Abstract
Ca(2+)-mobilizing hormones induce oscillations in the cytoplasmic concentration of free Ca2+ ('free Ca') (spikes) in many cells. In hepatocytes the frequency of spiking depends on agonist dose, but the time course of an individual spike does not change with agonist concentration. Interestingly, the time course of individual spikes does depend on the hormone species, but the cellular mechanisms underlying this agonist-specificity are not understood. Here we show that ryanodine, which blocks the muscle Ca2+ channel responsible for Ca(2+)-induced Ca2+ release ('CICR') in the open conformation, has almost no effect on phenylephrine-induced spikes, but does, in contrast, inhibit vasopressin- or angiotensin II-induced spikes. We also show that ryanodine has no effect either on the increase in frequency or on the elevated peak free Ca induced by increased cyclic AMP on phenylephrine spikes. In contrast, ryanodine truncates the prolonged falling phases of spikes induced by vasopressin or angiotensin II in the presence of elevated cyclic AMP. A working hypothesis is proposed in which vasopressin- or angiotensin II-induced spikes consist of an Ins(1,4,5)P3-mediated symmetrical spike, identical in time course and mechanism with those induced by phenylephrine, followed by a 'tail' that represents CICR. The data hint at the existence of a novel signalling pathway.
Full text
PDF
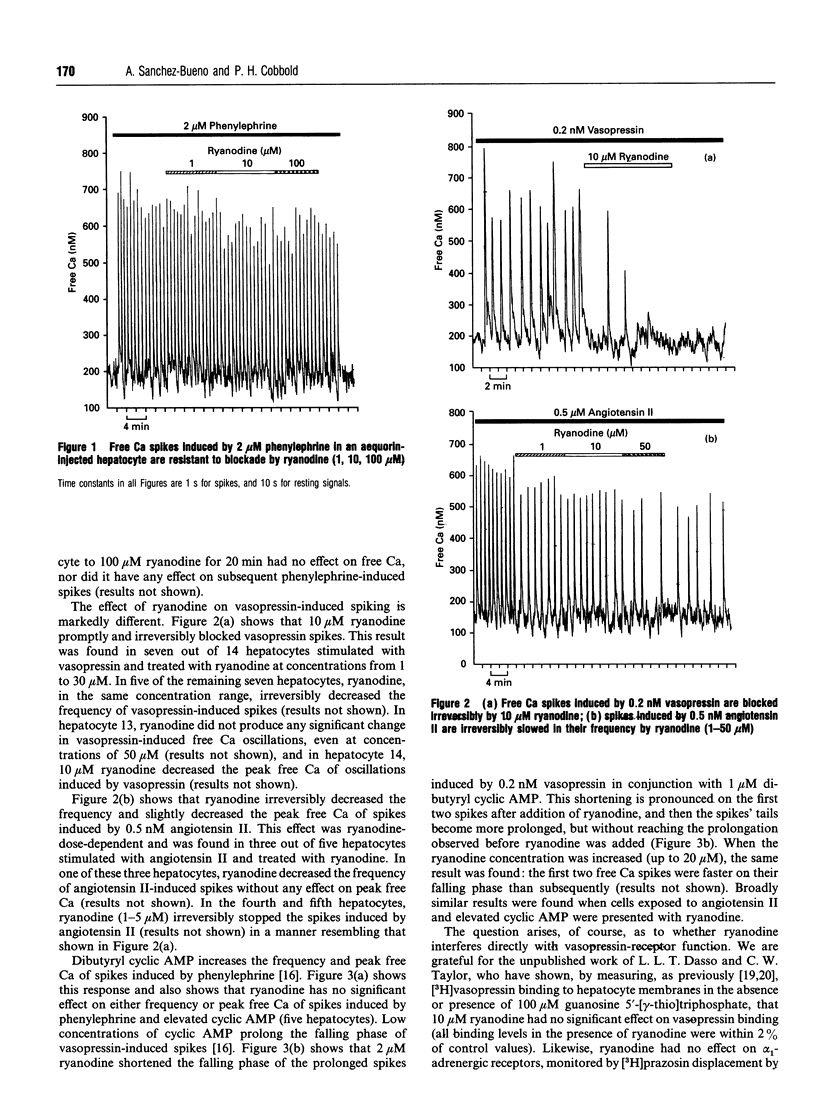
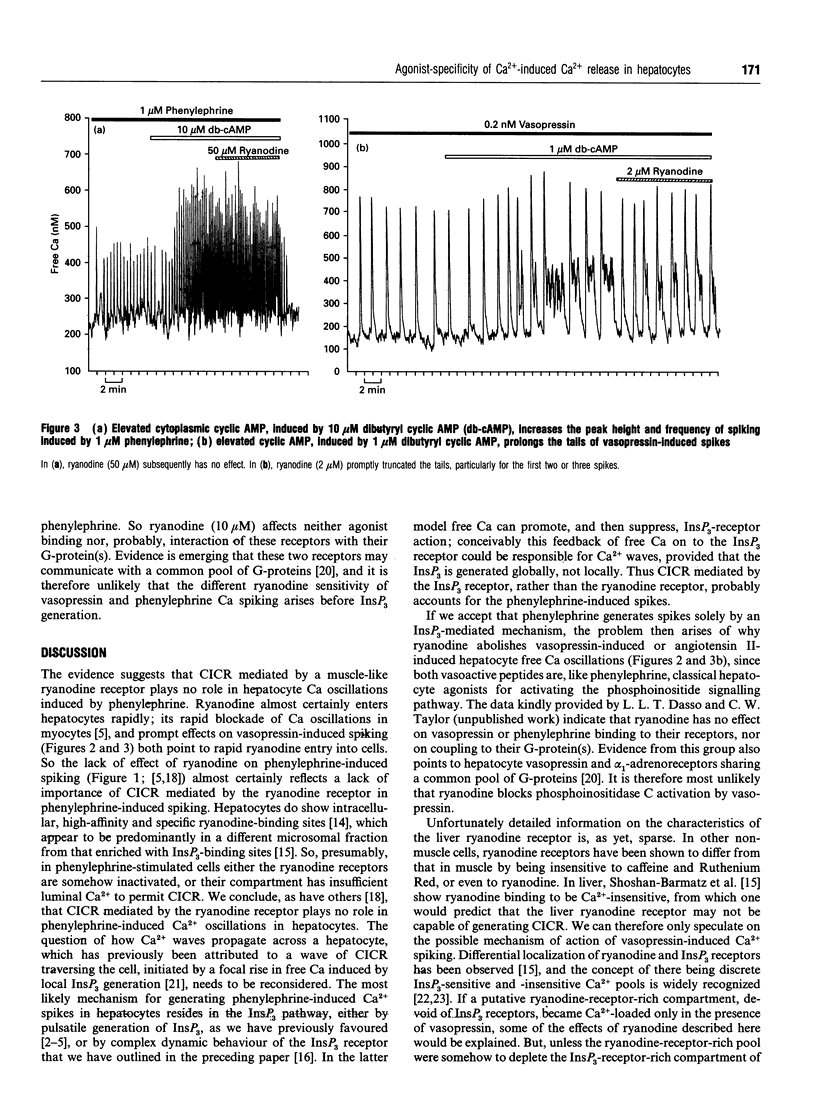

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Berridge M. J., Cobbold P. H., Cuthbertson K. S. Spatial and temporal aspects of cell signalling. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):325–343. doi: 10.1098/rstb.1988.0080. [DOI] [PubMed] [Google Scholar]
- Cobbold P. H., Sanchez-Bueno A., Dixon C. J. The hepatocyte calcium oscillator. Cell Calcium. 1991 Feb-Mar;12(2-3):87–95. doi: 10.1016/0143-4160(91)90011-3. [DOI] [PubMed] [Google Scholar]
- Dasso L. L., Taylor C. W. Different calcium-mobilizing receptors share the same guanine nucleotide-binding protein pool in hepatocytes. Mol Pharmacol. 1992 Sep;42(3):453–457. [PubMed] [Google Scholar]
- Dasso L. L., Taylor C. W. Heparin and other polyanions uncouple alpha 1-adrenoceptors from G-proteins. Biochem J. 1991 Dec 15;280(Pt 3):791–795. doi: 10.1042/bj2800791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galione A., Lee H. C., Busa W. B. Ca(2+)-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science. 1991 Sep 6;253(5024):1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- Iino M., Kobayashi T., Endo M. Use of ryanodine for functional removal of the calcium store in smooth muscle cells of the guinea-pig. Biochem Biophys Res Commun. 1988 Apr 15;152(1):417–422. doi: 10.1016/s0006-291x(88)80730-7. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Characteristics of inositol trisphosphate-mediated Ca2+ release from permeabilized hepatocytes. J Biol Chem. 1986 Nov 5;261(31):14658–14664. [PubMed] [Google Scholar]
- Ma J., Fill M., Knudson C. M., Campbell K. P., Coronado R. Ryanodine receptor of skeletal muscle is a gap junction-type channel. Science. 1988 Oct 7;242(4875):99–102. doi: 10.1126/science.2459777. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Fesce R., Meldolesi J. Spontaneous [Ca2+]i fluctuations in rat chromaffin cells do not require inositol 1,4,5-trisphosphate elevations but are generated by a caffeine- and ryanodine-sensitive intracellular Ca2+ store. J Biol Chem. 1990 Feb 25;265(6):3005–3008. [PubMed] [Google Scholar]
- Meldolesi J., Madeddu L., Pozzan T. Intracellular Ca2+ storage organelles in non-muscle cells: heterogeneity and functional assignment. Biochim Biophys Acta. 1990 Nov 12;1055(2):130–140. doi: 10.1016/0167-4889(90)90113-r. [DOI] [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. Agonist-induced cytosolic calcium oscillations originate from a specific locus in single hepatocytes. J Biol Chem. 1990 Jun 25;265(18):10792–10796. [PubMed] [Google Scholar]
- Sanchez-Bueno A., Dixon C. J., Woods N. M., Cuthbertson K. S., Cobbold P. H. Inhibitors of protein kinase C prolong the falling phase of each free-calcium transient in a hormone-stimulated hepatocyte. Biochem J. 1990 Jun 15;268(3):627–632. doi: 10.1042/bj2680627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Bueno A., Dixon C. J., Woods N. M., Cuthbertson K. S., Cobbold P. H. The hepatocyte calcium oscillator. Adv Second Messenger Phosphoprotein Res. 1990;24:115–121. [PubMed] [Google Scholar]
- Sanchez-Bueno A., Marrero I., Cobbold P. H. Different modulatory effects of elevated cyclic AMP on cytosolic Ca2+ spikes induced by phenylephrine or vasopressin in single rat hepatocytes. Biochem J. 1993 Apr 1;291(Pt 1):163–168. doi: 10.1042/bj2910163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan-Barmatz V. High affinity ryanodine binding sites in rat liver endoplasmic reticulum. FEBS Lett. 1990 Apr 24;263(2):317–320. doi: 10.1016/0014-5793(90)81403-b. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V., Zhang G. H., Garretson L., Kraus-Friedmann N. Distinct ryanodine- and inositol 1,4,5-trisphosphate-binding sites in hepatic microsomes. Biochem J. 1990 Jun 15;268(3):699–705. doi: 10.1042/bj2680699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi R., Stucki J. W. Hormone-induced calcium oscillations in liver cells can be explained by a simple one pool model. J Biol Chem. 1991 Jun 15;266(17):11068–11077. [PubMed] [Google Scholar]
- Thomas A. P. Enhancement of the inositol 1,4,5-trisphosphate-releasable Ca2+ pool by GTP in permeabilized hepatocytes. J Biol Chem. 1988 Feb 25;263(6):2704–2711. [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987 Feb;8(1):79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]


