greatest medical breakthroughs of the 20th century was the introduction of antibiotics into clinical medicine. Unfortunately, their widespread use has led to the rapid emergence of antimicrobial resistance [1], which is currently regarded as one of the biggest threats to human health, especially multidrug-resistant pathogens, such as Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterococcus faecium, and Staphylococcus aureus [2]. Bacterial infection is a common clinical disease that breaches the innate immune barriers or uncoordinates adaptive immune response. The pathogenicity of bacterial infection depends on the ability of bacterial adhesion, invasion and toxins [3]. When bacteria invade the body, they grow, multiply, and release toxins and affect a variety of organs and tissues, including skin, mucosa, gut, lungs, heart, brain, blood and so on. Antimicrobial-resistant bacterial infection is predicted to be the leading cause of death by the year 2050 if no action is taken [4]. The development of new antibiotics is unable to keep up with the pace at which antibiotic resistance is increasing [5]. Only less than 20 % of the antibiotics undergoing phase I trials are estimated to be approved ultimately, while the cost behind their discovery and development is as high as $500 million [6]. All these factors have caused this research field to gradually decline [7]. Thus novel antimicrobials and strategies must be explored to tackle the crisis of growing antimicrobial resistance.
Phages are viruses that naturally predate on bacteria. They cohabitate the same environments as their prey, including the human body and our food and water sources [8]. There are two types of phages classified based on their life cycle. Temperate phages replicate often through a lysogenic cycle or sometimes through a lytic cycle. Lytic phages, also called virulent phages, replicate exclusively through a lytic cycle and trigger the death of the host bacteria, releasing many copies of themselves. The main difference between temperate and virulent phages is that virulent phages exclusively undergo the lytic cycle whereas temperate phages frequently undergo the lysogenic cycle. Using lytic phage therapy to treat bacterial infections is an old idea. The English microbiologist Frederick Twort [9] and the French–Canadian biomedical scientist Felix d’He'relle [10] discovered phages independently. Pioneered by Bruynoghe and Maisin in 1921 [11], the potential of phage therapy to treat infectious diseases was soon recognized. Bruynoghe and Maisin used phage to treat a skin infection patient successfully. In 1924, Smith used B. typhosus phage to explore the treatment outcome of seven typhoid fever cases. After the administration of phages, the temperature of five patients then subsided, and the examination results of the faeces were negative with B. typiosus [12]. However, the discovery of antibiotics and the outbreak of World War II stunted its development and application. Currently, clinical and public health is seriously threatened by the emergence of antibiotic-resistant bacteria [13], especially multidrug-resistant Gram-negative bacteria, such as the third-generation cephalosporin-resistant and carbapenem-resistant Enterobacteriaceae and carbapenem-resistant A. baumannii and P. aeruginosa [14]. Furthermore, phages do not lyse human cells in vitro [15]. They can specifically and effectively kill almost any bacteria without affecting the normal bacterial flora. The killing mechanisms include adsorption, nucleic acid injection, virion assembly, virion release and further transmission [16]. Moreover, they can be easily isolated and cheaply cultivated. Consequently, interest in phage therapy to treat bacterial infections has rejuvenated in the last 20 years.
The main aim of this mini-review was to compare the use of lytic phages and chemical antibiotics for treating bacterial infections. We focus on the advantages and limitations of phage therapy with the intention of exploiting the advantages and overcoming the limitations. Phage therapy is associated with distinct advantages. It is aligned with the concept of evolutionary medicine. Moreover, it modulates the immune system and regulates anti-inflammatory responses. Some successful phase 1 and 2 trials over the last decade have testified to its efficacy (Table 1). The disadvantages of phage therapy include the narrow spectrum of available host strains, the special pharmacokinetics and pharmacodynamics in vivo, immune responses, and phage resistance hurdles.
Table 1.
Summary of the last five years phage therapy clinical trials.
| Year | Country | Patients/participants | Phage therapy | Methodology | Results | References |
|---|---|---|---|---|---|---|
| 2019 | France and Belgium | burn wounds infected by P.aeruginosa (PhagoBurn) | PP1131 | Randomised, controlled, double-blind phase 1/2 trial | The primary endpoint was reached slower in the PP1131 group than in the standard of care group. The maximal bacterial burden of participants was higher in the PP1131 group that in the standard of care group. The adverse events were lower in the PP1131 group that in the standard of care group. | [17] |
| 2019 | USA | healthy adults | LH01, LL5, T4D, and LL12 | Randomized, double-blind, placebo-controlled crossover intervention | There were no effects of treatment sequence on comprehensive metabolic panel outcomes | [18] |
| 2019 | Australian | recalcitrant chronic rhinosinusitis due to S aureus | AB-SA01 | Phase 1, first-in-humans, open-label clinical trial | Intranasal irrigation with AB-SA01 for 14 days was safe and well tolerated, with promising preliminary efficacy observations. | [19] |
| 2020 | Australian | severe S. aureus infections | AB-SA01 | Single-arm non-comparative trial | Phage therapy was safety and tolerability. No adverse reactions were reported. | [20] |
| 2021 | Georgia | urinary tract infections in patients undergoing transurethral resection of the prostate | Pyophage | Randomised, placebo-controlled, double-blind clinical trial | Intravesical phage therapy was non-inferior to standard-of-care antibiotic treatment in terms of efficacy or safety in treating UTIs in patients undergoing TURP. Phage safety profile seems to be favorable. | [21] |
2. Advantages of Phage therapy
2.1. Advantages over antibiotics
The inappropriate and massive use of antibiotics in medical and livestock settings has resulted in the increasing incidence of antibiotic resistance, rendering most antibiotics impotent. Thus there is urgent need of developing alternative drugs for antibiotics. Lytic phages may be an alternative for the treatment of bacterial infections and they enjoy some advantages over chemical antibiotics (Table 2). Phages are widely existed in nature and usually are isolated from the sewage samples. The filtered sewage solution is utilised to prepare liquid medium and the logarithmic phase host bacteria are added and mixed. After incubation at 37 °C for 18 h, the supernatant is used to purify phage through double-layer agar plate method. Phage is purified until the plaques are uniform in size [22]. Phage lysates is filtered and the production of phage therapy products should follow the Expert Consensus Quality and Safety Requirements for Sustainable Phage Therapy Products [23].
Table 2.
Comparison of chemical antibiotics and lytic phages in the treatment of bacterial infections.
| Aspect | Chemical antibiotic | Lytic phage |
|---|---|---|
| Belongs to | Chemicals | Biologicals |
| Evolution | Unable evolution | Co-evolve with bacteria |
| Discovery process | Difficult | Easy |
| Mechanism | Inhibition of bacteria wall synthesis, DNA replication, or protein synthesis | Infection and lysis of bacteria |
| Specificity | Broad spectrum: Gram-positive or Gram-negative species or both | Narrow spectrum: one or limited to a bacterial species |
| Interaction with bacteria | Bacteriostatic or Bacteriacidal | Bacteriacidal |
| Dosage | Continually dosed to clear infection | Fewer doses needed |
| Affect of microbiota | Yes | No |
| pharmacokinetics | Release controlable | Concentration rely on replication |
| Half-time life | Several hours up to 1 d | Several hours to weeks |
| Innate immune responses | No direct effect on innate immune cells | Phages interact with innate immune cells |
| Adaptive immune responses | Deactivate adaptive immune cell and no antibody induction | Activate adaptive immune cell and produce anti-phage antibodies |
| Reduced virulence of bacteria | No | Yes |
| Impaired antibiotic efflux | No | Yes |
| Treat biofilms | Yes (a few antibiotics) | Yes (many phages) |
| Treating intracellular bacterial pathogens | Some antibiotics | Can not entry into eukaryotic cells |
| Neutralizing antibodies | No | may be |
| Generation of endotoxins during therapy | No | Yes |
| Natural resistance | Yes (target missing) | Yes (nonsusceptible strains) |
| Acquired resistance | Yes (mutations) | Yes (CRISPR-Cas system, target modification, etc.) |
| Costs | Low production costs High development costs |
High production costs Low development costs |
| Regulatory frameworks | Approval process well established | Regulatory guidelines not fully established |
Phages represent large, replication-competent, constantly evolving biological agents. Phages and bacteria co-exist and co-evolve because their best chance for survival is to adapt to each other. If bacteria develop novel defense mechanisms, phages evolve new strategies (e.g. RNA-based anti-CRISPRs [24], phage-inducible chromosomal islands [25]) to attack the bacteria. In the lytic life cycle, receptor recognition is an important initial step. Phages recognize and adsorb to a specific receptor on the bacterial cell surface; they fail to lyse bacteria if the cell lacks the specific receptor. If the surface receptor has undergone some changes, the phage will alter the base plate spikes and/or tail fibers to match strain-specific variants of the receptor and establish an infection. Thus, a continuous evolutionary and dynamic balance exists between phages and bacteria. A single amino acid substitution in the major capsid protein F of an Escherichia coli phage led to significantly slower adsorption to host cells, but their burst size or lysis time remained unchanged [26]. The bacterial resistance and phage host range of P. fluorescens SBW25 and its lytic phage, Φ2, were observed to increase over coevolutionary time [27]. Horizontal gene transfer is especially remarkable among temperate phages. Homologous recombination is crucial for accelerated phage evolution [28].
Unlike antibiotics, phage therapy does not require frequent doses because a large burst size in the lytic cycle can produce and release abundant progeny in situ. Theoretically, only one phage can target a single host bacterium, likening it to act as a self replicating pharmaceutical. Hence, a single low dose of phage will replicate by itself at the infection site, continuously adsorbing to and killing bacteria [29]. In a randomized phase 1/2 trial regarding the treatment of burn wound infections using phage therapy, 26 patients were randomly administered (1:1) either a cocktail of 12 lytic anti-P. aeruginosa phages (PP1131) or the standard of care (1 % sulfadiazine silver emulsion cream) as a daily topical treatment for 7 days. After 2 weeks of follow-up, the daily bacterial burden in the phage group had successfully reduced by two quadrants or more compared with that in the standard of care group, although the median time to achieve this endpoint was significantly longer [17]. In a murine model of lethal E. coli septicemia infection, administering a single dose of 1 × 108 plaque-forming units (PFU)/mouse of ΦIK1 phage suspensions at 10 and 60 min post-bacterial infection rescued 100 % and 95 % of the mice, respectively [30]. Xu et al. [31] also used a single dose of phage KPP10 to treat P. aeruginosa strain D4-induced pneumonia murine models. After 2 h and 8 h of bacterial inoculation, KPP10, at a multiplicity of infection (MOI) of 80, was administered via nasal inhalation. Phage-treated mice had a significantly higher survival rate and a significantly lower number of viable bacteria in their lungs compared with control mice. They also showed lesser bleeding, lesser infiltration of inflammatory cells, and lesser mucus secretion in the lung interstitium upon pathological examination of lung tissues. The level of interleukin-1β (IL-1β) in the lungs of phage-treated mice was much lower than that in the control mice.
As antibacterial agents, phages themselves hardly have side effects on humans. The main components of phages are nucleic acids and proteins. Phages can be purified using the modified streak-for-isolation technique suggested by Peters et al. [32] or the double-layer agar plate method [22]. Through filtration, ultrafiltration, ultracentrifugation and endotoxin removal columns, phages are non-toxic [23]. Furthermore, since eukaryotic cell surface receptors and intracellular machinery are different from their bacterial counterparts, phages cannot infect eukaryotic cells. According to Nguyen et al. [8], about 31 billion phage particles access the human body daily through the gut epithelium. Subsequently, phages permeate into most organs of the body, including the kidney, spleen, lung, urinary tract, liver, and brain [33,34]. The human gut microbiota plays an important role in physiology, metabolism, and health [35]. Lytic phages can selectively reduce pathogenic bacteria in the gut. Except a few broad spectrum phages and polyvalent phages, usually, the spectrum of many phages is very narrow and limits to only a few strains. Compared with antibiotics, phages have little effect on beneficial natural microbiota due to their specificity. Thus, they may modulate the composition and diversity of the gut microbiota. Besides, they travel throughout the human body without exerting any side effects and were eliminated by the reticuloendothelial system.
Phage therapy is highly likely to be a novel treatment for sepsis. Lytic phages can help attenuate bacteremia that accompanies a syndrome while limiting excessive inflammatory responses. Petrovic et al. [20] conducted a single-arm non-comparative trial that included 13 participants aged 21–87 years old in an Australian hospital, suffering from at least two consecutive days of S. aureus bacteremia. The participants were intravenously administered a commercial cocktail of three Myoviridae phages (AB-SA01) twice daily for 2 weeks. The general clinical, hematological, and blood biochemical parameters of the participants were monitored for 90 days and no adverse reactions were recorded, vouching for the safety of these intravenously injected phages. A randomized, placebo-controlled, clinical trial in Georgia found that intravesical phage therapy is non-inferior to systemically applied antibiotics in the treatment of urinary tract infections. The authors recruited 113 patients, who were allocated in a 1:1:1 ratio by block randomization to the pyophage, placebo, and antibiotic treatment groups. After 7 days of treatment, the intravesical phage group did not differ from the other two groups. Moreover, adverse events were less frequent in the phage group (21 %) than in the placebo (41 %) and antibiotic groups (30 %). Thus, the safety profile of phage therapy seems to be favorable [21].
Most antibiotics have no or limited activity against bacterial biofilms, but phages can eradicate them. The S. aureus-specific phage Sb-1 can eradicate biofilms alone and in combination with antibiotics, such as fosfomycin, daptomycin, vancomycin, rifampin, and ciprofloxacin. Another instance is that phage ϕAB182 has high synergy with colistin, polymixin B, ceftazidime and cefotaxime to eradicate the biofilms formed by A. baumannii [36]. Matrix exopolysaccharide gets reduced in a dose-dependent manner [37]. Ryan et al. [38] also found that phages and antibiotics synergistically eradicate E. coli biofilms in vitro. When in vitro biofilms of P. aeruginosa PA14 were treated with phages alone, antibiotics alone, or a phage–antibiotic combination, the latter reduced bacterial densities more than the individual treatments could. The phage–antibiotic combination is more potent probably because of the higher diffusion of antibiotics in biofilms that are already disaggregated by the phages [39]. Depolymerases and lysins play a vital role in clearing the biofilm extracellular polymeric matrix [22,40]. As early as the year 1998, Hughes et al. found phage-borne polysaccharide depolymerase which derived from phage SF153b could degrade exopolysaccharide (EPS) of Enterobecter agglomerans strain 53b [41].
When phage and sublethal doses of some antibiotics were added to bacterial cultures, the diameters of phage plaques were bigger than those obtained after only phage treatment [42]. Phage–antibiotic synergy has been studied for a long time. Gram-negative and Gram-positive bacteria, in the presence of phages, were found to swell extensively, leading to an increase in the amounts of DNA and phage protein-encoding mRNAs. Lysis is delayed in swollen bacterial cells due to the relative shortage of holin [43]. Another study, investigating the synergy between the phage PEV20 and five different antibiotics against three P. aeruginosa strains, revealed that the ciprofloxacin–PEV20 combination was the most potent [44]. Similarly, the synergistic relationship between E. coli phage ΦHP3 and antibiotics has been evaluated [45]. When a single treatment of ΦHP3 or its combination with an ineffective low dosage of antibiotics was administered, the E. coli JJ2528 strain was revived after 8 h. As the concentration of antibiotics increased to intermediate doses, the revival of JJ2528 was inhibited by all antibiotics except trimethoprim and ciprofloxacin. High concentrations of antibiotics combined with ΦHP3 completely prevented the revival of the bacteria. Under certain conditions, phages can lower the minimum inhibitory concentration of antibiotic-resistant strains. The phage–antibiotics combination could reduce the development of antibiotic resistance and the dose of antibiotics during treatment [46].
An electromagnetic field (EMF) can influence the life cycle of phage, including shifting phages from lysogenic to lytic growth, increasing phage adsorption rate, burst rate and lytic efficiency, reducing the latent period of phage and phage-resistant bacterial mutants [47]. Recently, Grygorcewicz et al. [48] found the adsorption rate of E. coli T4 phage increased from 3.13 × 10−9 to 1.64 × 10−8 mL min−1 in the electromagnetic field, as well as the adsorption rate of S. aureus phage vb_SauM_A increased from 4.94 × 10−9 to 7.34 × 10−9 mL min−1.
2.2. Immunomodulatory and anti-inflammatory properties
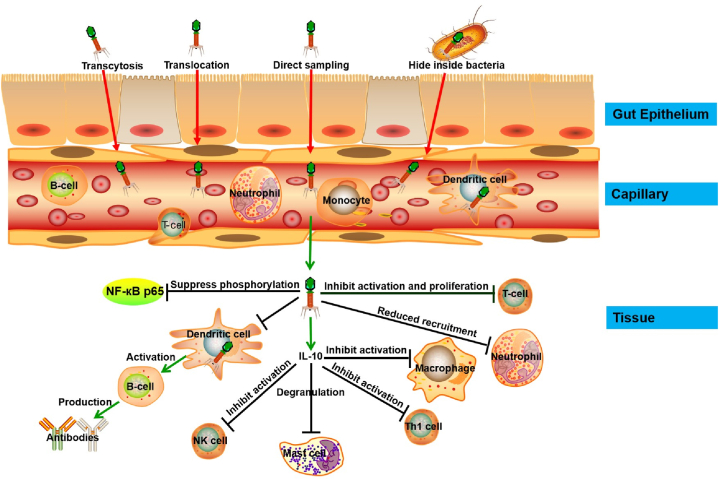
Although phages as a treatment for bacterial infections have been studied for almost 100 years, their interactions with the immune system are only recently being elucidated. Phages play an important role in maintaining the diversity, balance, and resilience of the gut microbiome. Like probiotics, they exert immunomodulatory effects in the intestines [49]. Barr et al. [50] observed that phages in the mucosal layer interacted with bacterial hosts, increasing the phage-to-bacteria ratios and subsequently, protecting the underlying epithelium from bacterial invasion. Phages share a symbiotic relationship with their metazoan hosts, which limits microbial colonization and pathology. The adherence of phages to the mucosal layer is mediated through displayed immunoglobulin (Ig)-like domains in phage glycans and capsid proteins. Fig. 1 shows how phages can migrate across epithelial cell layers and invade capillaries and tissues. In the blood, they are taken up by macrophages or dendritic cells and encounter other immune cells.
Fig. 1.
Migration of phages across epithelial cell layers and interaction with immune cells.
Phages adopt a non-specific transcytosis mechanism to migrate across epithelial cell layers. When orally administered, they can be detected in the urine and blood [51]. They are endocytosed by epithelial cells within 10 min, trafficked via the Golgi apparatus, and exocytosed by the basal cells. Approximately 31 billion phage particles are transcytosed daily across the gut epithelium into the human body, including the lymph, organs, blood, and even the brain [8]. After entering the bloodstream, phages encounter and interact with immune cells. For instance, the T4 phage expresses the Lys-Arg-Gly motif in its capsid protein, gp24. This motif can bind β3 integrins and downregulate the activities of β3+ cells (monocytes, platelets, lymphocytes, cancer cells) by inhibiting integrin functions [52]. Barfoot et al. [53] observed that dendritic cells could rapidly trap T4 phages. Despite multiple hypotheses for phages penetrating the epithelial barrier, including transcytosis by epithelial cells, translocation through the damaged, direct sampling by intestinal dendritic cells, and transport via bacteria, the precise mechanism remains unknown.
Neutrophils and granulocytes are vital immune cells that help defend against invading bacteria and viruses. Once activated, they secrete an array of cytokines, which influence the course of the immune response. Experimental data have shown that phages possess strong anti-inflammatory properties. Phage preparations were administered orally and/or locally to 37 patients with either osteomyelitis or infections of the skin, soft tissue, or lower respiratory tract. Nine days later, the mean C-reactive protein levels and leukocyte counts were found to be decreased significantly. The former, especially, decreased from 50 mg/L to 5 mg/L during 2–3 weeks despite the infection still existing [54]. The immune responses of human peripheral blood mononuclear cells to five different phages were comparable and mainly anti-inflammatory: IL-1A, IL-1B, IL-1RN, IL-6, CXCL1, CXCL5, SOCS3, and tumor necrosis factor-α (TNF-α) were upregulated, while LYZ and TGFBI were downregulated [55]. In order to reduce phage elimination by the reticuloendothelial system, Merril et al. [56] isolated long-circulating mutants of E. coli phage A and of S. typhimurium phage P22. Through the comparison of the parental and mutant A capsid proteins, the major phage head protein E was found to alter. Moreover, adding endotoxins to the purified phages did not induce additional immune responses. Sun et al. [57] also found that the inflammatory response elicited by the M13 phage was alleviated 7 days later, with increased IL-10 and decreased TNF-α and IL-6 levels. IL-10 is a regulatory cytokine that can inhibit allergic and inflammatory responses. It also contributes toward stabilizing mast cells from degranulation [58], and inhibits the activities of macrophages, natural killer cells, and Th1 cells. Similarly, the A3R phage is unlikely to induce human neutrophil degranulation [59]. Hence, besides their well-known antibacterial activity, phages exhibit anti-inflammatory properties as well.
Gorski's group proved that phages can not only inhibit human T-cell activation and proliferation but can also diminish the activation of nuclear factor kappa B, the master regulator controlling the expression of proinflammatory cytokines, chemokines, and adhesion molecules. Besides, phage administration reduces the infiltration of allogeneic skin allograft cells in vivo [60]. The filamentous Pf Pseudomonas phage was shown to inhibit bacterial invasion of lung airway epithelial cultures using a murine pneumonia model. The phage by itself could lower cytokine levels, reduce neutrophil recruitment, and alleviate lung injury [61]. Pabary et al. [62] demonstrated that treatment with phage PAO1 reduced the levels of inflammatory cytokines and neutrophils in bronchoalveolar lavage fluid in a P. aeruginosa pneumonia model. Thus, phages can attenuate the inflammatory response, both in vitro and in vivo.
Lipopolysaccharide (LPS)-binding phage proteins can also modulate the immune response. Gp12, belonging to the phage tail fiber proteins, can bind the surface proteins or LPS of E. coli. Recombinantly expressed gp12 still retains its ability to bind LPS without affecting the proliferation and viability of fibroblasts and endothelial cells. When administered to mice, gp12 almost completely diminished IL-1α levels in the circulation and reduced IL-6 levels by approximately 50 % in the serum. Besides, gp12 inhibited leukocytic infiltration into the lungs, liver, and spleen while producing no inflammatory effects in control animals [63].
Phage therapy does not affect the ability of phagocytes to kill bacteria. A study on 51 patients in Wroclaw ailing from chronic bacterial infections showed that the administration of phages did not significantly affect the ability of the patients’ polymorphonuclear neutrophils and peripheral blood monocytes to kill bacteria, including both the standard strains and the clinical isolates [64]. Therefore, phages can be used in immunosuppressed patients. A5/L phages administered to immunosuppressed mice infected with a sublethal or lethal dose of S. aureus strain L significantly reduced the bacterial load (>90 %) in the spleen and liver. About 72 % of the phage-treated mice attained long-term survival. The leukocyte and neutrophil count increased in the circulation while the proportion of myelocytic cells increased in the bone marrow. The bacteriolytic effect and stimulation of myelopoiesis, both, probably contribute to the effects elicited by phage therapy in immunocompromised patients [65].
Allergy is one of the greatest challenges to medicine and society. Allergic reactions are defined as IgE-mediated hypersensitivity reactions. As early as the 1950s and 1960s, the American physician Baker suggested that phages may help control asthma and allergy [66,67]. To relieve allergy, an anti-IgE antibody (BSW17) was produced through peptide libraries displayed on filamentous phages. BSW17 can efficiently recognize receptor-bound IgE without inducing the release of mediators from human mast cells or basophils. Therefore, such anti-IgE antibodies might be used to control allergic reactions in vivo [68].
The ability of the S. aureus phage vB_SauM_JS25 to inhibit inflammation has been demonstrated previously. MAC-T bovine mammary epithelial cells were infected with S. aureus JYG2, and highly purified vB_SauM_JS25 was added 1 h later. At 6 h post-treatment, the levels of IL-1β and TNF-α began to decrease in the phage-treated group compared with those in the S. aureus alone group. vB_SauM_JS25 was found to suppress the phosphorylation of the p65 subunit of nuclear factor kappa B [69]. On the other hand, phages also display an immunosuppressive effect. T4 phages can bind to mouse lymphocytes while retaining their biological activity. The authors suggested that these phages may produce immunosuppressive effects. Indeed, the phages significantly improved the survival rate of allogeneic skin allograft mice. Thus, phages may act as an additional treatment in clinical transplantation to suppress the immune system [70].
3. Limitations of Phage therapy
3.1. Pharmacological limitations
The molecular weight of phages is a million times higher than that of antibiotics, resulting in a lower uptake and transportation rate for them. Also, their shells package several different proteins, which can be recognized by the human mononuclear phagocytic system, leading to rapid elimination of the phages. Thus, phages exhibit complex pharmacokinetics and pharmacodynamics.
The most convenient and common administration route for any drug is oral application, and the same has been demonstrated for phage therapy. In Bangladesh, 15 healthy adult volunteers, who either received a nine-phage cocktail at a dose of 3 × 107 and 3 × 109 PFUs or a placebo control, showed no adverse responses. Moreover, the composition of their fecal microbiota was unaffected [71]. Four years later, the authors conducted another randomized clinical trial in children with acute bacterial diarrhea [72], wherein the patients were given a commercial Russian coliphage product. These results showed that the oral delivery of phages is safe. However, the prognosis of diarrhea did not improve.
Usually, the oral bioavailability of phages is very low [73]. Their titer and activity is also lower after oral application than after intravenous, intramuscular, or intraperitoneal administration in mice [74]. Usually, most phages are sensitive to an acidic environment: they get inactivated at pH values below 3 [75]. Understandably, phage titers will reduce in the stomach upon exposure to gastric juice.
MOI is the most important ratio defining the phage–bacteria interaction. In mouse models, the most effective MOI is below 0.1 [76], while in humans, it is 10 or higher [77]. In severe sepsis, bacterial concentrations in the blood are in the range of 101–105 (usually < 103) colony-forming units per milliliter [78]. The total volume of blood in a human is about 5 L. Therefore, the expected MOIinput is over 20 for an intravenous dose of 108 PFUs. Phage titers were observed to drop after intraperitoneal injection in rat models. A lytic phage, EC200 (PP), was intraperitoneally injected into sepsis rat models at a dose of 1 × 108 PFUs. The phage concentration in the blood decreased to about 107 PFU/mL after 2 h and 104 PFU/mL after 24 h [79]. Similarly, the titer of intravenously administered antipseudomonas phage cocktail declined from 1010 PFU/mL to 105 PFU/mL within 24 h in rats [80]. Thus, the dose at the infection site substantially drops to lower levels with time in phage therapy.
The delivery of phage to the target site also faces challenges. Phage particles have immunogenicity and can easily be cleared by the immune system, as well as phage capsid proteins can be inactivated by the low pH in the stomach or degraded by enzymes. Thus, it is necessary to develop some techniques to deliver phages. Excitedly, there are some successful methods to protect phages from enzymatic and chemical degradation, such as liposomes, hydrogels, fibrin glue. Phages can be encapsulated in liposomes to increase the stability of phages in different environments, especially the gastric fluid. The titer of three encapsulated Salmonella phages (UAB_Phi20, UAB_Phi78, and UAB_Phi87) decreased by 3.7–5.4 log units whereas the titer of nonencapsulated phages decreased by 5.7–7.8 log units. Additionally, the encapsulated phages in liposomes extended residence time in chickens [81]. Phages can be delivered via hydrogels. Due to the protection of hydrogels against the acidic stomach pH compared to free phage, Staphylococcal phage K showed high antibacterial activity [82]. The fibrin glue can also deliver phages. Fibrin glue polymerized P. aeruginosa phage PA5 sustainably released high titers of phages during 11 days and effectively killed bacteria [83].
3.2. Immune response
Phages consist of DNA or RNA inside the head and a protein coat on the outside. The natural immunogenicity of phages can give rise to interactions with the innate and adaptive immune systems. As early as 1964, Aronov et al. found phagocytic cells ingested phages during phagocytosis. The phagocytosis of the E. coli T2 phage by macrophages and neutrophils was observed. Moreover, these intracellular phage particles disintegrated after 15 min' incubation [84]. Similarly, T4 phage was observed to be phagocytosed by dendritic cells in vitro [53]. Anti-phage antibodies have been observed as early as 1987 [85], but their incidence depends on the site of infection and the route of administration. Oral and topical application of phages has not elicited any immunological complications in humans [86,87]. Intravenous administration, however, can activate the innate and adaptive immune systems [88]. Moreover, the neutralizing antibodies against phages are detected in the sera of humans and animals [89].
Although the phages used to treat human infections are rigorously purified and free from any allergens, immune responses may still be triggered. After phages cross the epithelial barrier, they reach the lymphoid organs, where they are processed and presented to T-cells by antigen presenting cells. Subsequent injections of phages result in an increase in non-neutralizing antibodies, IgM, and later, IgG. Researchers have verified that phage therapy can induce anti-phage antibodies. In a study in Wroclaw, levels of anti-phage antibodies were evaluated in the sera of 19 patients, who received Staphylococcal phages, and 20 other healthy volunteers using enzyme-linked immunosorbent assay [90]. After the third injection of the ENB6 phage, the titers of anti-phage IgM and IgG increased above background by 5-fold and 3800-fold, respectively [91]. T4-like phage-specific antibodies are estimated to be present in 81 % of the investigated sera in humans. Besides, the elevated levels of IgG and IgM are induced mainly by the major head protein (gp23) and the highly antigenic outer capsid protein (Hoc). An increase in the amount of anti-Hoc and anti-gp23 antibodies leads to a decrease in the activity of T4 phage in vitro and, to a certain degree, in vivo. Thus, the T4 phage-mediated protection against E. coli infection is lost in mice [92]. Another study investigated the long-term induction of antibodies in mice after the oral administration of the T4 phage. Serum anti-phage IgG was analyzed after 36 days, and secretory anti-phage IgA in the gut was analyzed after 79 days. IgA levels dropped to insignificant levels when the administration of the T4 phage was stopped, while it was induced faster than before when the T4 phage was administered a second time. Thus, the increased level of secretory IgA prevents gut transiting active phage [93]. The elevated levels of anti-phage IgG in the serum result in the elimination of phages from blood and the tissues [94]. On the other hand, the varphiX 174 phage is detected in circulation for 7 weeks without any detectable antibody in immunodeficient patients, while healthy patients clear the phage normally and produce antibodies [95]. Taken together, these studies indicate that phages can trigger the immune system to produce anti-phage-specific antibodies.
Immune responses involve cellular as well as humoral immunity. Many studies have reported the interactions between phages and phagocytes. Kantoch [96] discovered that guinea pig leukocytes could bind phages in a time-, concentration-, and temperature-dependent manner [97], following which the phages were internalized and eventually eliminated. Nelstrop et al. [98] demonstrated that phages could be phagocytosed by peritoneal macrophages without the assistance of humoral factors like antibodies and complements. The generation of phage neutralizing antibodies can interfere with their therapeutic efficacy. This can especially be a concern for patients with chronic infections, who need repeated administration of phages. Phage neutralizing antibodies were found to react with the distal phage tail as early as 1966 [99]. However, another study reported that the level of anti-phage IgG, IgM, and IgA did not increase in the serum after oral administration of E. coli T4 phage for 1 month [100]. Similarly, the level of anti-phage antibodies did not increase in the sera of 20 patients that were orally and/or locally administered a Staphylococcal phage cocktail. Even in those individual cases with increased IgG and IgM, the antibodies did not affect unsatisfactory clinical outcomes. Counterintuitively, some patients with relatively weak production of anti-phage antibodies suffered negative outcomes during phage therapy. Overall, the production of anti-phage antibodies does not seem to be an obstacle to phage therapy [101].
3.3. Phage resistance
Although the pharmacokinetics and pharmacodynamics of phages may differ from those of antibiotics, the evolution of bacterial resistance is inevitable. The co-evolution between phages and bacteria will eventually lead to the latter developing phage resistance.
Bacteria can develop resistance against phage infection via diverse mechanisms. In a study investigating phenotypic shift as one of the mechanisms, P. fluorescens SBW25 and the lytic phage phi2 were co-cultured in soil environments and nutrient broths. The presence of mucoid phenotypes of P. fluorescens were found to be associated with the phages [102]. Another study showed that the clustered regularly interspaced short palindromic repeats–Cas (CRISPR–Cas) system will be selected if the same P. aeruginosa cell often meets the same phage, which can influence the sensitivity of the bacteria to the phage [103,104]. The P. aeruginosa phage OMKO1 can bind to the outer membrane protein M, inducing lysis of the infected cells. Protein M is part of the mexAB- and mexXY-multidrug efflux systems. Binding of the phage and lysis will simultaneously direct the selection pressure toward evolving phage resistance in P. aeruginosa. Consequently, the bacteria will harbor mutated efflux pumps, which the phages cannot recognize [105]. Besides these, other unknown bacterial defense mechanisms may be found hiding in their genomes [106].
Another mechanism of phage resistance involves point mutations in some structures on the surface of bacteria that serve as receptors for phages. In a study, the authors sequenced four candidate genes from eight phages with unique phenotypes and identified eight evolved phages. The genotype of each evolved phage was related to a unique set of mutations. Crucially, most mutations were found in the phage tail fiber gene, which participates in the adsorption of the phage to the bacterial cell surface [27]. Another study demonstrated that the methionine aminopeptidase gene from Streptococcus thermophilus SMQ-301 is necessary for the lytic cycle of the phage DT1. A single mutation in this gene resulted in the emergence of strong resistance against this phage. The DT1-sensitivity phenotype was restored once the single mutation strain was complemented with the wild-type gene. When the same mutation was introduced into another strain (S. mutans), phage DT1 also developed resistance toward the host [107]. Moreover, Alseth et al. demonstrated that microbial community composition can drive the evolution of CRISPR–Cas adaptive immunity [108]. The CRISPR system captures foreign DNA as a memory. When the same foreign DNA is encountered again, the bacteria recognizes and cleaves it, ultimately resulting in phage resistance [109]. Additionally, the CRISPR–Cas system is deemed to be selected or maintained when the phage frequently interacts with the same host [103].
Other common mechanisms of phage defense includes restriction modification, DNA sensing immunity complexes like RecBCD and its homologues, and abortive infection and toxin-antitoxin system. The restriction-modification (RM) systems consist of a methyltransferase and a restriction endonuclease. The methyltransferase methylates adenine or cytosine in bacterial own DNA, while the restriction endonuclease recognizes short DNA motifs and cuts the unmethylated phage DNA. According to the mechanism of action and subunit composition, RM systems are classified into four major types: type I, type II, type III and type IV systems [110]. The RecBCD complex can recognize free DNA ends and entered into the bacterial cell [111]. The abortive infection and toxin-antitoxin system can disturb the phage life cycle and prevent progeny phage release through stressing the infecting bacteria [112].
In order to prevent or induce the emergence of phage resistance, some strategies may be adopted. Some phages can synergise with antibiotics to kill bacteria. For instance, P. aeruginosa phage combined with ceftazidime or ciprofloxacin to treat the infection of P. aeruginosa. The results show that the bacterial densities reduced, compared with the best single treatment [113]. In addition to the use of single types of phages as therapeutic agents, phage cocktails provide an alternative type of therapeutic agent. The phage cocktails strategy is not only enlarge the host range of phages but also reduces the possibility of phage resistance. The 61-year-old patient was diagnosed with severe abdominal sepsis accompanied by diaphragmatic hernia and disseminated intravascular coagulation. He was infected with P. aeruginosa septicemia with acute kidney injury and increasing serum creatinine levels. A purified phage cocktail (BFC1) was administered through a 6-h intravenous infusion for 10 days. Subsequently, his kidney function recovered and his blood cultures did not show further bacterial growth [114].
3.4. Lack of standard regulatory framework
The Food and Drug Administration and the European Medicines Agency do not approve phage therapy for humans, the main hurdles being the increased standards in the regulatory and legal framework. Currently, no explicit legal framework exists for the same, except in Poland, Russia, and Georgia, where phage therapy has been authorized for medicinal use [115].
Phage therapy belongs to a class of customized drugs and differs from conventional medicinal products in many aspects. Conventional drugs have a fixed predetermined composition, while phage cocktails vary tremendously in their constitution. Additionally, phages sustain evolution, which is a unique challenge for regulators [116]. Thus, conventional drugs match the current regulatory framework, while phages do not. In this respect, phage therapy is a type of personalized medicine. Obtaining marketing authorization for phage products is a huge challenge. Researchers engaged in this field have advocated for a specific regulatory framework [117,118]. To obtain regulatory approvals, many researchers have conducted extensive animal studies and clinical trials to explore roadblocks [119,120]. However, the Food and Drug Administration has only approved phages for non-medicinal applications, including food decontamination, environmental prophylaxis, and dietary supplements [121]. Phage therapy is an intriguing concept that must be studied further.
To be gratified, the European Union has previously funded a multinational multi-center clinical trials on burn patients (PhagoBurn Program). Between July 22, 2015, and Jan 2, 2017, scientists in France, Belgium and the Netherlands have recruited 27 patients to evaluate the efficacy and tolerability of a cocktail of 12 natural lytic anti-P. aeruginosa phages (PP1131) for the treatment of patients with infected burn wounds. The results of that study showed that very low concentrations of PP1131 reduced bacterial burden in burn wounds [17]. BX001 is the three-phage cocktail which specifically targets Cutibacterium acnes (C. acnes). In the phase 1 cosmetic randomized, controlled clinical trial, BX001 is safety, tolerability and efficacy. It has been shown to control the skin microbiome and reduced the facial burden of C. acnes [122]. A commercial phage based product called PreforPro® was used to examin the effects on gut microbiota and markers of intestinal and systemic inflammation in a healthy human population in a double-blinded, placebo-controlled crossover trial. After 28 days of oral administration, the populations of E. coli reduced in fecal. Moreover, the alpha and beta diversity of gut microbiota were no significant changes, suggesting that oral phages did not disrupt the microbiota [123]. There are other commercial phage preparations, such as Bakteriofag stafi lokokovyj and Sextaphag® produced by Russia, Staphylococcal bacteriophage and Pyo-bacteriophage produced by Georgia [124]. Thus, it shows that the policys and regulations in Eastern Europe are favorable for phage research. At present, there is still a lack of uniform standards of phage therapy around the world.
4. Conclusion and perspectives
In the mini-review, we summarized the advantages and limitations of lytic phages compared with chemical antibiotics to combat bacterial infections. Phage therapy would be beneficial to patients with bacterial infection in the aspect of reducing antibiotics side effects, reducing the costs of antibiotics development and production, possessing immunomodulatory and anti-inflammatory properties, eradicating bacterial biofilms. Of course, phage therapy has its own limitations, such as narrow spectrum of available host strains, the special pharmacokinetics and pharmacodynamics in vivo, immune responses, and phage resistance hurdles. Phage therapy has been employed before the advent of antibiotics in Western medicine, and recent clinical trials and case reports have yielded promising results. Several studies have demonstrated the potency of phages to treat infections associated with wounds and those of the gastrointestinal, respiratory, and urinary tracts. Recently, some researchers continue to make efforts to overcome these limitations of phage therapy. Phage therapy will be a welcome addition to the gamut of options available for treating antibiotic-resistant bacterial infections. In my opinion, it is more than very likely that phage therapy can be approved to combat bacterial infections by Food and Drug Administration in the future.
Ethics statement
Review and approval by an ethics committee was not needed for this study because it was a literature review, and no applicants/patients were enrolled.
Consent to participate (include appropriate statements)
Not applicable.
Data availability statement
No additional data was used for the research described in the review article.
CRediT authorship contribution statement
Zhimin Guo: Writing – review & editing, Methodology, Funding acquisition, Conceptualization. Mengyao Yuan: Writing – original draft, Methodology, Investigation, Data curation. Jiannan Chai: Writing – original draft, Methodology, Investigation, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81802056), China Postdoctoral Science Foundation (No. 2019M651215), the Scientific Research Fundation of the Education Department of Jilin Province (No. JJKH20231216KJ), the Achievement Transformation Project of the First Hospital of Jilin University (No. JDYYZH-2022006, No. JDYY2021-A0012, No. yjs2022007 and No. 2020-ZL-05) and the Science and Technology Innovation Platform of Jilin Province (YDZJ202202CXJD050).
References
- 1.Morrison L., Zembower T.R. Antimicrobial resistance. Gastrointest Endosc Clin N Am. 2020;30:619–635. doi: 10.1016/j.giec.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 2.De Oliveira D., Forde B.M., Kidd T.J., Harris P., Schembri M.A., Beatson S.A., Paterson D.L., Walker M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020;33 doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao Y., Cai W. Autophagy and bacterial infection. Adv. Exp. Med. Biol. 2020;1207:413–423. doi: 10.1007/978-981-15-4272-5_29. [DOI] [PubMed] [Google Scholar]
- 4.Listed N.A. United Nations meeting on antimicrobial resistance. Bull. World Health Organ. 2016;94:638–639. doi: 10.2471/BLT.16.020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cars O. Securing access to effective antibiotics for current and future generations. Whose responsibility? Ups. J. Med. Sci. 2014;119:209–214. doi: 10.3109/03009734.2014.912700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coates A., Hu Y., Holt J., Yeh P. Antibiotic combination therapy against resistant bacterial infections: synergy, rejuvenation and resistance reduction. Expert Rev. Anti Infect. Ther. 2020;18:5–15. doi: 10.1080/14787210.2020.1705155. [DOI] [PubMed] [Google Scholar]
- 7.Hutchings M.I., Truman A.W., Wilkinson B. Antibiotics: past, present and future. Curr. Opin. Microbiol. 2019;51:72–80. doi: 10.1016/j.mib.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen S., Baker K., Padman B.S., Patwa R., Dunstan R.A., Weston T.A., Schlosser K., Bailey B., Lithgow T., Lazarou M., Luque A., Rohwer F., Blumberg R.S., Barr J.J. Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers. mBio. 2017;8 doi: 10.1128/mBio.01874-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Twort F.W. An investigation on the nature of ultra-microscopic viruses. Lancet. 1915;186:1241–1243. doi: 10.1017/s0022172400043606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felix D. Sur un microbe invisible antagoniste des bacilles dysente'riques., CR Acad. Sci. Paris. 1917;165:373–375. [Google Scholar]
- 11.Bruynoghe R., Maisin J. ''Essais the'rapeutiques au moyen du bacte'riophage du staphylocoque.''. Comptes Rendus des séances de la Société de Biologie et de ses filiales. 1921;85:1120–1121. [Google Scholar]
- 12.Smith J. The bacteriophage in the treatment of typhoid fever. Br. Med. J. 1924;2:47–49. doi: 10.1136/bmj.2.3315.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aslam B., Wang W., Arshad M.I., Khurshid M., Muzammil S., Rasool M.H., Nisar M.A., Alvi R.F., Aslam M.A., Qamar M.U., Salamat M., Baloch Z. Antibiotic resistance: a rundown of a global crisis. Infect. Drug Resist. 2018;11:1645–1658. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., Ouellette M., Outterson K., Patel J., Cavaleri M., Cox E.M., Houchens C.R., Grayson M.L., Hansen P., Singh N., Theuretzbacher U., Magrini N. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 15.Di Giovine M., Salone B., Martina Y., Amati V., Zambruno G., Cundari E., Failla C.M., Saggio I. Binding properties, cell delivery, and gene transfer of adenoviral penton base displaying bacteriophage. Virology. 2001;282:102–112. doi: 10.1006/viro.2000.0809. [DOI] [PubMed] [Google Scholar]
- 16.Guo Z., Lin H., Ji X., Yan G., Lei L., Han W., Gu J., Huang J. Therapeutic applications of lytic phages in human medicine. Microb. Pathog. 2020;142 doi: 10.1016/j.micpath.2020.104048. [DOI] [PubMed] [Google Scholar]
- 17.Jault P., Leclerc T., Jennes S., Pirnay J.P., Que Y.A., Resch G., Rousseau A.F., Ravat F., Carsin H., Le Floch R., Schaal J.V., Soler C., Fevre C., Arnaud I., Bretaudeau L., Gabard J. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019;19:35–45. doi: 10.1016/S1473-3099(18)30482-1. [DOI] [PubMed] [Google Scholar]
- 18.Gindin M., Febvre H.P., Rao S., Wallace T.C., Weir T.L. Bacteriophage for gastrointestinal health (PHAGE) study: evaluating the safety and tolerability of supplemental bacteriophage consumption. J. Am. Coll. Nutr. 2019;38:68–75. doi: 10.1080/07315724.2018.1483783. [DOI] [PubMed] [Google Scholar]
- 19.Ooi M.L., Drilling A.J., Morales S., Fong S., Moraitis S., Macias-Valle L., Vreugde S., Psaltis A.J., Wormald P.J. Safety and tolerability of bacteriophage therapy for chronic rhinosinusitis due to staphylococcus aureus. JAMA Otolaryngol Head Neck Surg. 2019;145:723–729. doi: 10.1001/jamaoto.2019.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrovic F.A., Lin R., Ho J., Maddocks S., Ben Z.N., Iredell J.R. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol. 2020;5:465–472. doi: 10.1038/s41564-019-0634-z. [DOI] [PubMed] [Google Scholar]
- 21.Leitner L., Ujmajuridze A., Chanishvili N., Goderdzishvili M., Chkonia I., Rigvava S., Chkhotua A., Changashvili G., Mccallin S., Schneider M.P., Liechti M.D., Mehnert U., Bachmann L.M., Sybesma W., Kessler T.M. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomised, placebo-controlled, double-blind clinical trial. Lancet Infect. Dis. 2021;21:427–436. doi: 10.1016/S1473-3099(20)30330-3. [DOI] [PubMed] [Google Scholar]
- 22.Guo Z., Huang J., Yan G., Lei L., Wang S., Yu L., Zhou L., Gao A., Feng X., Han W., Gu J., Yang J. Identification and characterization of Dpo42, a novel depolymerase derived from the Escherichia coli phage vB_EcoM_ECOO78. Front. Microbiol. 2017;8:1460. doi: 10.3389/fmicb.2017.01460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirnay J.P., Blasdel B.G., Bretaudeau L., Buckling A., Chanishvili N., Clark J.R., Corte-Real S., Debarbieux L., Dublanchet A., De Vos D., Gabard J., Garcia M., Goderdzishvili M., Gorski A., Hardcastle J., Huys I., Kutter E., Lavigne R., Merabishvili M., Olchawa E., Parikka K.J., Patey O., Pouilot F., Resch G., Rohde C., Scheres J., Skurnik M., Vaneechoutte M., Van Parys L., Verbeken G., Zizi M., Van den Eede G. Quality and safety requirements for sustainable phage therapy products. Pharm. Res. (N. Y.) 2015;32:2173–2179. doi: 10.1007/s11095-014-1617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camara-Wilpert S., Mayo-Munoz D., Russel J., Fagerlund R.D., Madsen J.S., Fineran P.C., Sorensen S.J., Pinilla-Redondo R. Bacteriophages suppress CRISPR-Cas immunity using RNA-based anti-CRISPRs. Nature. 2023;623:601–607. doi: 10.1038/s41586-023-06612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fillol-Salom A., Rostol J.T., Ojiogu A.D., Chen J., Douce G., Humphrey S., Penades J.R. Bacteriophages benefit from mobilizing pathogenicity islands encoding immune systems against competitors. Cell. 2022;185:3248–3262. doi: 10.1016/j.cell.2022.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Roychoudhury P., Shrestha N., Wiss V.R., Krone S.M. Fitness benefits of low infectivity in a spatially structured population of bacteriophages. Proc. Biol. Sci. 2014;281 doi: 10.1098/rspb.2013.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scanlan P.D., Hall A.R., Lopez-Pascua L.D., Buckling A. Genetic basis of infectivity evolution in a bacteriophage. Mol. Ecol. 2011;20:981–989. doi: 10.1111/j.1365-294X.2010.04903.x. [DOI] [PubMed] [Google Scholar]
- 28.De Paepe M., Hutinet G., Son O., Amarir-Bouhram J., Schbath S., Petit M.A. Temperate phages acquire DNA from defective prophages by relaxed homologous recombination: the role of Rad52-like recombinases. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payne R.J., Phil D., Jansen V.A. Phage therapy: the peculiar kinetics of self-replicating pharmaceuticals. Clin. Pharmacol. Ther. 2000;68:225–230. doi: 10.1067/mcp.2000.109520. [DOI] [PubMed] [Google Scholar]
- 30.Schneider G., Szentes N., Horvath M., Dorn A., Cox A., Nagy G., Doffkay Z., Maroti G., Rakhely G., Kovacs T. Kinetics of targeted phage rescue in a mouse model of systemic escherichia coli k1. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/7569645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X., Haque A., Matsuzaki S., Matsumoto T., Nakamura S. The efficacy of phage therapy in a murine model of pseudomonas aeruginosa pneumonia and sepsis. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.682255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters D.L., Harris G., Davis C.M., Dennis J.J., Chen W. Bacteriophage isolation, purification, and characterization techniques against ubiquitous opportunistic pathogens. Curr Protoc. 2022;2:e594. doi: 10.1002/cpz1.594. [DOI] [PubMed] [Google Scholar]
- 33.Frenkel D., Solomon B. Filamentous phage as vector-mediated antibody delivery to the brain. Proc Natl Acad Sci U S A. 2002;99:5675–5679. doi: 10.1073/pnas.072027199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miedzybrodzki R., Klak M., Jonczyk-Matysiak E., Bubak B., Wojcik A., Kaszowska M., Weber-Dabrowska B., Lobocka M., Gorski A. Means to facilitate the overcoming of gastric juice barrier by a therapeutic staphylococcal bacteriophage a5/80. Front. Microbiol. 2017;8:467. doi: 10.3389/fmicb.2017.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bretin A., Gewirtz A.T., Chassaing B. Microbiota and metabolism: what's new in 2018? Am. J. Physiol. Endocrinol. Metab. 2018;315:E1–E6. doi: 10.1152/ajpendo.00014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vashisth M., Yashveer S., Jaglan A.B., Virmani N., Bera B.C., Vaid R.K., Anand T. Synergy of a virulent phage (phiAB182) with antibiotics leading to successful elimination of biofilms formed by MDR Acinetobacter baumannii. Can. J. Microbiol. 2022;68:731–746. doi: 10.1139/cjm-2022-0080. [DOI] [PubMed] [Google Scholar]
- 37.Tkhilaishvili T., Lombardi L., Klatt A.B., Trampuz A., Di Luca M. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int. J. Antimicrob. Agents. 2018;52:842–853. doi: 10.1016/j.ijantimicag.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Ryan E.M., Alkawareek M.Y., Donnelly R.F., Gilmore B.F. Synergistic phage-antibiotic combinations for the control of Escherichia coli biofilms in vitro. FEMS Immunol. Med. Microbiol. 2012;65:395–398. doi: 10.1111/j.1574-695X.2012.00977.x. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhry W.N., Concepcion-Acevedo J., Park T., Andleeb S., Bull J.J., Levin B.R. Synergy and order effects of antibiotics and phages in killing pseudomonas aeruginosa biofilms. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y., Cheng M., Zhang H., Dai J., Guo Z., Li X., Ji Y., Cai R., Xi H., Wang X., Xue Y., Sun C., Feng X., Lei L., Han W., Gu J. Antibacterial effects of phage lysin LysGH15 on planktonic cells and biofilms of diverse staphylococci. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00886-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes K.A., Sutherland I.W., Jones M.V. Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology (Read.) 1998;144(Pt 11):3039–3047. doi: 10.1099/00221287-144-11-3039. [DOI] [PubMed] [Google Scholar]
- 42.Comeau A.M., Tetart F., Trojet S.N., Prere M.F., Krisch H.M. Phage-Antibiotic Synergy (PAS): beta-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One. 2007;2:e799. doi: 10.1371/journal.pone.0000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim M., Jo Y., Hwang Y.J., Hong H.W., Hong S.S., Park K., Myung H. Phage-antibiotic synergy via delayed lysis. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.02085-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin Y., Chang R., Britton W.J., Morales S., Kutter E., Chan H.K. Synergy of nebulized phage PEV20 and ciprofloxacin combination against Pseudomonas aeruginosa. Int J Pharm. 2018;551:158–165. doi: 10.1016/j.ijpharm.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu L.C., Green S.I., Min L., Clark J.R., Salazar K.C., Terwilliger A.L., Kaplan H.B., Trautner B.W., Ramig R.F., Maresso A.W. Phage-Antibiotic synergy is driven by a unique combination of antibacterial mechanism of action and stoichiometry. mBio. 2020;11 doi: 10.1128/mBio.01462-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X., He Y., Wang Z., Wei J., Hu T., Si J., Tao G., Zhang L., Xie L., Abdalla A.E., Wang G., Li Y., Teng T. A combination therapy of Phages and Antibiotics: two is better than one. Int. J. Biol. Sci. 2021;17:3573–3582. doi: 10.7150/ijbs.60551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jablonska J., Dubrowska K., Glizniewicz M., Paszkiewicz O., Augustyniak A., Grygorcewicz B., Konopacki M., Markowska-Szczupak A., Kordas M., Dolegowska B., Rakoczy R. The use of the electromagnetic field in microbial process bioengineering. Adv. Appl. Microbiol. 2022;121:27–72. doi: 10.1016/bs.aambs.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Grygorcewicz B., Rakoczy R., Roszak M., Konopacki M., Kordas M., Piegat A., Serwin N., Cecerska-Heryc E., El F.M., Dolegowska B. Rotating magnetic Field-Assisted reactor enhances mechanisms of phage adsorption on bacterial cell surface. Curr. Issues Mol. Biol. 2022;44:1316–1325. doi: 10.3390/cimb44030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lusiak-Szelachowska M., Weber-Dabrowska B., Jonczyk-Matysiak E., Wojciechowska R., Gorski A. Bacteriophages in the gastrointestinal tract and their implications. Gut Pathog. 2017;9:44. doi: 10.1186/s13099-017-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barr J.J., Auro R., Furlan M., Whiteson K.L., Erb M.L., Pogliano J., Stotland A., Wolkowicz R., Cutting A.S., Doran K.S., Salamon P., Youle M., Rohwer F. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc. Natl. Acad. Sci. USA. 2013;110:10771–10776. doi: 10.1073/pnas.1305923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barr J.J. A bacteriophages journey through the human body. Immunol. Rev. 2017;279:106–122. doi: 10.1111/imr.12565. [DOI] [PubMed] [Google Scholar]
- 52.Gorski A., Dabrowska K., Switala-Jelen K., Nowaczyk M., Weber-Dabrowska B., Boratynski J., Wietrzyk J., Opolski A. New insights into the possible role of bacteriophages in host defense and disease. Med. Immunol. 2003;2:2. doi: 10.1186/1476-9433-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barfoot R., Denham S., Gyure L.A., Hall J.G., Hobbs S.M., Jackson L.E., Robertson D. Some properties of dendritic macrophages from peripheral lymph. Immunology. 1989;68:233–239. [PMC free article] [PubMed] [Google Scholar]
- 54.Miedzybrodzki R., Fortuna W., Weber-Dabrowska B., Gorski A. A retrospective analysis of changes in inflammatory markers in patients treated with bacterial viruses. Clin. Exp. Med. 2009;9:303–312. doi: 10.1007/s10238-009-0044-2. [DOI] [PubMed] [Google Scholar]
- 55.Van Belleghem J.D., Clement F., Merabishvili M., Lavigne R., Vaneechoutte M. Pro- and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci. Rep.-UK. 2017;7 doi: 10.1038/s41598-017-08336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merril C.R., Biswas B., Carlton R., Jensen N.C., Creed G.J., Zullo S., Adhya S. Long-circulating bacteriophage as antibacterial agents. Proc Natl Acad Sci U S A. 1996;93:3188–3192. doi: 10.1073/pnas.93.8.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Y., Li Y., Wu B., Wang J., Lu X., Qu S., Weng J., Feng B. Biological responses to M13 bacteriophage modified titanium surfaces in vitro. Acta Biomater. 2017;58:527–538. doi: 10.1016/j.actbio.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 58.Bundoc V.G., Keane-Myers A. IL-10 confers protection from mast cell degranulation in a mouse model of allergic conjunctivitis. Exp. Eye Res. 2007;85:575–579. doi: 10.1016/j.exer.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 59.Borysowski J., Miedzybrodzki R., Wierzbicki P., Klosowska D., Korczak-Kowalska G., Weber-Dabrowska B., Gorski A. A3R phage and staphylococcus aureus lysate do not induce neutrophil degranulation. Viruses. 2017;9 doi: 10.3390/v9020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gorski A., Kniotek M., Perkowska-Ptasinska A., Mroz A., Przerwa A., Gorczyca W., Dabrowska K., Weber-Dabrowska B., Nowaczyk M. Bacteriophages and transplantation tolerance. Transplant. Proc. 2006;38:331–333. doi: 10.1016/j.transproceed.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 61.Secor P.R., Michaels L.A., Smigiel K.S., Rohani M.G., Jennings L.K., Hisert K.B., Arrigoni A., Braun K.R., Birkland T.P., Lai Y., Hallstrand T.S., Bollyky P.L., Singh P.K., Parks W.C. Filamentous bacteriophage produced by pseudomonas aeruginosa alters the inflammatory response and promotes noninvasive infection in vivo. Infect. Immun. 2017;85 doi: 10.1128/IAI.00648-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pabary R., Singh C., Morales S., Bush A., Alshafi K., Bilton D., Alton E.W., Smithyman A., Davies J.C. Antipseudomonal bacteriophage reduces infective burden and inflammatory response in murine lung. Antimicrob. Agents Chemother. 2016;60:744–751. doi: 10.1128/AAC.01426-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miernikiewicz P., Klopot A., Soluch R., Szkuta P., Keska W., Hodyra-Stefaniak K., Konopka A., Nowak M., Lecion D., Kazmierczak Z., Majewska J., Harhala M., Gorski A., Dabrowska K. T4 phage tail adhesin gp12 counteracts LPS-Induced inflammation in vivo. Front. Microbiol. 2016;7:1112. doi: 10.3389/fmicb.2016.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jonczyk-Matysiak E., Lusiak-Szelachowska M., Klak M., Bubak B., Miedzybrodzki R., Weber-Dabrowska B., Zaczek M., Fortuna W., Rogoz P., Letkiewicz S., Szufnarowski K., Gorski A. The effect of bacteriophage preparations on intracellular killing of bacteria by phagocytes. J Immunol Res. 2015;2015 doi: 10.1155/2015/482863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zimecki M., Artym J., Kocieba M., Weber-Dabrowska B., Borysowski J., Gorski A. Prophylactic effect of bacteriophages on mice subjected to chemotherapy-induced immunosuppression and bone marrow transplant upon infection with Staphylococcus aureus. Med. Microbiol. Immunol. 2010;199:71–79. doi: 10.1007/s00430-009-0135-4. [DOI] [PubMed] [Google Scholar]
- 66.Baker A.G. Treatment of chronic bronchial asthma; Aerosol of staphylococcus bacteriophage lysate as an adjunct to systemic hyposensitization. Am. Pract. Dig. Treat. 1958;9:591–598. [PubMed] [Google Scholar]
- 67.Baker A.G. Staphyloccus bacteriophage lysate: topical and parenteral use in allergic patients. Pa. Med. J. 1963;66:25–28. [PubMed] [Google Scholar]
- 68.Rudolf M.P., Vogel M., Kricek F., Ruf C., Zurcher A.W., Reuschel R., Auer M., Miescher S., Stadler B.M. Epitope-specific antibody response to IgE by mimotope immunization. J. Immunol. 1998;160:3315–3321. [PubMed] [Google Scholar]
- 69.Zhang L., Hou X., Sun L., He T., Wei R., Pang M., Wang R. Staphylococcus aureus bacteriophage suppresses LPS-Induced inflammation in MAC-T bovine mammary epithelial cells. Front. Microbiol. 2018;9:1614. doi: 10.3389/fmicb.2018.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gorski A., Nowaczyk M., Weber-Dabrowska B., Kniotek M., Boratynski J., Ahmed A., Dabrowska K., Wierzbicki P., Switala-Jelen K., Opolski A. New insights into the possible role of bacteriophages in transplantation. Transplant. Proc. 2003;35:2372–2373. doi: 10.1016/s0041-1345(03)00811-x. [DOI] [PubMed] [Google Scholar]
- 71.Sarker S.A., Mccallin S., Barretto C., Berger B., Pittet A.C., Sultana S., Krause L., Huq S., Bibiloni R., Bruttin A., Reuteler G., Brussow H. Oral T4-like phage cocktail application to healthy adult volunteers from Bangladesh. Virology. 2012;434:222–232. doi: 10.1016/j.virol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Sarker S.A., Sultana S., Reuteler G., Moine D., Descombes P., Charton F., Bourdin G., Mccallin S., Ngom-Bru C., Neville T., Akter M., Huq S., Qadri F., Talukdar K., Kassam M., Delley M., Loiseau C., Deng Y., El Aidy S., Berger B., Brüssow H. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. EBioMedicine. 2016;4:124–137. doi: 10.1016/j.ebiom.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gorski A., Jonczyk-Matysiak E., Lusiak-Szelachowska M., Miedzybrodzki R., Weber-Dabrowska B., Borysowski J. The potential of phage therapy in sepsis. Front. Immunol. 2017;8:1783. doi: 10.3389/fimmu.2017.01783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geier M.R., Trigg M.E., Merril C.R. Fate of bacteriophage lambda in non-immune germ-free mice. Nature. 1973;246:221–223. doi: 10.1038/246221a0. [DOI] [PubMed] [Google Scholar]
- 75.Koo J., Depaola A., Marshall D.L. Impact of acid on survival of Vibrio vulnificus and Vibrio vulnificus phage. J Food Prot. 2000;63:1049–1052. doi: 10.4315/0362-028x-63.8.1049. [DOI] [PubMed] [Google Scholar]
- 76.Chan B.K., Sistrom M., Wertz J.E., Kortright K.E., Narayan D., Turner P.E. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci. Rep. 2016;6 doi: 10.1038/srep26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jung L.S., Ding T., Ahn J. Evaluation of lytic bacteriophages for control of multidrug-resistant Salmonella Typhimurium. Ann. Clin. Microbiol. Antimicrob. 2017;16:66. doi: 10.1186/s12941-017-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ginn A.N., Hazelton B., Shoma S., Cullen M., Solano T., Iredell J.R. Quantitative multiplexed-tandem PCR for direct detection of bacteraemia in critically ill patients. Pathology. 2017;49:304–308. doi: 10.1016/j.pathol.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 79.Pouillot F., Chomton M., Blois H., Courroux C., Noelig J., Bidet P., Bingen E., Bonacorsi S. Efficacy of bacteriophage therapy in experimental sepsis and meningitis caused by a clone O25b:H4-ST131 Escherichia coli strain producing CTX-M-15. Antimicrob. Agents Chemother. 2012;56:3568–3575. doi: 10.1128/AAC.06330-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oechslin F., Piccardi P., Mancini S., Gabard J., Moreillon P., Entenza J.M., Resch G., Que Y.A. Synergistic interaction between phage therapy and antibiotics clears pseudomonas aeruginosa infection in endocarditis and reduces virulence. J. Infect. Dis. 2017;215:703–712. doi: 10.1093/infdis/jiw632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Colom J., Cano-Sarabia M., Otero J., Cortes P., Maspoch D., Llagostera M. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl. Environ. Microbiol. 2015;81:4841–4849. doi: 10.1128/AEM.00812-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma Y., Pacan J.C., Wang Q., Sabour P.M., Huang X., Xu Y. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food Hydrocolloid. 2010;26:434–440. doi: 10.1016/j.foodhyd.2010.11.017. [DOI] [Google Scholar]
- 83.Rubalskii E., Ruemke S., Salmoukas C., Aleshkin A., Bochkareva S., Modin E., Mashaqi B., Boyle E.C., Boethig D., Rubalsky M., Zulkarneev E., Kuehn C., Haverich A. Fibrin glue as a local drug-delivery system for bacteriophage PA5. Sci. Rep. 2019;9:2091. doi: 10.1038/s41598-018-38318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aronow R., Danon D., Shahar A., Aronson M. Electron microscopy of in vitro endocytosis of t2 phage by cells from rabbit peritoneal exudate. J. Exp. Med. 1964;120:943–954. doi: 10.1084/jem.120.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kucharewicz-Krukowska A., Slopek S. Immunogenic effect of bacteriophage in patients subjected to phage therapy. Arch. Immunol. Ther. Exp. 1987;35:553–561. [PubMed] [Google Scholar]
- 86.Merabishvili M., Pirnay J.P., Verbeken G., Chanishvili N., Tediashvili M., Lashkhi N., Glonti T., Krylov V., Mast J., Van Parys L., Lavigne R., Volckaert G., Mattheus W., Verween G., De Corte P., Rose T., Jennes S., Zizi M., De Vos D., Vaneechoutte M. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mccallin S., Alam S.S., Barretto C., Sultana S., Berger B., Huq S., Krause L., Bibiloni R., Schmitt B., Reuteler G., Brussow H. Safety analysis of a Russian phage cocktail: from metagenomic analysis to oral application in healthy human subjects. Virology. 2013;443:187–196. doi: 10.1016/j.virol.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 88.Sokoloff A.V., Bock I., Zhang G., Sebestyen M.G., Wolff J.A. The interactions of peptides with the innate immune system studied with use of T7 phage peptide display. Mol. Ther. 2000;2:131–139. doi: 10.1006/mthe.2000.0110. [DOI] [PubMed] [Google Scholar]
- 89.Jerne N.K. Bacteriophage inactivation by antiphage serum diluted in distilled water. Nature. 1952;169:117–118. doi: 10.1038/169117b0. [DOI] [PubMed] [Google Scholar]
- 90.Lusiak-Szelachowska M., Zaczek M., Weber-Dabrowska B., Miedzybrodzki R., Klak M., Fortuna W., Letkiewicz S., Rogoz P., Szufnarowski K., Jonczyk-Matysiak E., Owczarek B., Gorski A. Phage neutralization by sera of patients receiving phage therapy. Viral Immunol. 2014;27:295–304. doi: 10.1089/vim.2013.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Biswas B., Adhya S., Washart P., Paul B., Trostel A.N., Powell B., Carlton R., Merril C.R. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun. 2002;70:204–210. doi: 10.1128/iai.70.1.204-210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dabrowska K., Miernikiewicz P., Piotrowicz A., Hodyra K., Owczarek B., Lecion D., Kazmierczak Z., Letarov A., Gorski A. Immunogenicity studies of proteins forming the T4 phage head surface. J. Virol. 2014;88:12551–12557. doi: 10.1128/JVI.02043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Majewska J., Beta W., Lecion D., Hodyra-Stefaniak K., Klopot A., Kazmierczak Z., Miernikiewicz P., Piotrowicz A., Ciekot J., Owczarek B., Kopciuch A., Wojtyna K., Harhala M., Makosa M., Dabrowska K. Oral application of t4 phage induces weak antibody production in the gut and in the blood. Viruses. 2015;7:4783–4799. doi: 10.3390/v7082845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hodyra-Stefaniak K., Miernikiewicz P., Drapala J., Drab M., Jonczyk-Matysiak E., Lecion D., Kazmierczak Z., Beta W., Majewska J., Harhala M., Bubak B., Klopot A., Gorski A., Dabrowska K. Mammalian Host-Versus-Phage immune response determines phage fate in vivo. Sci. Rep. 2015;5 doi: 10.1038/srep14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ochs H.D., Davis S.D., Wedgwood R.J. Immunologic responses to bacteriophage phi-X 174 in immunodeficiency diseases. J. Clin. Invest. 1971;50:2559–2568. doi: 10.1172/JCI106756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kantoch M., Skurski A., Wieczorek Z. In vitro blockade of bacterial phagocytosis of leukocytes by means of bacterial viruses. Schweiz Z Pathol Bakteriol. 1958;21:1106–1119. doi: 10.1159/000160571. [DOI] [PubMed] [Google Scholar]
- 97.Kurzepa A., Dabrowska K., Skaradzinski G., Gorski A. Bacteriophage interactions with phagocytes and their potential significance in experimental therapy. Clin. Exp. Med. 2009;9:93–100. doi: 10.1007/s10238-008-0027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nelstrop A.E., Taylor G., Collard P. Studies on phagocytosis. II. In vitro phagocytosis by macrophages. Immunology. 1968;14:339–346. [PMC free article] [PubMed] [Google Scholar]
- 99.Hajek P., Mandel L. Antibody response of young animals to bacteriophages of different immunological behaviour: phi X 174 and T2. Folia Microbiol (Praha) 1966;11:282–289. doi: 10.1007/BF02878898. [DOI] [PubMed] [Google Scholar]
- 100.Bruttin A., Brussow H. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob. Agents Chemother. 2005;49:2874–2878. doi: 10.1128/AAC.49.7.2874-2878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zaczek M., Lusiak-Szelachowska M., Jonczyk-Matysiak E., Weber-Dabrowska B., Miedzybrodzki R., Owczarek B., Kopciuch A., Fortuna W., Rogoz P., Gorski A. Antibody production in response to staphylococcal MS-1 phage cocktail in patients undergoing phage therapy. Front. Microbiol. 2016;7:1681. doi: 10.3389/fmicb.2016.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scanlan P.D., Buckling A. Co-evolution with lytic phage selects for the mucoid phenotype of Pseudomonas fluorescens SBW25. ISME J. 2012;6:1148–1158. doi: 10.1038/ismej.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chabas H., van Houte S., Hoyland-Kroghsbo N.M., Buckling A., Westra E.R. Immigration of susceptible hosts triggers the evolution of alternative parasite defence strategies. Proc. Biol. Sci. 2016;283 doi: 10.1098/rspb.2016.0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Laanto E., Hoikkala V., Ravantti J., Sundberg L.R. Long-term genomic coevolution of host-parasite interaction in the natural environment. Nat. Commun. 2017;8:111. doi: 10.1038/s41467-017-00158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chan B.K., Turner P.E., Kim S., Mojibian H.R., Elefteriades J.A., Narayan D. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol Med Public Health. 2018;2018:60–66. doi: 10.1093/emph/eoy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim J.S. Microbial warfare against viruses. Science. 2018;359:993. doi: 10.1126/science.aas9430. [DOI] [PubMed] [Google Scholar]
- 107.Labrie S.J., Mosterd C., Loignon S., Dupuis M.E., Desjardins P., Rousseau G.M., Tremblay D.M., Romero D.A., Horvath P., Fremaux C., Moineau S. A mutation in the methionine aminopeptidase gene provides phage resistance in Streptococcus thermophilus. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-49975-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alseth E.O., Pursey E., Lujan A.M., Mcleod I., Rollie C., Westra E.R. Bacterial biodiversity drives the evolution of CRISPR-based phage resistance. Nature. 2019;574:549–552. doi: 10.1038/s41586-019-1662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim J.S. Microbial warfare against viruses. Science. 2018;359:993. doi: 10.1126/science.aas9430. [DOI] [PubMed] [Google Scholar]
- 110.Rostol J.T., Marraffini L. (Ph)ighting phages: how bacteria resist their parasites. Cell Host Microbe. 2019;25:184–194. doi: 10.1016/j.chom.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Levy A., Goren M.G., Yosef I., Auster O., Manor M., Amitai G., Edgar R., Qimron U., Sorek R. CRISPR adaptation biases explain preference for acquisition of foreign DNA. Nature. 2015;520:505–510. doi: 10.1038/nature14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang T., Tamman H., Coppieters T.W.K., Kurata T., Leroux M., Srikant S., Brodiazhenko T., Cepauskas A., Talavera A., Martens C., Atkinson G.C., Hauryliuk V., Garcia-Pino A., Laub M.T. Direct activation of a bacterial innate immune system by a viral capsid protein. Nature. 2022;612:132–140. doi: 10.1038/s41586-022-05444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chaudhry W.N., Concepcion-Acevedo J., Park T., Andleeb S., Bull J.J., Levin B.R. Synergy and order effects of antibiotics and phages in killing pseudomonas aeruginosa biofilms. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jennes S., Merabishvili M., Soentjens P., Pang K.W., Rose T., Keersebilck E., Soete O., Francois P.M., Teodorescu S., Verween G., Verbeken G., De Vos D., Pirnay J.P. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury-a case report. Crit. Care. 2017;21:129. doi: 10.1186/s13054-017-1709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kutateladze M. Experience of the eliava institute in bacteriophage therapy. Virol. Sin. 2015;30:80–81. doi: 10.1007/s12250-014-3557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pelfrene E., Willebrand E., Cavaleiro S.A., Sebris Z., Cavaleri M. Bacteriophage therapy: a regulatory perspective. J. Antimicrob. Chemother. 2016;71:2071–2074. doi: 10.1093/jac/dkw083. [DOI] [PubMed] [Google Scholar]
- 117.Fauconnier A. Regulating phage therapy: the biological master file concept could help to overcome regulatory challenge of personalized medicines. EMBO Rep. 2017;18:198–200. doi: 10.15252/embr.201643250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Verbeken G., Pirnay J.P., Lavigne R., Jennes S., De Vos D., Casteels M., Huys I. Call for a dedicated European legal framework for bacteriophage therapy. Arch. Immunol. Ther. Exp. 2014;62:117–129. doi: 10.1007/s00005-014-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Petrovic F.A., Khalid A., Maddocks S., Ho J., Gilbey T., Sandaradura I., Lin R.C., Ben Z.N., Venturini C., Bowring B., Iredell J.R. Phage therapy for severe bacterial infections: a narrative review. Med. J. Aust. 2020;212:279–285. doi: 10.5694/mja2.50355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Anand T., Virmani N., Kumar S., Mohanty A.K., Pavulraj S., Bera B.C., Vaid R.K., Ahlawat U., Tripathi B.N. Phage therapy for treatment of virulent Klebsiella pneumoniae infection in a mouse model. J Glob Antimicrob Resist. 2020;21:34–41. doi: 10.1016/j.jgar.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 121.Sarhan W.A., Azzazy H.M. Phage approved in food, why not as a therapeutic? Expert Rev. Anti Infect. Ther. 2015;13:91–101. doi: 10.1586/14787210.2015.990383. [DOI] [PubMed] [Google Scholar]
- 122.Golembo M., Puttagunta S., Rappo U., Weinstock E., Engelstein R., Gahali-Sass I., Moses A., Kario E., Ben-Dor C.E., Nicenboim J., Ben D.H., Sudakov K., Cohen A., Bassan M., Zak N.B. Development of a topical bacteriophage gel targeting Cutibacterium acnes for acne prone skin and results of a phase 1 cosmetic randomized clinical trial. Skin Health Dis. 2022;2:e93. doi: 10.1002/ski2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Febvre H.P., Rao S., Gindin M., Goodwin N., Finer E., Vivanco J.S., Lu S., Manter D.K., Wallace T.C., Weir T.L. PHAGE study: effects of supplemental bacteriophage intake on inflammation and gut microbiota in healthy adults. Nutrients. 2019;11 doi: 10.3390/nu11030666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Straka M., Hubenakova Z., Lichvarikova A., Janosikova L., Markuskova B., Minich A., Liptakova A., Drahovska H., Slobodnikova L. Susceptibility of Staphylococcus aureus strains to commercial therapeutic phage preparations. Bratisl. Lek. Listy. 2022;123:724–729. doi: 10.4149/BLL_2022_116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data was used for the research described in the review article.